| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 5, May 2023, pages 149-154
A Rare Case of Congestive Heart Failure due to Isolated Aortic Valve Disease in a Middle-Aged Man Secondary to Rheumatic Fever
Varshitha Tumkur Pandurangaa, c, Asher Gorantlaa, b, Asad Ahmeda, b, Jacob Sabua, b, Mary Mallappallila, b, Sabu Johna, b
aDivision of Cardiology, Department of Medicine, Kings County Hospital and SUNY Downstate Medical Centre, Brooklyn NY, USA
bKings County Hospital and SUNY Downstate Medical Centre, Brooklyn, NY, USA
cCorresponding Author: Varshitha Tumkur Panduranga, St. George's University School of Medicine, Grenada, West Indies
Manuscript submitted April 7, 2023, accepted April 27, 2023, published online May 31, 2023
Short title: CHF due to AS Secondary to Rheumatic Fever
doi: https://doi.org/10.14740/jmc4090
| Abstract | ▴Top |
Rheumatic heart disease (RHD) is commonly seen in people from developing and low-income countries. More cases are being recorded in developed countries due to migration and globalization. RHD develops in people with a history of rheumatic fever; it is an autoimmune response to group A streptococcal infection due to similarities at the molecular level. Congestive heart failure, arrhythmia, atrial fibrillation, stroke, and infective endocarditis are a few of the many complications associated with RHD. Here we present a case of a 48-year-old male with a past medical history of rheumatic fever at the age of 12 years, who presented to the emergency room (ER) complaining of bilateral ankle swelling, dyspnea on exertion, and palpitations. The patient was tachycardic with a heart rate of 146 beats per minute and tachypneic with a respiratory rate of 22 breaths per minute. On physical exam, there was a harsh systolic and diastolic murmur at the right upper sternal border. A 12-lead electrocardiogram (EKG) revealed atrial flutter with a variable block. Chest X-ray revealed an enlarged cardiac silhouette with a pro-brain natriuretic peptide (proBNP) of 2,772 pg/mL (normal ≤ 125 pg/mL). The patient was stabilized with metoprolol and furosemide and was admitted to the hospital for further investigation. Transthoracic echocardiogram showed left ventricular ejection fraction (LVEF) of 50-55% with severe concentric hypertrophy of the left ventricle with a severely dilated left atrium. Increased thickness of the aortic valve with severe stenosis and a peak gradient of 139 mm Hg and a mean gradient of 82 mm Hg was noted. The valve area was measured to be 0.8 cm2. Transesophageal echocardiogram showed a tri-leaflet aortic valve with commissural fusion of valve cusps with severe leaflet thickening consistent with rheumatic valve disease. The patient underwent tissue aortic valve replacement with a bioprosthetic valve. The pathology report showed extensive fibrosis and calcification of the aortic valve. The patient came in for a follow-up visit 6 months later and expressed feeling better and more active.
Keywords: Rheumatic fever; Rheumatic heart disease; Aortic stenosis; Congestive heart failure
| Introduction | ▴Top |
Isolated aortic valve disease secondary to rheumatic fever is rare in a middle-aged person. Even though rheumatic heart disease (RHD) is rare in industrialized nations, we are seeing more cases due to migration from developing nations [1]. RHD is a sequela of rheumatic fever caused by Streptococcus pyogenes. It is an autoimmune inflammation of cardiac valves that can be concomitant with fever, arthritis, chorea, and skin manifestations. Sixty-five percent of the patients with a history of rheumatic fever develop RHD [2]. Pathogenesis of RHD involves structural similarities between the group A Streptococcus (GAS) antigen and host cardiac myosin and tropomyosin, leading to autoimmune inflammation of the heart involving both the humoral and cell-mediated immune system. An elevated level of anti-GAS antibodies is correlated with valvulitis [3]. RHD mainly affects the mitral valve causing stenosis or regurgitation. An observational echocardiographic study in Bangladesh recorded mitral stenosis to be the most common lesion followed by mitral regurgitation, tricuspid regurgitation, and aortic regurgitation [4]. Isolated aortic stenosis (AS) secondary to rheumatic fever is uncommon. Out of the 858 patients who had rheumatic aortic valve disease in a cross-sectional study, only 9.7% had isolated AS [5]. Pathological study of rheumatic valves revealed leaflet thickening, commissural fusion, and eventual calcification of cardiac valves [1]. It has been shown that valve replacement has led to decreased levels of serum anti-GAS which further reduces the risk of secondary valve abnormalities [3].
