| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 6, June 2024, pages 102-105
Eosinophilic Jejunitis Presenting as Intractable Vomiting, Persistent Leukocytosis, and Ascites in a Young Adult Patient
Dawood Tahira, c, Shravya Ginnarama, Erkanda Ikonomib
aDepartment of Internal Medicine, Jefferson Abington Hospital, Abington, PA, USA
bDepartment of Gastroenterology, Jefferson Abington Hospital, Abington, PA, USA
cCorresponding Author: Dawood Tahir, Department of Internal Medicine, Jefferson Abington Hospital, Abington, PA, USA
Manuscript submitted March 7, 2024, accepted April 30, 2024, published online May 25, 2024
Short title: Eosinophilic Jejunitis
doi: https://doi.org/10.14740/jmc4196
| Abstract | ▴Top |
Eosinophilic enteritis (EoN) poses a distinctive challenge, affecting individuals with various clinical presentations depending on the layer and extent of the bowel wall. We present a case of a 19-year-old female with abdominal pain, vomiting, and loose stools for 1 month. Labs were significant for persistent leukocytosis with peripheral eosinophilia. A computed tomography of the abdomen/pelvis demonstrated moderate abdominal ascites and moderately diffuse mucosal thickening of jejunal loops. A diagnostic paracentesis unveiled low serum ascites albumin gradient and 92% eosinophils. Push enteroscopy resulted in no significant biopsy findings, though a laparoscopic full-thickness jejunal biopsy exhibited increased eosinophils in the bowel wall. Intravenous steroid, proton pump inhibitor, and dietary changes resolved the symptoms and normalized the labs within a week. Our case report highlights a variable presentation of eosinophilic jejunitis uncommon in this disease population. EoN is an easily missed diagnosis and mandates frequent follow-up to prompt relevant investigations. Atopic clinical features are not prevalent in each case. While rare, EoN requires a strong clinical suspicion, even if endoscopic biopsies are unremarkable, prompting timely laparoscopic full-thickness biopsy. Per protocol, physicians must do the infectious and eosinophilia workup to rule out other etiologies. Our case also highlights that worsening clinical condition in EoN warrants early intravenous steroids with a favorable prognosis and considers a psychosocial aspect of the disease on the patient’s health.
Keywords: Eosinophilic jejunitis; Leukocytosis; Eosinophilia; Gastroenteritis; Ascites; Steroid
| Introduction | ▴Top |
Eosinophilic enteritis (EoN) poses a distinctive challenge, affecting individuals with various clinical presentations depending on the layer and extent of the gut wall [1]. The prevalence of the disease varies depending on the organ involved - eosinophilic gastritis 6/100,000, gastroenteritis 8/100,000, and colitis 3/100,000 [2]. Eosinophilic gastroenteritis typically occurs in the third through fifth decades, with a peak age of onset in the third decade [1]. The pathogenesis remains unclear but is attributed mainly to the allergy component, interleukin-5, which expresses food allergens and specific T helper-2 cells infiltrating the intestinal epithelium and causing disruption [1]. The entire gastrointestinal tract may be involved, often with patchy distribution, which explains why endoscopic biopsies may miss the diagnosis [1]. The pediatric group commonly endorses concomitant allergies [2]. About 45% of patients with gastroenteritis have co-existing allergies [2].
Our case aims to educate readers about the variable presentation of EoN in a young adult, especially when rarely presenting with persistent leukocytosis and dysphagia to both solids and liquids, as these are rarely witnessed in this disease population. It warrants ruling out malignancy, infectious, and other causes of eosinophilia. Our case also highlights that when the clinical condition worsens, administering high-dose intravenous steroids is useful when used earlier in the disease course with a favorable prognosis. Misdiagnosis is possible as these overlapping symptoms (nausea, vomiting, abdominal pain, and diarrhea) may be attributed to irritable bowel syndrome, inflammatory bowel disease, and (viral/bacterial/parasitic) gastroenteritis, and timely diagnosis may avoid legal action.
