| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 11, November 2023, pages 362-368
Percutaneous Intracardiac Mass Extraction in High Surgical-Risk Patients
Mariam Riada, Mustafeez Ur Rahmana, Rajasekhar Mulyalaa, Nadia Sayyedb, Danielle Bayera, Bassam Omara, c
aCardiology Division, University of South Alabama, Mobile, AL 36617, USA
bDepartment of Internal Medicine, Khyber Medical College, Peshawar, Pakistan
cCorresponding Author: Bassam Omar, Cardiology Division, University of South Alabama, Mobile, AL 36617, USA
Manuscript submitted October 19, 2023, accepted November 3, 2023, published online November 23, 2023
Short title: Percutaneous Intracardiac Mass Extraction
doi: https://doi.org/10.14740/jmc4150
| Abstract | ▴Top |
Large intracardiac masses including tumors, thrombi, and vegetations result in detrimental embolic or obstructive sequelae and present a management dilemma. Open heart surgery, the traditional approach, may not be an option for many patients with a prohibitive surgical risk due to multiple comorbidities. Recently, percutaneous options have emerged with reported success in extracting such intracardiac masses. A 42-year-old female with history of advanced primary sclerosing cholangitis with decompensated liver cirrhosis causing ascites and variceal bleed presented to the emergency department with fatigue, subjective fevers, chills and melena. Laboratory results revealed neutrophil-predominant leukocytosis and normocytic anemia, and blood cultures were positive for Candida albicans. Electrocardiography showed sinus tachycardia. Chest X-ray was unremarkable. She underwent packed red blood cell transfusion and esophageal banding for variceal bleeding. Transthoracic echocardiogram revealed normal left ventricular ejection fraction and no wall motion abnormalities. A right atrial mobile mass measuring approximately 1.0 × 3.0 cm was noted. Multidisciplinary heart team discussion concluded that while the mass posed a high embolic risk, the patient had a prohibitive risk for surgical intervention. Successful percutaneous removal of the mass using Penumbra system device (Penumbra Incorporated, Alameda, CA) was accomplished. This case report details the procedure and outcomes, as well as presents a literature review.
Keywords: Intracardiac mass; Penumbra device; High surgical risk; Candidemia; Vegetation
| Introduction | ▴Top |
Intracardiac masses are diverse and their clinical presentation is usually a reflection of the specific pathogenic potential of the involved mass [1]. They are often incidental findings on imaging studies [2]. They present a management dilemma due to their potential detrimental effect [3] in patients who otherwise may have multiple comorbidities and may not be suitable candidates for surgical intervention.
The recent emergence of percutaneous options dedicated to the extraction of intracardiac masses [4] provides promise for a nonsurgical option of timely removal or debulking, thereby mitigating the hazard of prohibitive surgical risk or watchful waiting of medical therapy. We present in this report an intriguing case of a highly mobile right atrial mass with an increased risk for embolization to the pulmonary circulation, in the setting of prohibitive surgical risk. Successful percutaneous extraction of the mass resulted in early patient recovery and discharge in a stable condition.
| Case Report | ▴Top |
Investigations
A 42-year-old female with a history of advanced primary sclerosing cholangitis with decompensated liver cirrhosis due to ascites, variceal bleeding, and ulcerative colitis presented to the emergency department with fatigue, subjective fevers, chills and melena. Physical examination was remarkable for tachycardia with normal S1 and S2 and no murmurs. She otherwise had diffuse abdominal tenderness and distention. Laboratory results revealed unremarkable complete metabolic panel except for an elevated alkaline phosphatase of 589 U/L and a total bilirubin of 4.3 mg/dL. Complete blood count was notable for leukocytosis, with white blood cell count of 1.704 × 104/µL which rapidly increased to 3.38 × 104/µL, and anemia, with hemoglobin of 7.8 g/dL which rapidly declined to 6.3 g/dL within 2 days. Initial electrocardiogram (Fig. 1) revealed sinus tachycardia with a rate of 116 bpm. The patient underwent prompt transfusion and esophagogastroduodenoscopy (EGD), which revealed bleeding varices that were successfully banded.