| Case Report | ▴Top |
Investigations
A 48-year-old male with a past medical history of rheumatic fever at age of 12 years presented to the emergency department (ED) complaining of bilateral ankle swelling and shortness of breath on exertion. The lower extremity edema had started 2 weeks before his arrival at the ED and had been progressively worsening. Shortness of breath was not present at rest. The patient mentioned that he was able to walk several blocks before but had been experiencing dyspnea recently with walking only a few steps. He also experienced dyspnea while supine and described it as “like I am not connected to my lungs”. Additionally, the patient also complained of palpitations. He had been working as a construction site worker for more than 20 years, but he had been unable to work due to his symptoms. The patient was originally from Jamaica and had been living in the USA for the past 26 years. The patient was also a sickle cell carrier. He denied vision abnormalities, skin changes, bone or joint pain, headache, or dizziness.
In the ED, the patient was afebrile with a heart rate of 146 beats/minute, respiratory rate of 22 breaths/min, and oxygen saturation of 97% on room air. On physical exam, a harsh systolic murmur was recorded at the right upper sternal border along with a diastolic murmur. There was also a systolic murmur at the apex. There was bilateral lower extremity pitting edema. Electrocardiogram (EKG) revealed wide complex tachycardia with right bundle branch block and a repeat EKG confirmed atrial flutter with a variable block (1:2 or 1:3). Laboratory results revealed significantly elevated pro-brain natriuretic peptide (proBNP) to 2,772 pg/mL (normal ≤ 125 pg/mL) which is consistent with heart failure (HF) as shown in Table 1.
 Click to view | Table 1. Initial Laboratory Values on Admission |