| Case Report | ▴Top |
Investigations
A 19-year-old female presented to the emergency department with chief complaints of abdominal pain, vomiting, and loose, watery stools for 1 month. The abdominal pain was 6 on 10 on a pain scale, diffuse, radiated to the back, and worsened with food intake, prompting her to vomit repeatedly. Simultaneously, she reported dysphagia to solids and liquids. The rest of the review of systems was unremarkable. Previously evaluated in the clinic twice, she had an elevated white blood cell count of 17 × 109/L (4.0 - 11.0 × 109/L), normal neutrophils of 67% (40.0-73.0%) in the absence of fever; she tried omeprazole 40 mg twice daily for a month but to no relief. Medical history was negative for asthma, eczema, or drug-induced hypersensitivity. The patient noted no recent dine-out, travel, nonsteroidal anti-inflammatory drug (NSAID), or alcohol use history. She endorsed allergic rhinitis, which is allergic to pollen exposure. Her family history was positive for pancreatic cancer in her maternal grandmother. On hospital admission, vital signs were notable for temperature of 99.7 °F, heart rate of 80 beats per minute, blood pressure of 124/80 mm Hg, respiratory rate of 15 breaths per minute, and oxygen saturation of 97% on room air.
Diagnosis
She had a diffusely tender abdomen on physical examination without guarding or rebound. Bowel sounds were positive in all four quadrants. The cardiopulmonary examination was unremarkable. No skin rash was appreciated on the exam.
Labs were significant for persistent elevated white blood cell count, slightly increased from outpatient labs to 20.4 × 109/L (4.0 - 11.0 × 109/L) inpatient, with new onset peripheral eosinophilia - absolute eosinophils of 7.98 × 109/L (0.00 - 0.70 × 109/L) with relative eosinophils elevated at 42% (0.0-6.0%). The comprehensive metabolic panel was unremarkable. Blood cultures showed no organism growth, and stool culture was negative for Salmonella, Shigella, and Campylobacter. Clostridium difficile antigen also resulted in negative. Stool Giardia lamblia, Shiga toxins (one and two), and Cryptosporidium antigens were also negative. Lactate dehydrogenase (LDH) was normal at 187 IU/L (125 - 240 IU/L), and erythrocyte sedimentation rate (ESR) was < 1 mm/h (0 - 20 mm/h). Peripheral blood smear revealed numerous mature eosinophils and was negative for parasites. Human immunodeficiency virus (HIV)-1 and HIV-2 antibodies/antigens were non-reactive, and immunoglobulins IgA, IgG, and IgM were within normal limits. Vitamin B12 was 380 pg/mL (normal 210 - 950 pg/mL).
A computed tomography (CT) of abdomen and pelvis with intravenous contrast demonstrated moderate abdominal ascites and moderately diffuse mucosal thickening of jejunal loops (Fig. 1). A right upper quadrant ultrasound revealed ascites around the liver (Fig. 2), prompting a therapeutic (removal of 3,100 mL fluid) and diagnostic paracentesis, which unveiled low serum ascites albumin gradient (SAAG), high protein, 92% eosinophils, and negative for malignant cells. Flow cytometric analysis of ascitic fluid disclosed no monoclonal B-cell or abnormal T-cell populations. Push enteroscopy grossly unmasked a normal mucosa, and biopsies were taken. Stomach antrum and body revealed benign gastric mucosa with reactive gastropathy and was negative for intestinal metaplasia, dysplasia, Helicobacter pylori immunostain, eosinophilic infiltrates, granulomata, helminth eggs, and larvae. Duodenum and jejunum biopsies were negative for villar blunting or increased intra-epithelial lymphocytes.