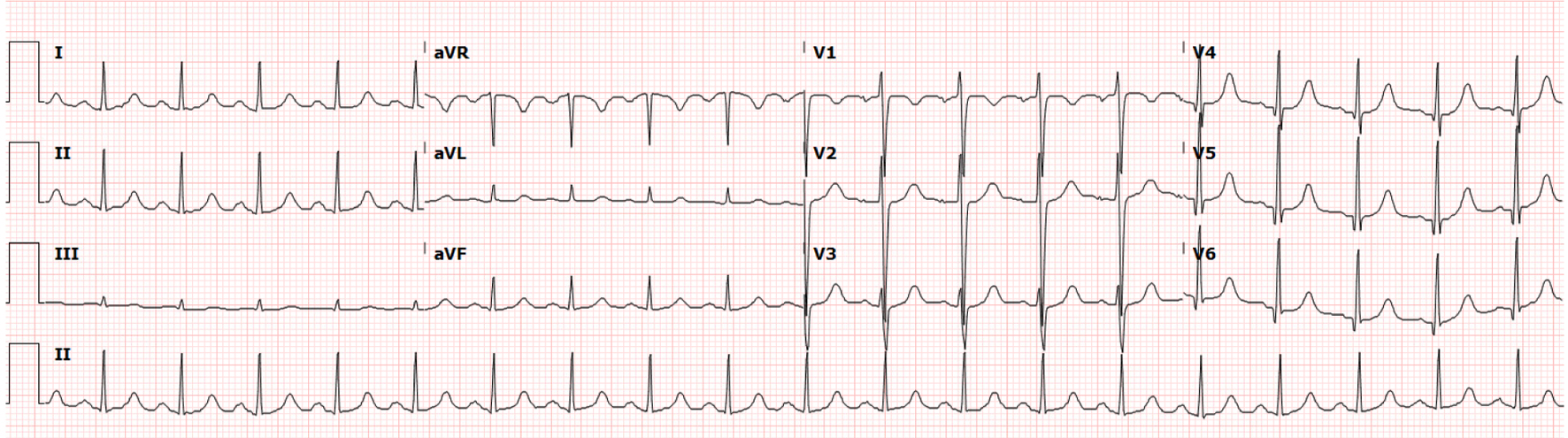 Click for large image | Figure 1. Electrocardiogram at time of presentation to the emergency department. |
Diagnosis
She developed febrile episodes during her hospitalization and her blood cultures grew Candida albicans; thus, intravenous (IV) anti-fungal treatment with micafungin was started. As part of her infectious work-up, a transthoracic echocardiogram was performed which revealed normal left ventricular ejection fraction without segmental wall-motion abnormalities. There was a large right atrial mobile mass measuring approximately 1.0 × 3.0 cm, likely representing thrombus versus vegetation (Fig. 2). Trace tricuspid regurgitation was reported.
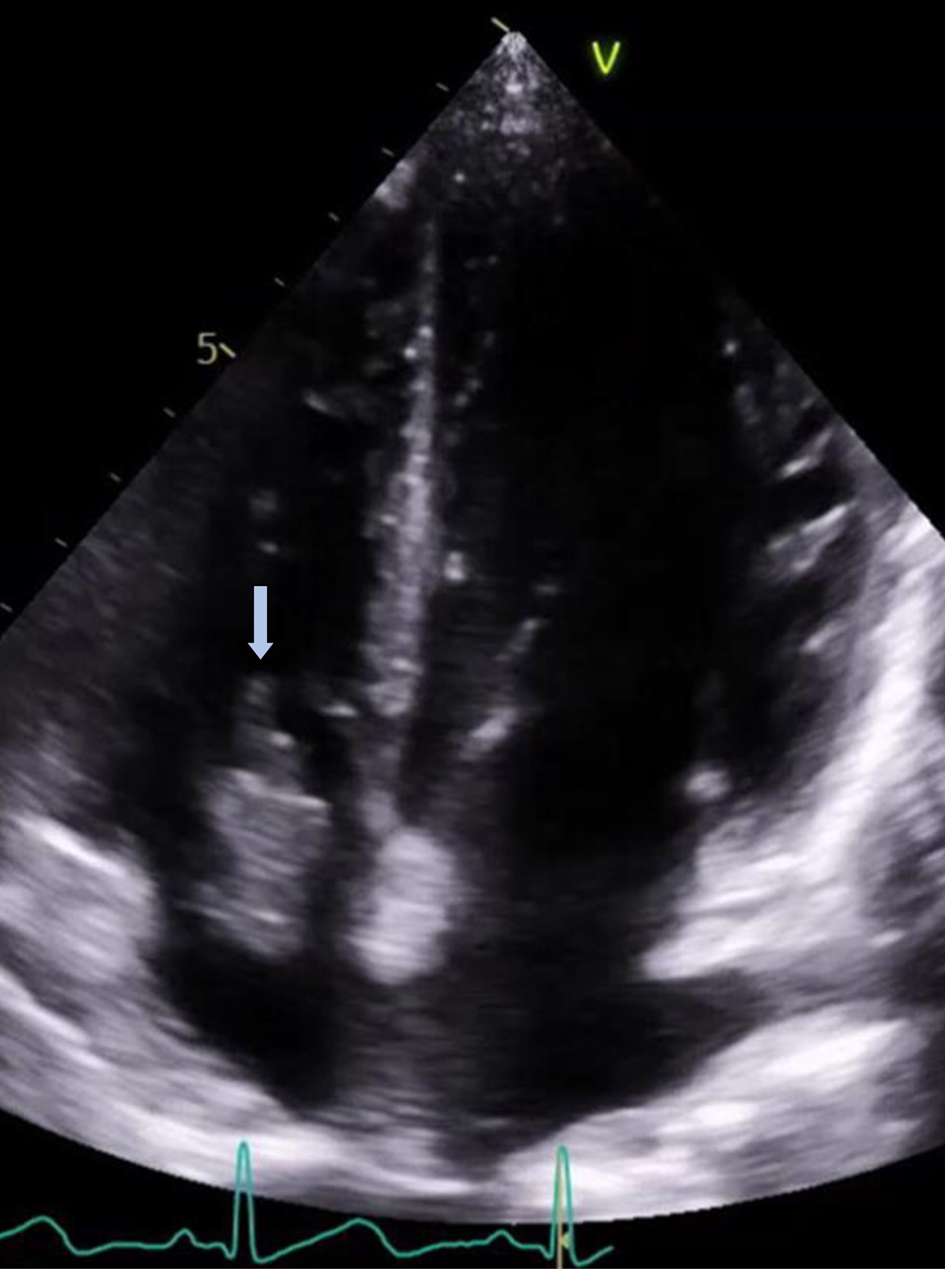 Click for large image | Figure 2. Transthoracic echocardiogram showing the large right atrial echodensity (arrow) protruding through the tricuspid valve. |
Treatment
The patient was continued on IV anti-fungal therapy without resolution of the right atrial mass after 1 week of treatment. A multidisciplinary heart team meeting was convened and concluded that she was at a prohibitively elevated risk for surgical removal of the mass given her multiple comorbidities, variceal bleeding, and ongoing septicemia, with the recommendation to pursue a percutaneous alternative. Based on the clinical presentation and the imaging studies, it was felt that it would be insufficient to continue anti-fungal therapy alone without percutaneous intervention. The patient agreed to undergo percutaneous mass extraction.