Chest X-ray revealed an enlarged cardiac silhouette. The patient was initially stabilized with metoprolol 50 mg and furosemide 20 mg, but the heart rate did not improve. The patient was then given an esmolol drip which reduced the heart rate to normal. The patient was transferred to the critical care unit for further investigation and monitoring.
Diagnosis
Transthoracic echocardiogram showed 50-55% ejection fraction, severe concentric left ventricular hypertrophy, and a severely dilated left atrium. The aortic valve was extensively thickened and calcified with severe stenosis as shown in Figures 1 and 2. Additionally, severe aortic regurgitation was also recorded as shown in Figure 3. Right ventricular systolic function was low with mild dilation of the cavity. The aortic valve area was recorded to be 0.8 cm2. Findings point towards blood backing up in the left atria due to severe AS causing atrial dilation and flutter. Long-standing stenosis has led to the development of concentric left ventricle hypertrophy.
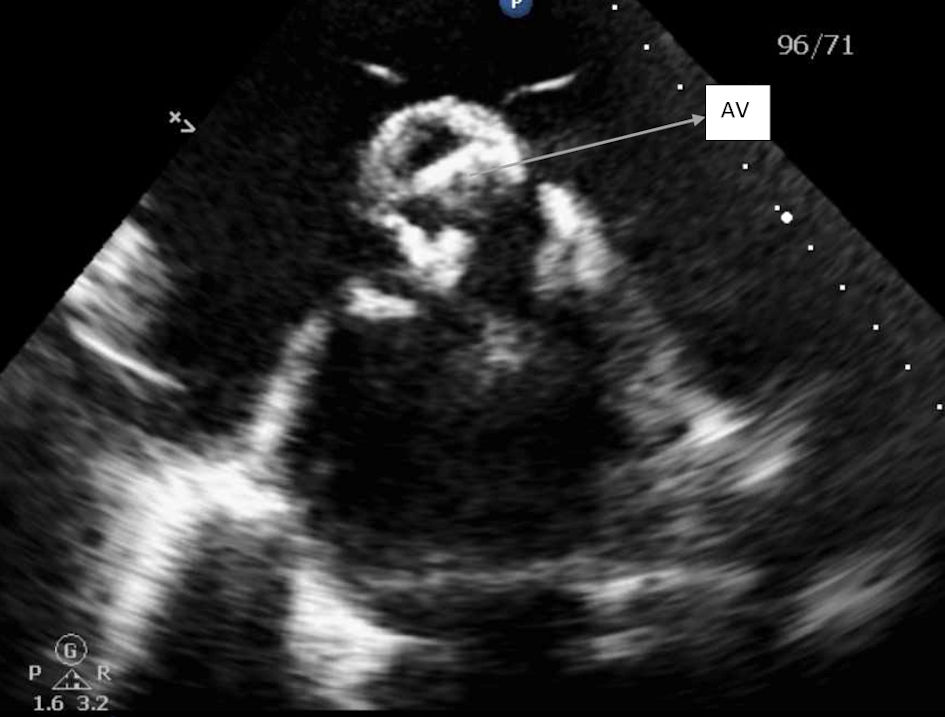 Click for large image | Figure 1. Parasternal short axis view of aortic valve, showing severely calcified aortic leaflets. AV: aortic valve. |
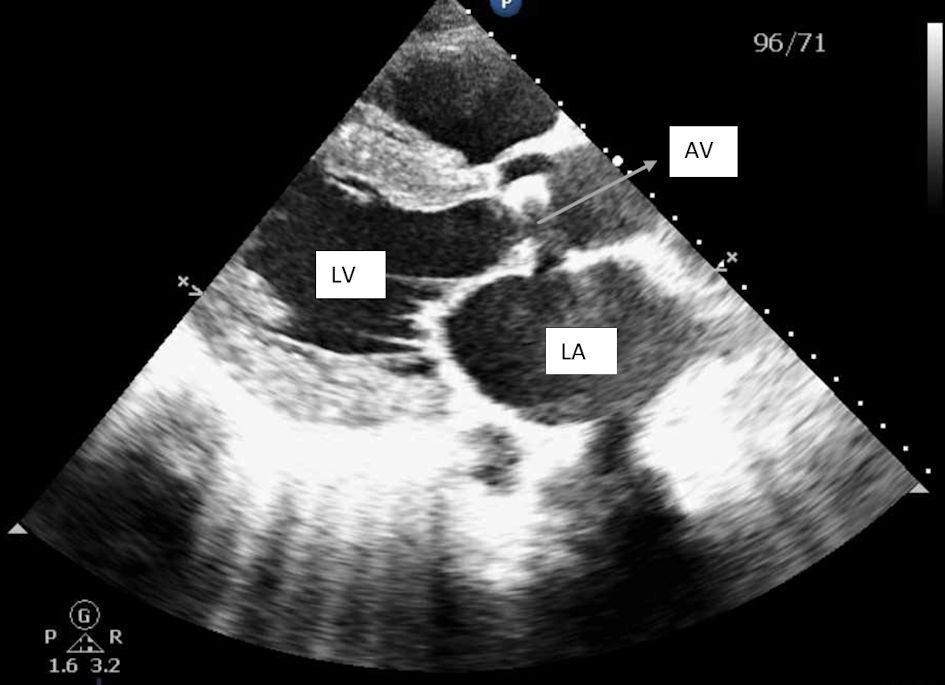 Click for large image | Figure 2. Parasternal long axis view showing left ventricular hypertrophy and severely dilated left atrium and calcified aortic valves. LV: left ventricle; LA: left atrium; AV: aortic valve. |
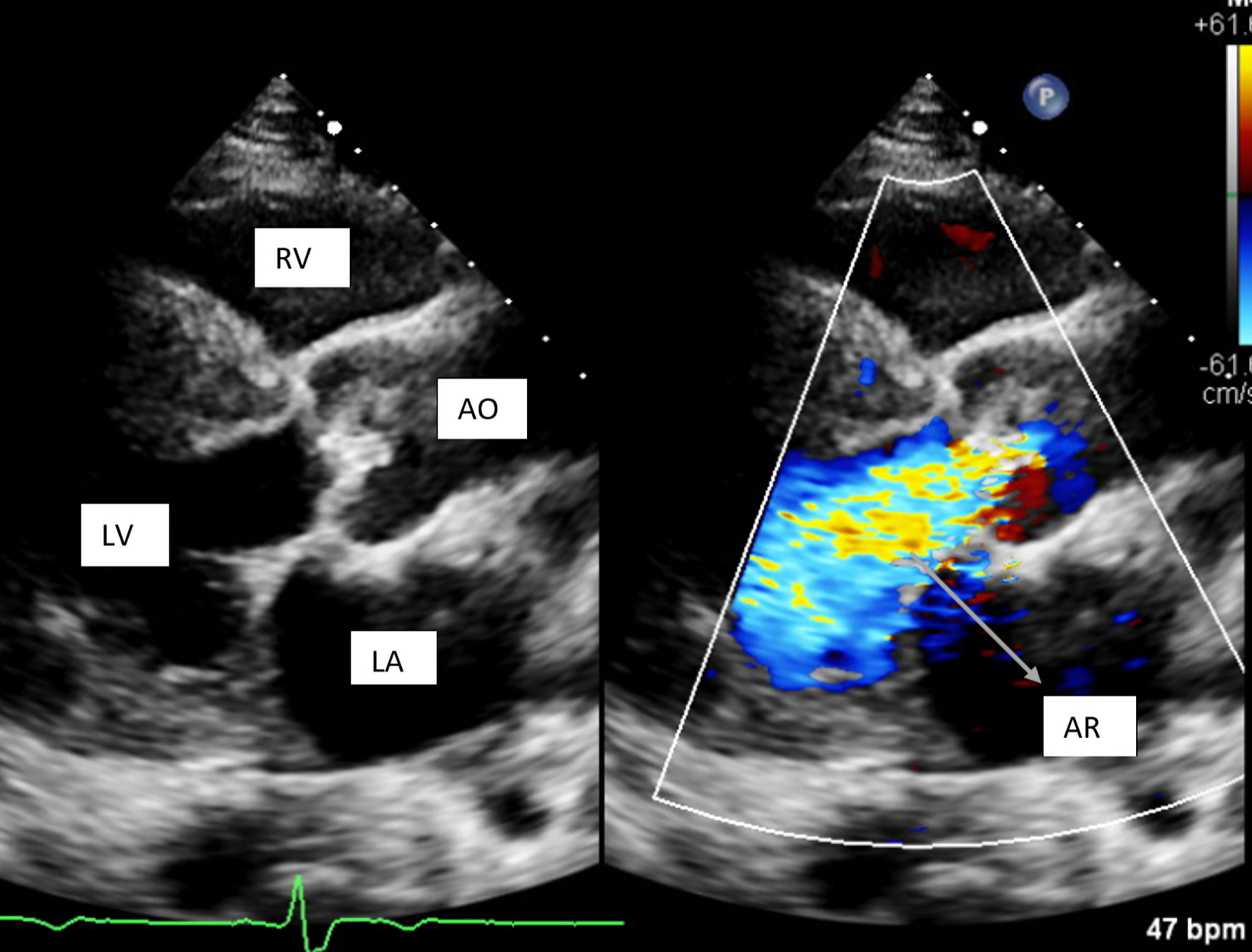 Click for large image | Figure 3. Parasternal long axis view showing severe aortic regurgitation. RV: right ventricle; LV: left ventricle; LA: left atrium; AO: aorta; AR: aortic regurgitation. |
Transoesophageal echocardiogram showed a tri-leaflet aortic valve with commissural fusion of the cusps and leaflet thickening consistent with rheumatic valve changes. The valve area calculated by planimetry was 1.0 cm2 as shown in Figure 4. The peak and mean aortic valve gradients were recorded to be 139 mm Hg and 82 mm Hg, respectively. Calculated valve area by continuity equation was 0.8 cm2.
