| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 12, December 2023, pages 419-425
Technetium-99-Guided Axillary Lymph Node Identification: A Case Report of a Novel Technique for Targeted Lymph Node Excision Biopsy for Node Positive Breast Cancer After Neoadjuvant Chemotherapy
Jason E. Copelanda, b, c, d, Cherian J. Cheriana, b, c, Matthew A. Lyewa, b
aDepartment of Surgery, Anaesthesia, Radiology and Emergency Medicine, University of the West Indies, Mona, Jamaica
bDepartment of General Surgery, Kingston Public Hospital, Kingston, Jamaica
cThe Breast Health & Oncology Care Centre at the Andrews Memorial Hospital, Kingston, Jamaica
dCorresponding Author: Jason E. Copeland, Department of Surgery, Kingston Public Hospital, Kingston, Jamaica
Manuscript submitted November 14, 2023, accepted December 18, 2023, published online December 29, 2023
Short title: Tc-99m-Guided Axillary Lymph Node Identification
doi: https://doi.org/10.14740/jmc4172
| Abstract | ▴Top |
Targeted axillary lymph node identification for breast cancer involves localization and removal of previously marked metastatic lymph nodes after the completion of neoadjuvant chemotherapy (NACT), when clinical and radiological complete responses of the axillary nodes are achieved. Traditionally, axillary lymph node dissection is performed for patients with node positive disease, but the high rates of pathological complete responses now seen after NACT have ushered in lower morbidity techniques such as sentinel lymph node excision biopsies, targeted axillary lymph node dissection and targeted axillary lymph node identification (clip node identification) in node positive disease which has converted to clinical/radiologically node negative. The latter two techniques often require the use of expensive seeds and advanced localization techniques. Here we describe the case of a 59-year-old woman who was diagnosed with node positive invasive breast cancer who was sequenced with NACT. We developed a novel technique, where technetium-99m was injected directly into a previously clipped metastatic axillary lymph node which was then localized with the Neoprobe gamma detection system intra-operatively and removed. This is a relatively low-cost technique that can be easily introduced in limited resourced health systems where radio-guided sentinel lymph node biopsies are already being performed.
Keywords: Targeted axillary node dissection; Post-neoadjuvant chemotherapy; Axillary lymph node surgery; Technetium-99; Breast cancer in Jamaica
| Introduction | ▴Top |
Axillary lymph node dissection (ALND) has been the standard procedure used in the node positive axilla for pathological disease staging and loco-regional control [1-3]. This procedure is however associated with high rates of lymphoedema and post-mastectomy pain syndromes [4, 5]. Recently, there has been a paradigm change in the sequencing of therapy in node positive breast cancer patients with neoadjuvant chemotherapy (NACT) being recommended for patients with triple negative or human epidermal growth factor receptor 2 (HER2) overexpressed invasive breast cancers who have high risks of disease recurrence [6, 7]. With the increased use of NACT, a significant percentage of node positive breast cancer patients will be converted to node negative statuses prior to undergoing surgery. Sentinel lymph node biopsy (SLNB) has replaced ALND as the standard of care surgical procedure for axillary staging in node negative patients [8]. However, the use of SLNB after NACT may be associated with high false negatives rates [9-11]. The identification and removal of clipped/marked previously positive axillary nodes, with or without sentinel lymph node excision, can significantly decrease the false negative rates associated with post-NACT axillary staging. Many methods have been described for marking/identifying positive axillary nodes such as charcoal tattooing, magnetic seed placement, wire localization, radiofrequency identification tags and most commonly, iodine 125 [12-15]. We report on the successful technique of direct injection of technetium-99m (Tc-99m) into a previously clipped axillary lymph node after neoadjuvant therapy that allowed accurate intra-operative localization with a gamma detection system.
The primary objective was to ascertain if the previously biopsied and clipped axillary lymph node could be correctly localized and retrieved intra-operatively using a gamma detection localization system, after ultrasound-guided injection of Tc-99m into the clipped node. Our secondary objective was to evaluate the pathological correlation between the retrieved node and the remaining axillary nodes via an ALND.
| Case Report | ▴Top |
Investigations
A 59-year-old post-menopausal female presented with a 1-month history of a painless left breast lump. She had no family history of breast or ovarian cancer and her last screening mammogram was 2 years ago. On clinical examination, she had bilateral grade 2 ptotic breasts with a solitary 4 × 4 cm mass in her left breast at the 3 o’clock position. A large left axillary lymph node was also palpated, but the right breast and axilla were normal.