In the catheterization lab, bilateral femoral veins were accessed using Seldinger technique with ultrasound guidance, as a precaution in case of massive pulmonary embolization. Venogram performed through a 6-F Pigtail catheter revealed no masses extending into either the superior or inferior vena cava. Venous access was secured using 18-F Gore DrySeal Flex Sheath (W. L. Gore & Associates, Newark, DE) inserted over a Supra Core guidewire (Abbott Cardiovascular, Plymouth, MN). Penumbra Lightning 12 aspiration catheter (Fig. 3) was advanced into a Flex sheath, and with echo guidance was anchored at the right atrial mass. Aspiration was applied using the Lightning Intelligent Aspiration System. The Penumbra catheter was then withdrawn to the tip of the Flex catheter and both were retracted en block through the Gore DrySeal sheath along with the adherent mass. The Gore DrySeal was removed and a previously placed PerClose device (Abbott Cardiovascular, Plymouth, MN) was deployed with adequate hemostasis; a suture was placed for closure of the venotomy.
 Click for large image | Figure 3. Components of the Penumbra Lightning 12 Intelligent Aspiration System used in our case. |
Follow-up and outcomes
The pathologic examination of the right atrial mass (Fig. 4) was consistent with Candida albicans vegetation. The patient continued to recover well and the plan was to administer IV anti-fungal therapy for 6 weeks. Post-procedure transthoracic echocardiogram (TTE) revealed resolution of the right atrial mass (Fig. 5); mild tricuspid regurgitation was reported. She was discharged home and set up for IV anti-fungal therapy. She followed up with infectious disease service, and a subsequent TTE about 10 days following the procedure revealed no recurrence of the atrial mass with continued clinical stability.