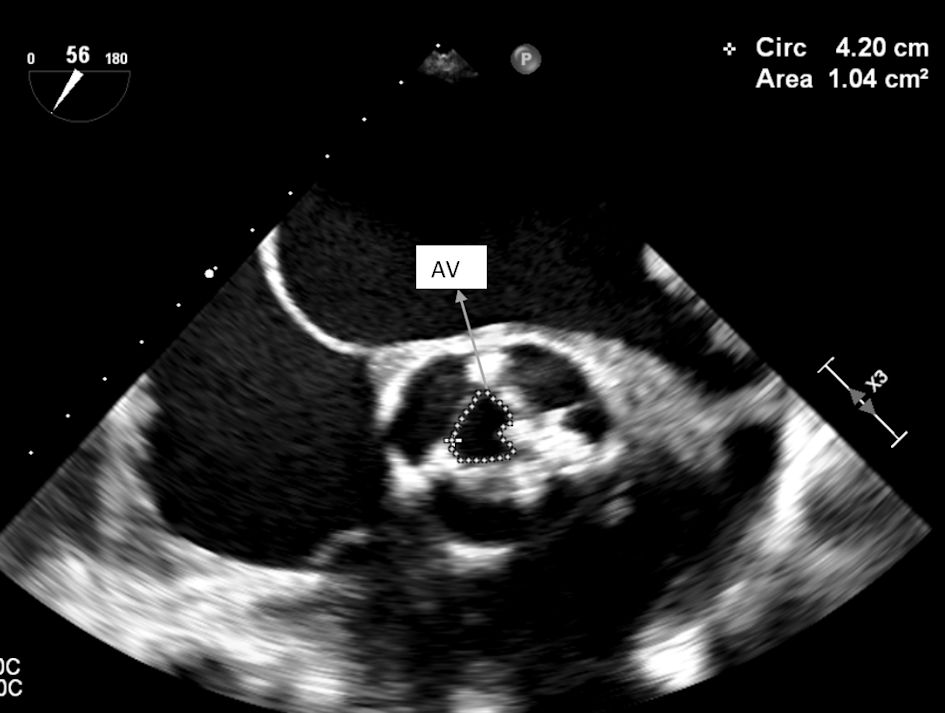 Click for large image | Figure 4. TEE showing aortic valve area by planimetry (1.0 cm2). TEE: transesophageal echocardiography; AV: aortic valve. |
Computed tomography (CT) chest without contrast showed cardiomegaly with bilateral atrial enlargement. Severe aortic calcifications with mild ascending aortic ectasia measuring 42 mm in diameter were also observed. Carotid duplex revealed less than 50% stenosis in both right and left proximal internal carotid artery and distal internal carotid artery. The vertebral artery showed anterograde flow. Cardiac catheterization showed minimal non-obstructive coronary artery disease. A diagnosis of acute congestive HF due to AS was established. Surgical pathology report recorded extensive fibrosis of the aortic valve along with calcification and necrosis. The structural and pathological changes in the aortic valve are consistent with the changes seen in rheumatic aortic valve disease.
Treatment
The patient was initially stabilized with metoprolol 50 mg and furosemide 20 mg, but the heart rate remained high. The patient was then started on an esmolol drip which brought the heart rate to normal. The patient was also fully anticoagulated with enoxaparin and referred to cardiothoracic surgery for aortic valve replacement. The patient underwent tissue aortic valve replacement with a 25-mm Inspiris Resila bioprosthetic valve. Bilateral pulmonary vein isolation using ArtiCure radiofrequency ablation was also done, along with clipping of the left atrial appendage with #45 ArtiClip. The patient’s symptoms resolved and was discharged home on aspirin 81 mg and apixaban.
Follow-up and outcomes
The patient came in for a follow-up visit a few months later to the cardiology clinic and expressed that he felt much better. He was active and able to function normally without any shortness of breath. He was able to resume his work. The patient was advised to stop apixaban but continue aspirin 81 mg daily.
| Discussion | ▴Top |