Diagnosis
Digital mammography and breast ultrasound confirmed an irregular left breast mass at the 3 o’clock position. A single enlarged left level I axillary lymph node was also identified. Additionally, a suspicious mass was detected in her contralateral (right) breast on magnetic resonance imaging (MRI), which was not noticed on mammography or breast ultrasound, in addition to the palpable left breast mass and enlarged axillary node (Fig. 1a, b). Ultrasound-guided core needle biopsies of the left breast mass and the axillary lymph node were performed which confirmed a modified Scarff-Bloom-Richardson grade II, T2 N1 triple negative invasive carcinoma with left axillary nodal metastasis. There were no clinical or radiological evidence of distant metastases on contrast-enhanced computed tomography (CT) of chest, abdomen and pelvis or on bone scan. The previously biopsied left axillary node was subsequently clipped with an ultrasound detectable Mammawire Fix Breast clip (breast tissue marker) in a separate procedure. We were unable to make a biopsy of the suspicious right breast lesion seen on breast MRI since it was not seen on focused breast ultrasound and we do not possess MRI-guided biopsy capabilities. Her breast cancer genetic panel testing did not reveal any genetic mutation.
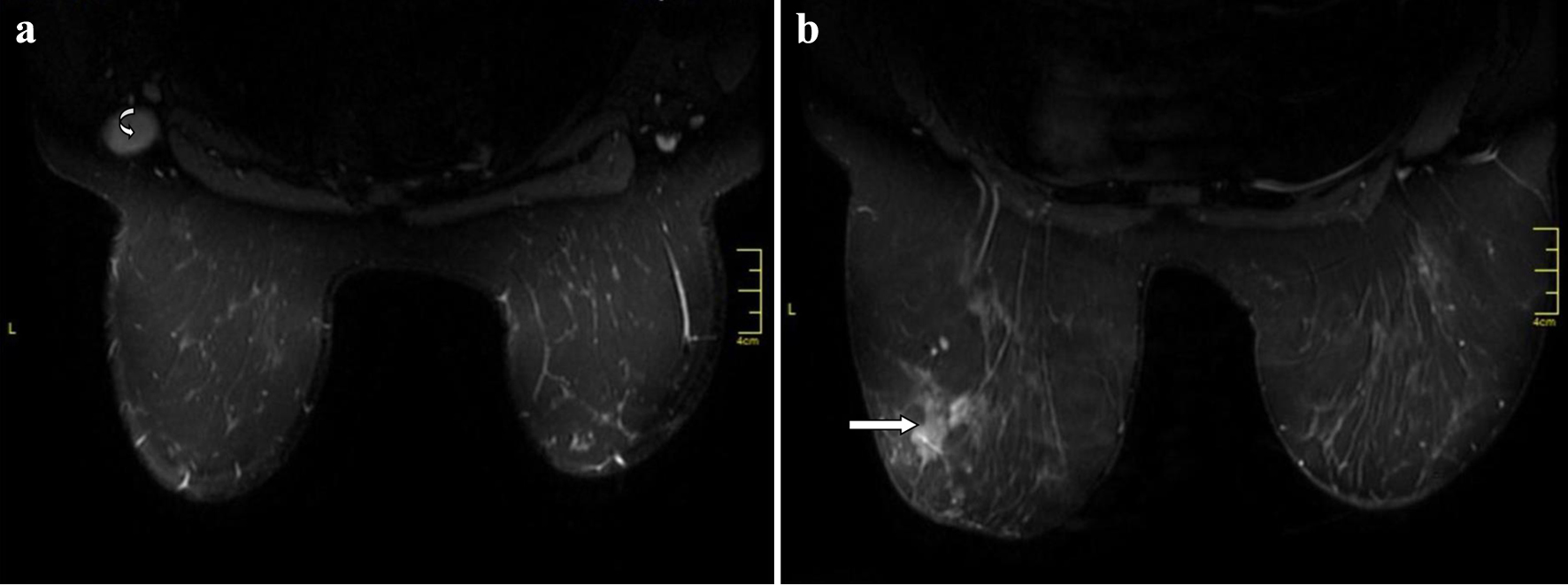 Click for large image | Figure 1. Contrast-enhanced breast magnetic resonance imaging. (a) The curved arrow identifies metastatic left axillary lymph node prior to placement of the Mammawire Fix Breast clip. (b) The straight arrow identifies 4 × 4 cm carcinoma of the left breast. |
Treatment
She was sequenced with neoadjuvant dose-dense systemic chemotherapy, to which she had a complete clinical response in the axilla but only a partial response in the breast. Bilateral nipple-sparing mastectomies were performed, with immediate direct-to-implant breast reconstruction, plus right Tc-99m SLNB and left radio-guided axillary lymph node identification with Tc-99m. Since the use of direct nodal injection of Tc-99m for clip node localization was being investigated for the first time in this procedure, a planned level I/II left axillary lymph node dissection was also performed to pathologically correlate the status of the Tc-99m localized clipped node and the rest of the axillary lymph nodes.