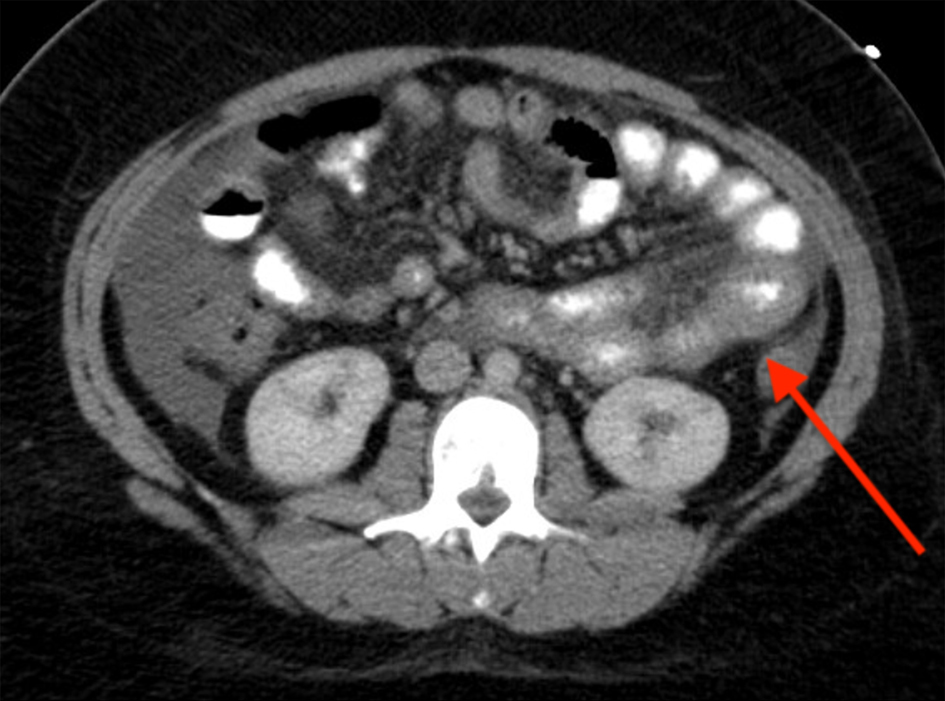 Click for large image | Figure 1. CT of abdomen/pelvis with IV contrast: abdominal ascites and moderately diffuse mucosal thickening of jejunal loops (arrow). CT: computed tomography; IV: intravenous. |
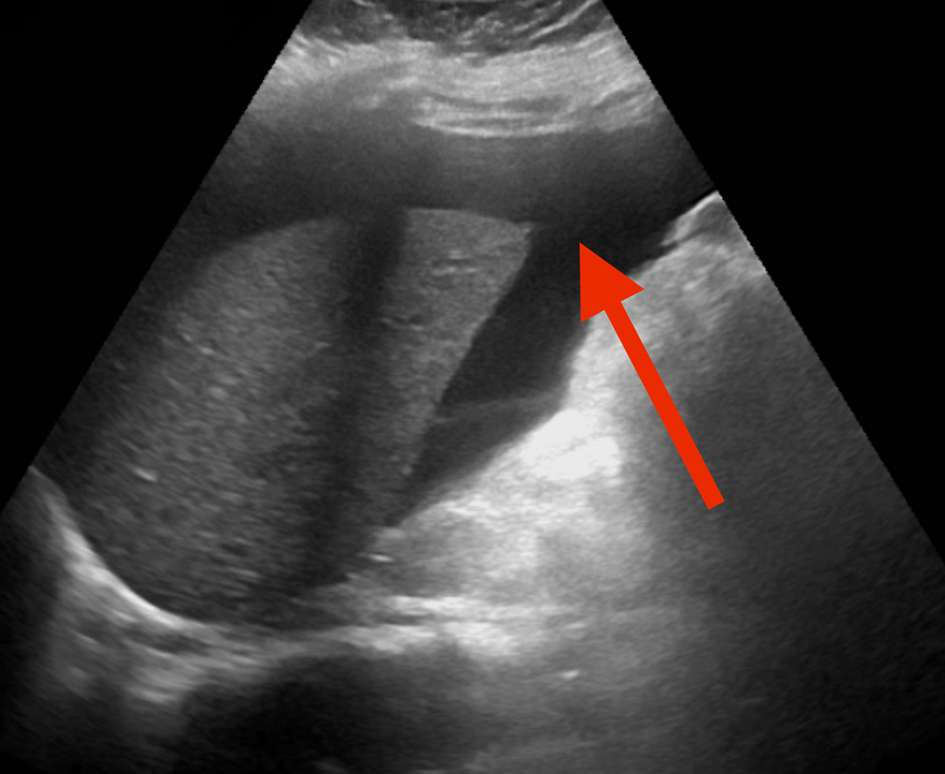 Click for large image | Figure 2. RUQ US revealing ascites around the liver (arrow). RUQ: right upper quadrant; US: ultrasound. |
Despite this, diarrhea, vomiting, and abdominal pain worsened, necessitating a laparoscopic full-thickness jejunal biopsy (Fig. 3) that showed increased eosinophils in the lamina propria, submucosal edema with sheets of eosinophils, and muscularis propria with infiltrating eosinophils, consistent with eosinophilic jejunitis.