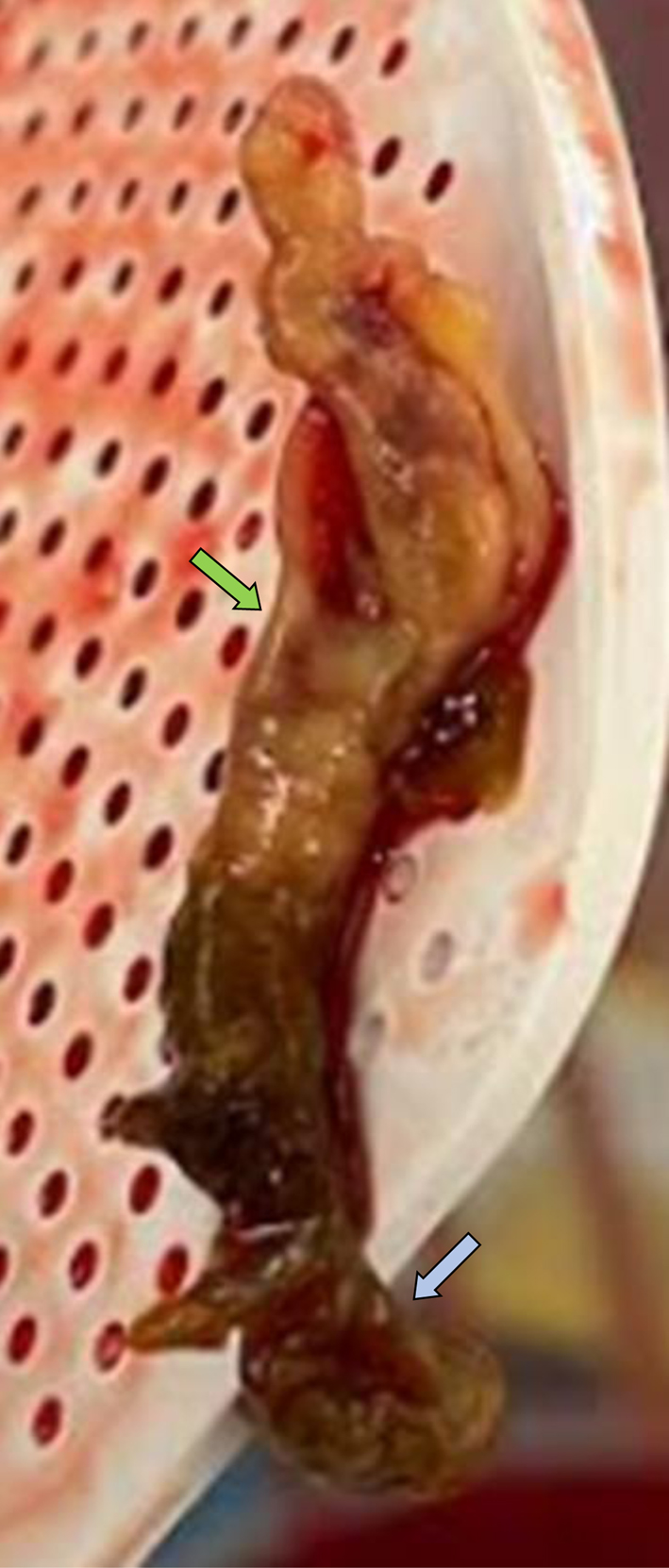 Click for large image | Figure 4. Right atrial mass (Candida vegetation) gross appearance. The green arrow shows the body of the mass, while the blue arrow shows the stalk. |
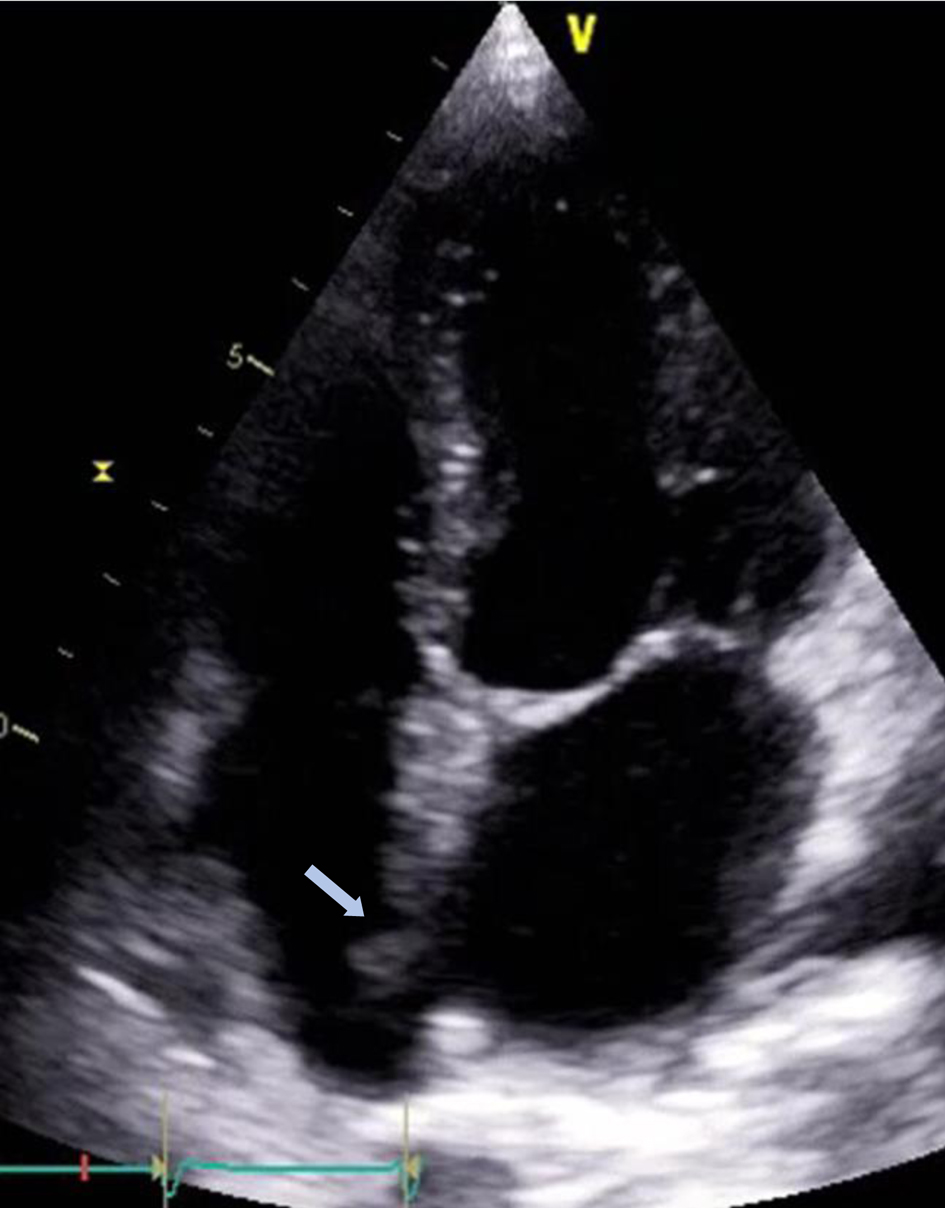 Click for large image | Figure 5. Follow-up transthoracic echocardiogram showing resolution of the right atrial mass (arrow) following percutaneous extraction. |
| Discussion | ▴Top |
Cardiac masses are heterogeneous in pathogenesis and presentation [5] and include primary and secondary cardiac tumors [6], intracardiac thrombi [7], large vegetations [8] and calcified lesions [9]. They are associated with variable manifestations and symptoms due to flow obstructive [10], neurologic sequelae [11], peripheral embolization [12], inflammatory response [13] and electrical disturbance [14]. Although the general incidence of cardiac masses is not clear, a 12-year single-center experience [15] reported an overall echocardiographic prevalence, confirmed by other modalities, of 0.2%, with 0.05% primary benign tumors, 0.004% primary malignant tumors and 0.03% secondary tumors, in addition to 0.1% thrombi.
A TTE is the first-line imaging modality in the diagnosis of intracardiac masses [16], often with the aid of contrast enhancement [17], three-dimensional (3D) TTE [18], transesophageal echocardiography (TEE) [19], and 3D TEE [20], which is especially intraoperatively and in the volumetric assessment of masses [21]. Intracardiac echocardiography has also been employed to guide biopsy of an intracardiac mass [22]. Spectral Doppler imaging, especially pulsed wave tissue Doppler imaging (TDI), for measuring an intracardiac mass mobility, can also be useful in the embolic risk stratification [23]. Computed tomography (CT) is useful as an adjunctive or alternative method for diagnosing and characterizing