On the day of surgery, an axillary ultrasound was performed by the in-house radiologist to identify the previously biopsied and clipped axillary node (Fig. 2a). Once the node was positively identified, 0.2 mL of Tc-99m human serum albumin micro aggregate was injected into the lymph node (Fig. 2b). The Neoprobe gamma detection system (Neo2000, Neoprobe Corporation, Dublin, OH) on the Tc setting was used to detect the hottest count in the axilla immediately after the Tc-99m injection and the overlying skin was marked. The patient was then transferred to the operating room. At the time of axillary surgery, the skin incision was made at the previously marked area and the Neoprobe gamma detection system was used to intra-operatively identify the node previously injected with Tc-99m. The axillary node was sent for X-rays to confirm that the excised node contained the tissue marker/clip (Fig. 3) and planned levels I and II axillary lymph node dissection was performed to establish correlation between the localized node and the rest of the axillary lymph nodes.
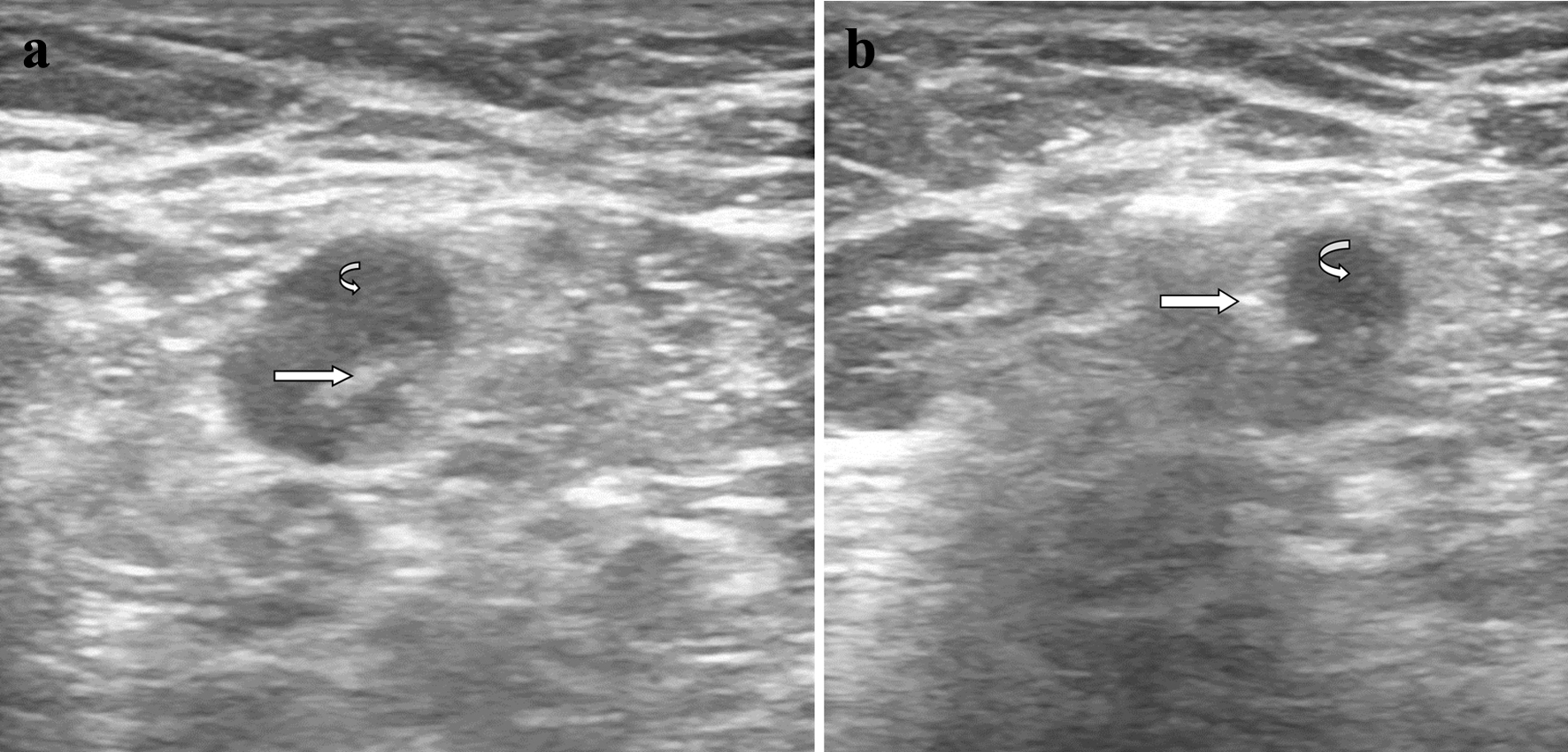 Click for large image | Figure 2. (a) Ultrasound of metastatic left axillary lymph node. The metastatic left axillary lymph node is shown with the curved arrow. The straight arrow identifies the Mammawire Fix Breast clip, successfully deployed into the lymph node. (b) Ultrasound-guided injection of technetium-99m into the metastatic left axillary lymph node. The metastatic left axillary lymph node is shown with the curved arrow. The straight arrow identifies the tip of the needle injecting technetium-99m successfully into the lymph node. |