The prevalence of RHD, which is the most common valvular heart disease, is increasing in developing nations and amongst impoverished and indigenous populations of developed nations. This might be due to the increased survival of patients and the availability of diagnostic methods like echocardiography for the detection of RHD [6]. RHD is irreversible damage to heart valves caused secondary to acute rheumatic fever (ARF). ARF is an autoimmune response to the pharyngeal infection caused by Streptococcus pyogenes, also called GAS. RHD involves various bacterial antigens like M, T, and R proteins, as well as N-acetylglucosamine, which are structurally similar to cardiac myosin, vimentin, laminin, and tropomyosin in hosts [3]. The antigen-presenting cells recognize and process bacterial antigens leading to antibody production. These antibodies cross-react with human antigens due to their similar structures causing long-term damage to cardiac valves [7].
AS in RHD involves extensive leaflet thickening along with calcification. Autoimmune-induced inflammation and increased expression of vascular endothelial growth factor (VEGF) lead to neo-vascularization. This causes the deposition of minerals leading to calcification [7]. The affected valves also show commissural fusion and fibrosis [8]. A study conducted in China collected aortic valve samples from RHD patients undergoing valve replacement to study the pathogenesis of rheumatic heart valves. Histopathology of the valves showed hyaline degeneration and infiltration of broken matrix fibers suggesting fibro-myxomatous degeneration. Significant infiltration of monocytes and macrophages was noted on immunostaining along with increased inflammatory gene expression in the rheumatic aortic valves. Additionally, the serum level of N-terminal proBNP, a cardiac function marker was also found to be high. This indicated poor left ventricular and aortic valve function further supporting the results of increased left atrial diameter, left ventricular end-diastolic diameter, and aortic mean gradient seen in these patients [9].