cardiac masses [24]. Cardiac magnetic resonance imaging can provide further clarity to details not obvious by echocardiography or cardiac CT [25]. All three modalities, when available, may provide complementary information essential in the management of cardiac masses [26]. A potential role for nuclear imaging with metabolic agents (tagged fluorodeoxyglucose) combined with CT ([18F]FDG-PET/CT) has also been demonstrated [27]. Multimodality imaging is often needed for better delineation of the anatomic and physiologic aspects of an intracardiac mass and helps plan management [28].
Cardiac masses, especially malignant tumors, carry a high mortality rate as they often are diagnosed in patients with multiple advanced comorbidities and malignancies [29]. The embolic potential and mortality of predominantly left-sided tumors were attributed to the histological type rather than implantation site of the tumor in one study [30]. Surgical resection remains a desired curative and often well-tolerated treatment when medical treatment alone is not expected to provide sufficient or timely resolution of an intracardiac mass [31]. Surgical intervention however may not be an ideal option for some patients when their risk of open heart surgery is very high or prohibitive, and medical management alone is not anticipated to alleviate the potential obstructive, embolic, infectious or arrhythmic sequelae of the mass. Percutaneous intravascular and intracardiac treatment options have been gradually gaining momentum and currently offer an attractive alternative of care in many high-risk patients. With the increase in the use of percutaneous cardiac interventional options, there have been several studies evaluating the role of catheter-based devices such as AngioVac, Penumbra and Inari in the aspiration of intracardiac masses [32], as compared in Table 1.