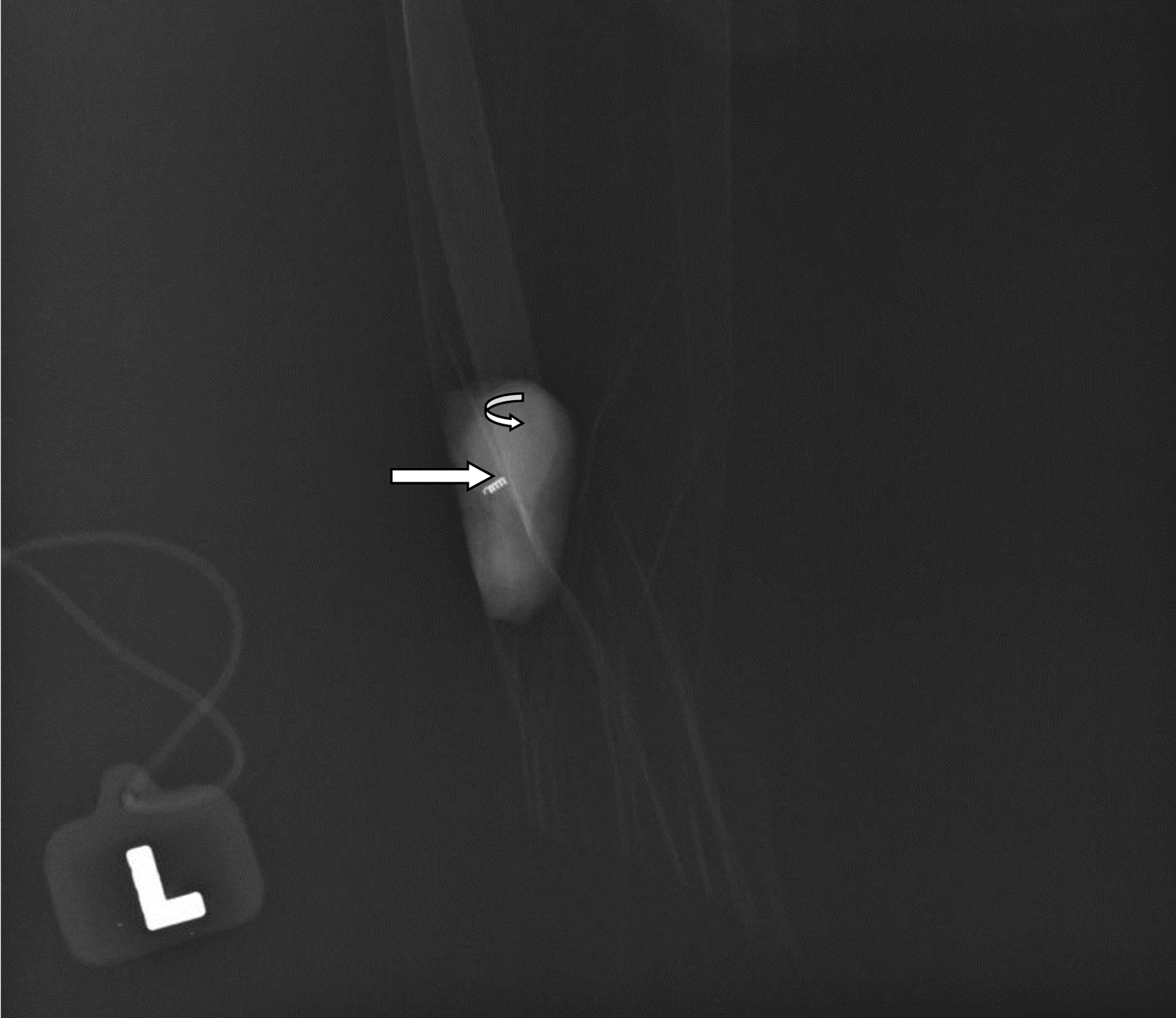 Click for large image | Figure 3. X-ray image of the technetium-99m localized metastatic left axillary lymph node. Curved arrow identifies the metastatic left axillary lymph node, while the straight arrow signifies the Mammawire Fix Breast clip within the lymph node. |
Follow-up and outcomes
The previously biopsied and clipped right axillary lymph node was positively identified on pre-operative ultrasound and the Tc-99m was successfully injected into the node. The lymph node was successfully localized intra-operatively using the Neoprobe gamma detection system. On final pathology, the Tc-99m injected node was positive for metastases, but the remaining 26 lymph nodes from the ALND were negative for metastases. There was pathological complete response (pCR) in the left breast and no malignancy was found in the right breast.
| Discussion | ▴Top |
The type of axillary lymph node surgery that is performed after NACT remains controversial. The procedure that we have described was successfully utilized to localize a previously clipped, biopsy-confirmed metastatic axillary lymph node following NACT. It is a simple technique to incorporate, especially in units that are already using gamma detection systems for SLNB or radio-guided occult lesion localization. To our knowledge, our group is the first to report on the use of direct nodal injection of Tc-99m as a method for targeted intra-operative axillary lymph node identification.
In Jamaica, breast cancer accounts for the highest cancer-related morbidity and mortality in women. This is partly due to the high rates of triple negative molecular subtypes within our population, which is more commonly associated with early treatment failures and overall worse prognosis [16-19]. Triple negative breast cancers are however associated with high rates of pCR when sequenced with NACT [20-22].
There has been a paradigm change in the use of NACT in patients with invasive breast cancer, especially for those with stage II-III disease [23]. While neoadjuvant sequencing of chemotherapy does not, by itself, improve disease-free or overall survival when compared to adjuvant sequencing, it does offer the possibility of down staging the primary breast and axillary disease thereby facilitating less morbid surgeries [24, 25]. The response of the primary breast cancer to neoadjuvant systemic chemotherapy is also a major prognostic parameter, with patients achieving complete pathological responses having better long-term survival outcomes compared to those with partial or no response [25-27]. In addition, recent prospective randomized trials have shown disease-free and overall survival benefit in patients with triple negative or HER2 positive invasive breast cancers who do not achieve a pCR with NACT/anti-HER2 therapy who are then treated with additional drug therapies compared to those who are not [28, 29].
Similar to the response of the primary cancer in the breasts, the biopsy-proven positive axillary lymph nodes may achieve a pCR to neoadjuvant systemic therapy. Rates of 47-79% axillary nodal pCR are commonly reported, especially in triple negative or HER2 overexpressed invasive breast cancer [30-34]. If axillary nodal pCR is suspected based on the clinical/radiological response, the options for axillary staging include ALND, SLNB, excision of marked axillary nodes and targeted ALND.