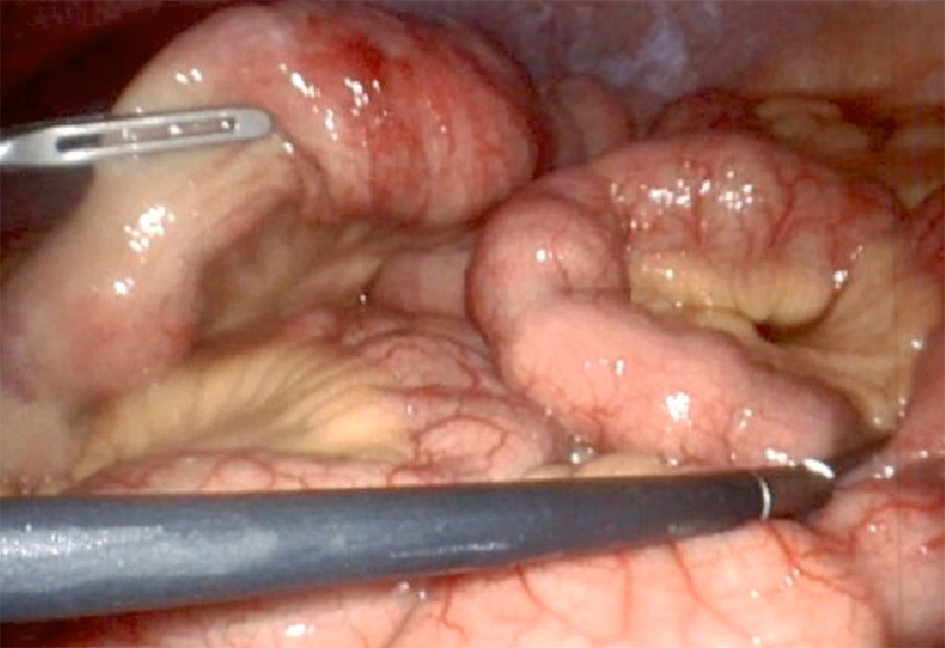 Click for large image | Figure 3. Laparoscopic jejunal gross view and proceeding with full thickness biopsies. |
Treatment
Intravenous steroids (methylprednisolone 60 mg on day 1 and 20 mg on day 2, then switched to prednisone 40 mg for a week), pantoprazole 40 mg once daily, and lactose and gluten-free diet changes resolved the symptoms and normalized the labs within a week.
Follow-up and outcomes
Post-intervention counseled the patient about nutritional changes and discharged on oral prednisone tapered off weekly for the next 4 weeks. There was no recurrence of gastrointestinal symptoms later, and labs remained unremarkable.
| Discussion | ▴Top |
EoN is an easily missed diagnosis and requires frequent follow-up to prompt relevant investigations [1, 3]. However, pathophysiology is linked with hypersensitivity reactions, atopic clinical features (including asthma, hay fever, drug sensitivity, or urticaria), and peripheral eosinophilia, which are not prevalent in each case [1, 2]. A clinicopathological study completed at Mayo Clinic revealed allergy history in half of the patients only and peripheral eosinophilia was absent in 22.5% of subjects [1]. Our patient lacked classical atopy features and did not have peripheral eosinophilia in initial clinic labs. Eosinophilia, if present, guides the treatment plan, but if absent, does not rule out the disease [4].