 Click to view | Table 1. Comparison of the AngioVac, Penumbra and Inari Systems |
AngioVac system (AngioDynamics Inc., Latham, NY, USA) employs a bypass circuit via extracorporeal membrane oxygenation (ECMO) veno-venous support to extract masses from the venous system through suction generated at the tip of the catheter by a centrifugal pump. This minimizes blood loss by reinfusing the blood back into the circuit while providing active clearance of debris. AngioVac was approved by FDA in 2009 for the removal of unwanted material from the vascular system and has been used as early as 2011 in the removal of complex masses from the right atrium [33]. In a meta-analysis of the AngioVac catheter device system use in caval thromboemboli, right-sided cardiac masses, catheter-related thrombosis, and pulmonary embolism, the success rate was 73.6%, with reported access site complications including bleeding, hematoma, and distal embolization [34]. Rajput et al reported 16 patients who had intracardiac masses aspirated using the AngioVac device catheter system; none had access site complications and all were successfully closed using Perclose device [35].
The Penumbra Lightning Aspiration System is another newer percutaneous catheter device system; it is a delivery system which does not require ECMO as in the AngioVac system. The Penumbra system was initially used in neuro-interventional procedures for cerebrovascular accidents. One study demonstrated it to be of use in debulking vegetations prior to lead extraction [36]. Another report showed an improved clinical outcome in patients suffering submassive pulmonary emboli [37].
The INARI CloTriever system (Inari Medical, Inc., CA, USA) is another system approved by the FDA in 2017 for retrieving clots from the venous system in the setting of deep vein thrombosis [38]. Success has also been reported using the Flowtriever system in the treatment of acute intermediate-risk pulmonary embolism [39]. Its use in the removal of tricuspid valve vegetation was also reported [40]. Furthermore, the system was employed in the aspiration of tumor embolization into the pulmonary artery, providing therapeutic and diagnostic benefit [41].
Our patient developed a relatively large right atrial mass following fungemia related to a previous catheter device. Although success has been reported in treating a similar case with medication [42], conservative management with IV anti-fungal and removal of the catheter alone would not have been expected to clear the infection in our patient. Successful surgical removal of a candida-infected thrombus from the right atrium was previously reported [43]; however, our patient was considered inoperable due to ongoing variceal bleeding necessitating multiple esophageal banding, in the setting of acutely decompensated liver cirrhosis. A multi-disciplinary meeting involving the primary cardiologist, interventional cardiologist, and cardiovascular surgeon was convened to entertain options, and a percutaneous mass removal was recommended. The patient underwent successful percutaneous mass extraction using a Penumbra catheter device.
In conclusion, intracardiac masses are often incidental findings on echocardiography performed in various clinical settings, with heterogeneous pathologies [44]. They carry an imminent threat for detrimental embolism or obstruction, and often portend a poor prognosis. While surgical therapy offers complete removal under direct visualization, such masses are increasingly encountered in patients who may not be good surgical candidates. The percutaneous approach of retrieving or debulking these masses offers a safer and less invasive alternative, perhaps yet another venue where cardiac surgery and interventional cardiology merge [45].
Learning points
Transcatheter therapeutics have progressed exponentially over the past few decades and currently offer a plausible alternative to the removal or debulking of intravascular thrombi and masses in patients deemed high risk for surgery. The recent approval of devices specifically designed for the extraction of intracardiac masses adds more tools to the armamentarium of the cardiologist, offering hope of cure or palliation to many patients previously considered inoperable. It is essential for the treating physician to be familiar with these procedures so that eligible patients can be referred and treated in a timely manner.
Acknowledgments
None to declare.
Financial Disclosure
This work has not received any financial support or funding.
Conflict of Interest
All authors declare no conflict of interest regarding this submission.
Informed Consent
Not applicable as no personal health information is included in the case report.
Author Contributions
Dr. Mariam Riad contributed to the main write-up of the abstract and case report. Dr. Mustafeez Ur Rahman contributed to the write-up of the case and collection of images and captions. Dr. Rajasekhar Mulyala contributed to the literature search and discussion write-up. Dr. Nadia Sayyed contributed to the image collection and literature review. Dr. Danielle Bayer contributed to the procedural details and gross specimen image and pathology. Dr. Bassam Omar contributed to the final critique, organization and finalization of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Tyebally S, Chen D, Bhattacharyya S, Mughrabi A, Hussain Z, Manisty C, Westwood M, et al. Cardiac tumors: JACC CardioOncology State-of-the-Art review. JACC CardioOncol. 2020;2(2):293g-311.
doi pubmed pmc - Grazzini G, Pradella S, Miele V. Incidental identification of right atrial mass. Heart. 2020;106(19):1502-1534.
doi pubmed - Dias RR, Stolf NA, Malbouisson LM, Fernandes F, Ramirez FJ, Mady C, Oliveira SA. Morbidity and embolic potential of left atrial cardiac tumors. Thorac Cardiovasc Surg. 2006;54(6):400-403.
doi pubmed - Hosoba S, Mori M, Furtado AD, Lattouf OM. Extraction of right-sided vegetation with use of an aspiration catheter system. Innovations (Phila). 2015;10(5):357-359.
doi pubmed - Basso C, Rizzo S, Valente M, Thiene G. Cardiac masses and tumours. Heart. 2016;102(15):1230-1245.