The use of ALND is associated with high rates of morbidities such as post-mastectomy pain syndrome, lymphoedema, sensory loss and shoulder stiffness when compared to SLNB [35, 36]. Published studies on the use of SLNB alone to assess axillary lymph node status after NACT have reported false negative rates between 12.6% and 14.2% [9-11]. These false negative rates are partly due to the finding that in 24% of patients, the clipped node was not a part of the sentinel lymph node. However, once the clipped node was removed, the false negative rate fell to 6.8%. A further analysis comparing the clipped node approach alone with the full targeted axillary dissection (TAD) revealed false negative rates of 4.2% and 1.4%, respectively [37].
TAD refers to the technique where previously biopsied and clipped axillary lymph nodes in node positive breast cancers that showed clinical/radiological response to NACT are localized and removed together with SLNB [38]. Based on the pathological assessment of the TAD specimen, a significant proportion of patients may avoid ALND (50-55%) [37, 39].
Targeted axillary lymph node excision biopsy refers to the localization and removal of the previously clipped positive axillary lymph node after NACT without the addition of the SLNB component [40]. One technique for performing axillary lymph node localization/identification is the use of the MARI procedure (marking the axillary lymph node with radioactive iodine seeds). The MARI node technique uses a titanium encapsulated I125 seed inserted into the previously biopsied axillary lymph node. After NACT, the MARI node is localized using a gamma detection system (Neoprobe). The procedure is associated with 97% nodal identification rates and false negative rates of only 7% [12, 41].
Localization of the marked node has been performed using other techniques such as wires, charcoal tattooing, magnetic and radiofrequency identification devices with varying success rates [14, 42, 43]. Our technique utilizes the same basic idea as the MARI technique, except for the use of Tc-99m rather than radioactive iodine seeds to localize the previously clipped node. Due to the comparatively short half-life of Tc-99m, the previously clipped axillary node needs to be imaged and injected within a few hours prior to the time of surgery. Conceptually, our technique of Tc-99m nodal localization bears similarities to the radio-guided occult lesion localization (ROLL) that was more widely used to localize non-palpable breast lesion prior to the widespread use of radioactive and magnetic seeds [44].
While the wire-guided technique for axillary lymph node localization is inexpensive, with good localization capabilities, it is more cumbersome and needs to be placed very close to the time of surgery [42]. The use of radioactive seeds is more commonly performed in well-resourced institutions but it is associated with rigorous radiation safety protocols, limited access, and higher setup costs than Tc-99m. It does have the advantage of being able to be positioned into the node weeks before the date of surgery which allows the procedure to be uncoupled from surgery [12, 37].
The more recently described magnetic and radiofrequency identification devices are promising. They allow for ease of insertion compared to wire techniques and uncoupling from the time of the surgical procedure. In the recently published MAGET study, where MAGSEED was used to mark the biopsy positive axillary lymph node, there was a 100% nodal retrieval rate using the Sentimag guidance with 0% false negative rate [13].
The use of carbon dye tattooing for TAD has shown promise, with moderate identification rates when single marking with carbon ink only is performed. This increases to 94% where doublemarking, with ink and clip, is employed [14]. Although the technique is simple and reproducible, it may be associated with the removal of excess nodes due to cardon ink migration out of the clipped node [14, 15].
Our procedure was proven to be successful in accurately indentifying the previosuly biopsied-clipped node and though it was pathologically positive for metastatic disease, the remaining axillary nodes were all negative for metastases. The accuracy and false negative rate of this technique would need to be established using a larger sample of patients. However, in light of the outcomes from the MARI node and other clip node localization techniques, the false negative rate of the our procedure is expected to be in keeping with these techniques.
The success of this technique is heavily reliant on good collaboration between the breast surgeon and the radiologist, to limit delays and logistic complexities. Alternatively, surgeon-directed intra-operative ultrasound with direct nodal injection of Tc-99m can be performed by a surgeon with the necessary ultrasound skill set.
Learning points
Tc-99m-injected axillary lymph nodes can be selectively localized and retrieved using a gamma detection localization method. This technique may be used in performing targeted axillary lymph node localization for previously biopsied and clipped axillary lymph nodes after NACT. The half-life of Tc-99m is approximately 6 h, therefore close collaboration between the breast surgeon and the radiologist is critical to prevent unwanted delays while ensuring clinical success. This technique can easily be introduced in facilities that are already performing Tc-99m-guided SLNB.