Calcific AS has two main etiologies in different age groups. Congenitally abnormal bicuspid aortic valve leads to the development of calcific AS in a younger population due to its abnormal valve geometry and mechanical stress. In contrast, calcific AS in the elderly is due to a degenerative process characterized by inflammation, atherosclerosis, calcium deposition, and ossification. Calcified AS in a middle-aged man without a congenitally abnormal valve is uncommon, unless due to a secondary pathology like RHD [7]. RHD predominately affects the mitral valve in almost all cases, but other valves also may be involved, like the aortic or tricuspid, either affected on their own or also in conjunction with the mitral valve. Mitral stenosis is the most common valvular lesion seen in RHD. Rheumatic aortic valve disease (predominately regurgitation) is commonly seen in combination with rheumatic mitral valve disease [7]. Isolated AS due to a rheumatic pathology is very uncommon. According to a large prospective study in Africa, rheumatic AS was recorded to be present in only 9% of the study population [10]. The overall prognosis of aortic valve disease is poor as it severely affects the left ventricular function and cardiac output.
A retrospective study in Kenya analyzed the echocardiogram of 582 RHD patients. The study revealed the overall mean age of RHD patients to be 21.1 ± 12 years, peaking at 14 - 18 years of age, emphasizing that RHD is commonly seen in children and young adults [11]. Although this is true in developing countries, the time between the initial episode of rheumatic fever and the onset of valve disease ranges from a few years to more than 20 years. A study in Brazil screened a total of 488 adults with a mean age of 40 ± 15 years, and a prevalence of 39/1,000 cases of RHD cases was recorded indicating that RHD can be seen in middle-aged patients [12].
RHD is one of the leading causes of HF in young and middle-aged patients. According to a retrospective study of HF patients, 35.5% of the total patients had RHD to be the underlying cause of their HF, as well as 12.8% and 61.6% of the young and middle-aged subgroups, respectively [13]. HF in young adults is infrequent since most patients present late in the course of the disease [13]. Symptoms of heart failure with preserved ejection fraction (HFpEF) include shortness of breath, chest pain, palpitation, and syncope which are related to diastolic dysfunction [14]. Concentric hypertrophy is observed in patients with AS which leads to diastolic dysfunction. This decreases anterograde blood flow and stagnation of blood in the heart chambers further leading to complications like atrial fibrillation and thrombus formation. A prospective cohort study recorded increased atrial fibrosis and collagen type III synthesis along with extracellular remodeling in patients with severe AS [15]. These changes can trigger the aberrant electrical activity of the atria leading to fibrillation and flutter.
The American Heart Association (AHA) describes the stages of AS as a continuum. Stage A includes those at risk of AS development; stage B is mild-to-moderate AS; stage C in patients with severe AS without symptoms and having a normal left ventricular ejection fraction (LVEF) at 50% or higher; stage D1 is symptomatic, severe, high-gradient AS; D2 is symptomatic low-gradient AS with LVEF less than 50%; and D3 is symptomatic severe, low-flow, low-gradient AS with LVEF more than or equal to 50%. To further elaborate on stage D1, which is exhibited by the patient discussed in this case report, it involves severe, high-gradient AS with severe calcific or rheumatic changes, reduced leaflet motion, Vmax more than 4 m/s, or mean transaortic pressure gradient at more than 40 mm Hg. According to AHA, these patients have 50% mortality at 1 year and 70-80% mortality at 2 years without aortic valve replacement [16]. Aortic valve replacement with a prosthetic valve is a recommended surgery performed in patients with rheumatic aortic valve disease. All patients, irrespective of age, should be offered valve replacement. It is indicated for improvement in symptoms, improved left ventricular function, and increased quality of life as patients were able to resume their normal routine activities [17]. It is also noted that serum levels of anti-GAS antibodies substantially decrease after valve replacement, indicating a low risk of secondary valvular abnormalities [3].
Conclusions
Even though RHD is rare in developed nations, more cases are being reported due to globalization. It is important to assess the history of rheumatic fever in patients presenting with valvular heart disease. Isolated AS and aortic regurgitation is a rare presentation of RHD in both developed and developing nations.