doi pubmed - Campisi A, Ciarrocchi AP, Asadi N, Dell'Amore A. Primary and secondary cardiac tumors: clinical presentation, diagnosis, surgical treatment, and results. Gen Thorac Cardiovasc Surg. 2022;70(2):107-115.
doi pubmed - Patel M, Wei X, Weigel K, Gertz ZM, Kron J, Robinson AA, Trankle CR. Diagnosis and Treatment of Intracardiac Thrombus. J Cardiovasc Pharmacol. 2021;78(3):361-371.
doi pubmed - Song MH, Usui M, Usui A, Watanabe T, Ueda Y. Giant vegetation mimicking cardiac tumor in tricuspid valve endocarditis after catheter ablation. Jpn J Thorac Cardiovasc Surg. 2001;49(4):255-257.
doi pubmed - Suetani Y, Arita Y, Tanaka K, Okada M, Ogasawara N. Multiple cardiac calcified amorphous tumors. JACC Case Rep. 2022;4(2):91-93.
doi pubmed pmc - Ohara Y, Hiasa Y, Hosokawa S, Yamaguchi K, Ogura R, Ogata T, Yuba K, et al. Primary cardiac tumor presenting as obstruction of the left ventricular outflow tract. Circ J. 2004;68(10):961-963.
doi pubmed - Roeltgen D, Kidwell CS. Neurologic complications of cardiac tumors. Handb Clin Neurol. 2014;119:209-222.
doi pubmed - Ho KKF, Barsoum R, Shepherd B, McGahan T. Bilateral acute lower limb ischemia secondary to complete embolization of cardiac myxoma. J Vasc Surg. 2020;71(5):1759-1761.
doi pubmed - Maze Y, Kajimoto M, Tenpaku H, Satou T. Left atrial myxoma with severe inflammatory response. Jpn J Thorac Cardiovasc Surg. 2004;52(4):221-223.
doi pubmed - Yamana F, Domae K, Shirakawa Y, Takahashi T, Hao H. Cardiac calcified amorphous tumor with sustained ventricular tachycardia. Asian Cardiovasc Thorac Ann. 2022;30(4):474-476.
doi pubmed - Bugra Z, Emet S, Umman B, Ozer PK, et al. Intracardiac masses: Single center experience within 12 years: I-MASS Study. Am Heart J Plus. 2021;28:100081.
- Peters PJ, Reinhardt S. The echocardiographic evaluation of intracardiac masses: a review. J Am Soc Echocardiogr. 2006;19(2):230-240.
doi pubmed - Walpot J, Al Mafragi A, Vermeiren GLJ. Intracardiac masses: utility of contrast-enhanced echocardiography. Neth J Med. 2017;75(4):172-173.
pubmed - Zaragoza-Macias E, Chen MA, Gill EA. Real time three-dimensional echocardiography evaluation of intracardiac masses. Echocardiography. 2012;29(2):207-219.
doi pubmed - Goldman JH, Foster E. Transesophageal echocardiographic (TEE) evaluation of intracardiac and pericardial masses. Cardiol Clin. 2000;18(4):849-860.
doi pubmed - Muller S, Feuchtner G, Bonatti J, Muller L, Laufer G, Hiemetzberger R, Pachinger O, et al. Value of transesophageal 3D echocardiography as an adjunct to conventional 2D imaging in preoperative evaluation of cardiac masses. Echocardiography. 2008;25(6):624-631.
doi pubmed - Ahmed S, Nanda NC, Miller AP, Nekkanti R, Yousif AM, Pacifico AD, Kirklin JK, et al. Volume quantification of intracardiac mass lesions by transesophageal three-dimensional echocardiography. Ultrasound Med Biol. 2002;28(11-12):1389-1393.
doi pubmed - Balla S, Berzingi C, Brown CM, Alkhouli M. Intracardiac echocardiography-guided biopsy of a left ventricular mass. JACC Case Rep. 2019;1(3):424-425.
doi pubmed pmc - Sonaglioni A, Nicolosi GL, Lombardo M, Anza C, Ambrosio G. Prognostic relevance of left ventricular thrombus motility: assessment by pulsed wave tissue doppler imaging. Angiology. 2021;72(4):355-363.
doi pubmed - Terry NLJ, Manapragada P, Aziz MU, Singh SP. Cardiac mass evaluation with cardiac computed tomography: A review. J Med Imaging Radiat Sci. 2021;52(3S):S78-S87.
doi pubmed - Sultan FAT, Ahmed SW. Cardiac magnetic resonance evaluation of cardiac masses in patients with suspicion of cardiac masses on echo or computed tomography. J Clin Imaging Sci. 2020;10:57.