Conclusion
The pathological assessment of the clipped axillary node is associated with low false negative rates and it is preferred to standard ALND in patients who have attained a complete radiological response after NACT, due to the lower rates of surgical morbidity. Tc-99m nodal injection appears to be an accurate method to identify these clipped nodes.
Acknowledgments
The authors wish to acknowledge Dr. Donald Ellis and the radiology staff of the Andrews Memorial Hospital for imaging contribution and the administrative staff of the Breast Health & Oncology Care Centre for their support in the conduction of this study.
Financial Disclosure
The authors have no relevant financial or funding disclosure at the time of submission of this case report.
Conflict of Interest
The authors have no conflict of interest related to this case report.
Informed Consent
Informed consent was obtained from this patient prior to the submission of the case report.
Author Contributions
Conceptualization: Jason E. Copeland. Writing review and editing: Jason E. Copeland, Cherian J. Cherian, and Matthew A. Lyew. All authors have read and agreed to the submitted version of the manuscript.
Data Availability
The supporting data for this case report are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Moore MP, Kinne DW. Axillary lymphadenectomy: a diagnostic and therapeutic procedure. J Surg Oncol. 1997;66(1):2-6.
doi pubmed - Konkin DE, Tyldesley S, Kennecke H, Speers CH, Olivotto IA, Davis N. Management and outcomes of isolated axillary node recurrence in breast cancer. Arch Surg. 2006;141(9):867-872; discussion 872-864.
doi pubmed - Marschall J, Nechala P, Colquhoun P, Chibbar R. Reassessing the role of axillary lymph-node dissection in patients with early-stage breast cancer. Can J Surg. 2003;46(4):285-289.
pubmed pmc - Larson D, Weinstein M, Goldberg I, Silver B, Recht A, Cady B, Silen W, et al. Edema of the arm as a function of the extent of axillary surgery in patients with stage I-II carcinoma of the breast treated with primary radiotherapy. Int J Radiat Oncol Biol Phys. 1986;12(9):1575-1582.
doi pubmed - Abass MO, Gismalla MDA, Alsheikh AA, Elhassan MMA. Axillary lymph node dissection for breast cancer: efficacy and complication in developing countries. J Glob Oncol. 2018;4:1-8.
doi pubmed pmc - Burstein HJ, Curigliano G, Thurlimann B, Weber WP, Poortmans P, Regan MM, Senn HJ, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216-1235.
doi pubmed pmc - Thomssen C, Balic M, Harbeck N, Gnant M. St. Gallen/Vienna 2021: a brief summary of the consensus discussion on customizing therapies for women with early breast cancer. Breast Care (Basel). 2021;16(2):135-143.
doi pubmed pmc - Krag DN, Julian TB, Harlow SP, Weaver DL, Ashikaga T, Bryant J, Single RM, et al. NSABP-32: Phase III, randomized trial comparing axillary resection with sentinal lymph node dissection: a description of the trial. Ann Surg Oncol. 2004;11(3 Suppl):208S-210S.
doi pubmed - Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, Meterissian S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258-264.
doi pubmed - Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455-1461.
doi pubmed pmc - Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, Lebeau A, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609-618.
doi pubmed - Donker M, Straver ME, Wesseling J, Loo CE, Schot M, Drukker CA, van Tinteren H, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378-382.
doi pubmed - Martinez M, Jimenez S, Guzman F, Fernandez M, Arizaga E, Sanz C. Evaluation of axillary lymph node marking with magseed(R) before and after neoadjuvant systemic therapy in breast cancer patients: MAGNET study. Breast J. 2022;2022:6111907.
doi pubmed pmc - Pinto D, Batista E, Gouveia P, Mavioso C, Anacleto J, Ribeiro J, Sousa B, et al. Targeted axillary dissection after chemotherapy: feasibility study with clip and carbon dye tattoo - neotarget trial. Breast Care (Basel). 2022;17(2):166-171.
doi pubmed pmc - Choy N, Lipson J, Porter C, et al. Initial results with preoperative tattooing of biopsied xillary lymph nodes and correlation to sentinel lymph nodes in breast cancer patients. Ann Surg Oncol. 2015;22:3777-382.