Learning points
Isolated rheumatic AS is rare but a potential complication of rheumatic fever. It mainly presents in middle-aged patients as calcific AS with severe left ventricular diastolic dysfunction. Surgical aortic valve replacement is the treatment of choice for rheumatic aortic valvopathy in all patient populations.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Case was identified by supervising author Dr. Sabu John. Initial manuscript was written by Varshitha Tumkur Panduranga. Editing was done by Jacob Sabu. The echocardiogram pictures were identified by Dr. Asher Gorantla and confirmed by Dr. Sabu John. Literature search and references were done by Dr. Mary Mallappallil, Dr. Asad Ahmed and Jacob Sabu. Final manuscript was reviewed by Varshitha Tumkur Panduranga, Dr. Sabu John, Jacob Sabu, Dr. Asher Gorantla, Dr. Mary Mallappallil and Dr. Asad Ahmed. Final manuscript was approved by supervising physician Dr. Sabu John.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Okor I, Bob-Manuel T, Garikapati K, Baldawi H, Gillies C, Ibebuogu UN. Transcatheter aortic valve replacement in rheumatic aortic stenosis: a comprehensive review. Curr Probl Cardiol. 2021;46(12):100843.
doi pubmed - Kumar RK, Tandon R. Rheumatic fever & rheumatic heart disease: the last 50 years. Indian J Med Res. 2013;137(4):643-658.
pubmed pmc - Passos LSA, Nunes MCP, Aikawa E. Rheumatic heart valve disease pathophysiology and underlying mechanisms. Front Cardiovasc Med. 2020;7:612716.
doi pubmed pmc - Singha CK, Khaled FI, Mahabub SM, Joarder AI, Arzu J, Adhikary DK, Banerjee SK, et al. Echocardiographic assessment of valvular involvement in chronic rheumatic heart disease in BSMMU, Bangladesh. Mymensingh Med J. 2022;31(1):149-153.
pubmed - Talwar S, Saikrishna C, Saxena A, Kumar AS. Aortic valve repair for rheumatic aortic valve disease. Ann Thorac Surg. 2005;79(6):1921-1925.
doi pubmed - Aluru JS, Barsouk A, Saginala K, Rawla P, Barsouk A. Valvular heart disease epidemiology. Med Sci (Basel). 2022;10(2):32.
doi pubmed pmc - Leal M, Passos LSA, Guarconi FV, Aguiar JMS, Silva R, Paula TMN, Santos RFD, et al. Rheumatic heart disease in the modern era: recent developments and current challenges. Rev Soc Bras Med Trop. 2019;52:e20180041.
doi pubmed - Zuhlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, Mauff K, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015;36(18):1115-1122a.
doi pubmed pmc - Xiao F, Zheng R, Yang D, Cao K, Zhang S, Wu B, Shao Y, et al. Sex-dependent aortic valve pathology in patients with rheumatic heart disease. PLoS One. 2017;12(6):e0180230.
doi pubmed pmc - Remenyi B, ElGuindy A, Smith SC, Jr., Yacoub M, Holmes DR, Jr. Valvular aspects of rheumatic heart disease. Lancet. 2016;387(10025):1335-1346.
doi pubmed - Koech MM, Barasa F, Ng'eno TK. Why prevention of rheumatic heart disease should be a component of primary healthcare. J Trop Pediatr. 2012;58(5):414-415.
doi pubmed - Wegener A, Holm AE, Gomes LC, Lima KO, Kaagaard MD, Matos LO, Vieira IVM, et al. Prevalence of rheumatic heart disease in adults from the Brazilian Amazon Basin. Int J Cardiol. 2022;352:115-122.
doi pubmed - Ogbemudia EJ, Umuerri EM. Relevance of rheumatic valvular heart disease in the aetiology of heart failure in contemporary times. West Afr J Med. 2021;38(3):241-245.
pubmed - Santos Mateo JJ, Sabater Molina M, Gimeno Blanes JR. Hypertrophic cardiomyopathy. Med Clin (Barc). 2018;150(11):434-442.
doi pubmed - Fragao-Marques M, Miranda I, Martins D, Barroso I, Mendes C, Pereira-Neves A, Falcao-Pires I, et al. Atrial matrix remodeling in atrial fibrillation patients with aortic stenosis. BMC Cardiovasc Disord. 2020;20(1):468.
doi pubmed pmc - Kanwar A, Thaden JJ, Nkomo VT. Management of patients with aortic valve stenosis. Mayo Clin Proc. 2018;93(4):488-508.
doi pubmed - Sampath Kumar A, Talwar S, Saxena A, Singh R. Ross procedure in rheumatic aortic valve disease. Eur J Cardiothorac Surg. 2006;29(2):156-161.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