doi pubmed pmc - Pergola V, Al-Admawi M, Fadel B, Di Salvo G. An unusual cardiac mass: Echocardiography, computed tomography, and magnetic resonance imaging. J Cardiol Cases. 2016;13(5):143-145.
doi pubmed pmc - Mallia A, Travaini L, Trifiro G, Paganelli G. Detection of a cardiac mass by [18F]FDG-PET/CT: a rare case. Ecancermedicalscience. 2009;3:152.
doi pubmed pmc - Wu CM, Bergquist PJ, Srichai MB. Multimodality imaging in the evaluation of intracardiac masses. Curr Treat Options Cardiovasc Med. 2019;21(10):55.
doi pubmed - Tasdemir A, Tuncay A, Karaman H, Canoz O, Asik R, Ozmen R, Elcik D. Cardiac masses: pathological and surgical features - a multicenter study. Braz J Cardiovasc Surg. 2021;36(5):656-662.
doi pubmed pmc - Dias RR, Fernandes F, Ramires FJ, Mady C, Albuquerque CP, Jatene FB. Mortality and embolic potential of cardiac tumors. Arq Bras Cardiol. 2014;103(1):13-18.
doi pubmed pmc - Yin L, He D, Shen H, Ling X, Li W, Xue Q, Wang Z. Surgical treatment of cardiac tumors: a 5-year experience from a single cardiac center. J Thorac Dis. 2016;8(5):911-919.
doi pubmed pmc - Moriarty JM, Rueda V, Liao M, Kim GHJ, Rochon PJ, Zayed MA, Lasorda D, et al. Endovascular removal of thrombus and right heart masses using the AngioVac system: results of 234 patients from the prospective, multicenter registry of AngioVac procedures in detail (RAPID). J Vasc Interv Radiol. 2021;32(4):549-557.e543.
doi pubmed - Todoran TM, Sobieszczyk PS, Levy MS, Perry TE, Shook DC, Kinlay S, Davidson MJ, et al. Percutaneous extraction of right atrial mass using the Angiovac aspiration system. J Vasc Interv Radiol. 2011;22(9):1345-1347.
doi pubmed - Enezate T, Alkhatib D, Raja J, Chinta V, Patel M, Omran J. Angiovac for minimally invasive removal of intravascular and intracardiac masses: a systematic review. Curr Cardiol Rep. 2022;24(4):377-382.
doi pubmed - Rajput FA, Du L, Woods M, Jacobson K. Percutaneous vacuum-assisted thrombectomy using AngioVac aspiration system. Cardiovasc Revasc Med. 2020;21(4):489-493.
doi pubmed - Richardson TD, Lugo RM, Crossley GH, Ellis CR. Use of a clot aspiration system during transvenous lead extraction. J Cardiovasc Electrophysiol. 2020;31(3):718-722.
doi pubmed - Leong DW, Ayadi B, Dexter DJ, Rosenberg M, Horowitz JM, Chuang ML, Dohad S. Continuous mechanical aspiration thrombectomy performs equally well in main versus branch pulmonary emboli: A subgroup analysis of the EXTRACT-PE trial. Catheter Cardiovasc Interv. 2023;101(2):468-475.
doi pubmed - Chan SM, Laage Gaupp FM, Mojibian H. ClotTriever system for mechanical thrombectomy of deep vein thrombosis. Future Cardiol. 2023;19(1):29-38.
doi pubmed - Tu T, Toma C, Tapson VF, Adams C, Jaber WA, Silver M, Khandhar S, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv. 2019;12(9):859-869.
doi pubmed - Becker MC, Reddy S, Miccio B, Pappas O. A novel technique for the percutaneous removal of tricuspid valve vegetations utilizing the Inari Flowtriever System. Catheter Cardiovasc Interv. 2022;100(2):261-265.
doi pubmed - Herron C, T JF, Kobayashi D. Diagnostic transcatheter aspiration for pulmonary artery tumor embolism using an INARI Triever aspiration catheter. Catheter Cardiovasc Interv. 2021;98(6):E828-E831.
doi pubmed - Jafry AH, Ijaz SH, Mazhar M, Shahnawaz A, Yousif A. Not "Much Room" in the heart: a rare case of a massive intracardiac candida mass. Case Rep Infect Dis. 2021;2021:9216825.
doi pubmed pmc - Akay MH, Sirlak M, Gregoric ID, Frazier OH. Infected right atrial thrombus after explantation of a left ventricular assist device. Tex Heart Inst J. 2012;39(3):390-392.
pubmed pmc - Lobo A, Lewis JF, Conti CR. Intracardiac masses detected by echocardiography: case presentations and review of the literature. Clin Cardiol. 2000;23(9):702-708.
doi pubmed pmc - Oesterle SN. Where cardiac surgery and interventional cardiology merge: the future of catheter-based interventions for cardiovascular disease. Heart Surg Forum. 2001;4(4):290-296.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