- Copeland J, Oyedeji A, Powell N, Cherian CJ, Tokumaru Y, Murthy V, Takabe K, et al. Breast cancer in Jamaica: stage, grade and molecular subtype distributions across age blocks, the implications for screening and treatment. World J Oncol. 2021;12(4):93-103.
doi pubmed pmc - Chin SN, Green C, Strachan GG, Wharfe G. Clinicopathologic characteristics of breast cancer in Jamaica. Asian Pac J Cancer Prev. 2014;15(7):3319-3322.
doi pubmed - Peters KA, Roberts PO, Cornwall DA, Mitchell DI, Thompson RK. Clinicopathological factors affecting breast cancer survival in jamaican women: a retrospective review. J Racial Ethn Health Disparities. 2023;10(2):844-858.
doi pubmed - Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674-690.
doi pubmed pmc - Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13-21.
doi pubmed pmc - von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747-756.
doi pubmed - Geyer CE, Sikov WM, Huober J, Rugo HS, Wolmark N, O'Shaughnessy J, Maag D, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol. 2022;33(4):384-394.
doi pubmed - Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, Khan SA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39(13):1485-1505.
doi pubmed pmc - Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB, Jr., et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483-2493.
doi pubmed - Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165-4174.
doi pubmed - Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778-785.
doi pubmed - van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224-4237.
doi pubmed - Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, Kuroi K, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159.
doi pubmed - von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, Wolmark N, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628.
doi pubmed - Zhang GC, Zhang YF, Xu FP, Qian XK, Guo ZB, Ren CY, Yao M. Axillary lymph node status, adjusted for pathologic complete response in breast and axilla after neoadjuvant chemotherapy, predicts differential disease-free survival in breast cancer. Curr Oncol. 2013;20(3):e180-192.
doi pubmed pmc - Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260(4):608-614; discussion 614-606.
doi pubmed pmc - Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, El-Tamer M, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol. 2016;23(11):3467-3474.
doi pubmed pmc - Al-Tweigeri T, Elshenawy M, Badran A, Omar A, Suleman K, Al Malik O, Anwar I, et al. Impact of pathologic complete response following neoadjuvant chemotherapy +/- trastuzumab in locally advanced breast cancer. J Oncol. 2021;2021:6639763.
doi pubmed pmc - Diego EJ, McAuliffe PF, Soran A, McGuire KP, Johnson RR, Bonaventura M, Ahrendt GM. Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol. 2016;23(5):1549-1553.
doi pubmed - Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599-609.
doi pubmed - Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, Skelly JM, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102(2):111-118.
doi pubmed pmc - Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, Bedrosian I, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. 2016;34(10):1072-1078.
doi pubmed pmc - Mittendorf EA, Caudle AS, Yang W, Krishnamurthy S, Shaitelman S, Chavez-MacGregor M, Woodward WA, et al. Implementation of the american college of surgeons oncology group z1071 trial data in clinical practice: is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014;21(8):2468-2473.
doi pubmed - Beniey M, Boulva K, Rodriguez-Qizilbash S, Kaviani A, Younan R, Patocskai E. Targeted axillary dissection in node-positive breast cancer: a retrospective study and cost analysis. Cureus. 2021;13(4):e14610.
doi pubmed pmc - Gurleyik G, Aksu SA, Aker F, Tekyol KK, Tanrikulu E, Gurleyik E. Targeted axillary biopsy and sentinel lymph node biopsy for axillary restaging after neoadjuvant chemotherapy. Ann Surg Treat Res. 2021;100(6):305-312.
doi pubmed pmc - Straver ME, Loo CE, Alderliesten T, Rutgers EJ, Vrancken Peeters MT. Marking the axilla with radioactive iodine seeds (MARI procedure) may reduce the need for axillary dissection after neoadjuvant chemotherapy for breast cancer. Br J Surg. 2010;97(8):1226-1231.
doi pubmed - Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018;44(9):1307-1311.
doi pubmed - Reitsamer R, Peintinger F, Forsthuber E, Sir A. The applicability of Magseed(R) for targeted axillary dissection in breast cancer patients treated with neoadjuvant chemotherapy. Breast. 2021;57:113-117.
doi pubmed pmc - Luini A, Zurrida S, Paganelli G, Galimberti V, Sacchini V, Monti S, Veronesi P, et al. Comparison of radioguided excision with wire localization of occult breast lesions. Br J Surg. 1999;86(4):522-525.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