It is uncommon to find leukocytosis in EoN [5]. A few EoN cases with leukocytosis have been reported [5]; our case highlights the variable presentation of it and reiterates ruling out infectious, inflammatory, and malignancy workup. It is also essential to rule out all other causes of peripheral eosinophilia [4]. Imaging is vital because a CT scan/magnetic resonance imaging (MRI) may reveal bowel wall thickening, nodularity, ascites, or luminal narrowing [6], hinting towards a specific bowel site, which further guides where to take biopsy samples.
Infectious cultures, cell count, and cytology should be tested in patients with ascites. There are no established diagnostic criteria for ascitic fluid eosinophilia; studies have reported elevated eosinophil counts (up to 88%) [7], consistent with our patient’s ascitic tap positive for 92% eosinophils. While rare, EoN requires a strong clinical suspicion. Endoscopic findings include erythema, gut wall erosions, and ulcerations. These are nonspecific specific findings. Upper endoscopic evaluation with a biopsy is diagnostic in at least 80% of the cases involving the mucosal layer [1]. As witnessed in our patient, endoscopic evaluation and histopathological biopsies may be unremarkable [1, 8]. This prompts a timely laparoscopic full-thickness biopsy, especially when endoscopic/enteroscopic biopsies are unremarkable and abdominal imaging reveals bowel obstruction and/or bowel wall thickening [8].
Dietary elimination and steroids are the mainstay treatments with favorable prognosis in 90% of cases [9]. Steroid induces eosinophils apoptosis and inhibits chemotaxis [9].
The usual dose of oral prednisone is 20 to 40 mg/day, and then it can be tapered over the next 2 - 4 weeks [9]. Our patient had worsening abdominal pain with nausea necessitating intravenous high-dose steroid administration early, intravenous methylprednisolone 60 mg on day 1 and 20 mg on day 2, then switched to prednisone 40 mg. This suggests that different steroid regimes and routes may play a role in effectively treating EoN - this can be further explored in future comparative studies.
Histological biopsy criteria are often used as normal eosinophils are present in the bowel wall in normal physiologic states throughout the gastrointestinal tract, except the esophagus [10]. Though there is no definite cut-off for the number of eosinophils present per high power field (HPF) to diagnose EoN, the experienced pathologist may assess if a certain/elevated number is present for a particular area [10]. These areas include the stomach with ≥ 30 eosinophils per HPF in 5 HPF, duodenum with ≥ 52 eosinophils per HPF, ileum with > 56 per HPF, right colon with > 100 per HPF, transverse and descending colon with > 84 per HPF, and rectosigmoid colon > 64 per HPF [10]. No definite criteria are mentioned for the number of eosinophils per HPF present in the jejunum. Our pathologists reviewed > 100 eosinophils per HPF in jejunum. There is substantial variability in histopathological biopsy practice patterns [10].
Considering the diagnosis’s impact on the patient’s psychological health, a study revealed significant signs of general mental distress in 36% of eosinophilic esophagitis patients with a threefold risk of substantial anxiety in those patients younger than 35 years [11]. Our 19-year-old female patient expressed concern over the diagnosis and was initially hesitant about dietary changes. With proper counseling and an explanation of the underlying disease, we regained her trust, and she started the treatment aptly and appropriately followed up in the clinic. The disease may influence social relationships, financial impact, and impact on the body, especially fatigue [11].
Follow-up biopsies - endoscopic or ultrasound-guided to assess histologic improvement [12] - assess the resolution of the disease. Other less invasive approaches include reducing peripheral eosinophilia and/or radiologic findings [12]. The choice of follow-up modality should be individualized based on the patient’s case. Our patient did not require repeat histological biopsies; her clinical symptoms improved, and peripheral eosinophilia resolved.
Our patient had a favorable response to intravenous steroid therapy. Emerging therapies are working on targeting monoclonal antibodies for interleukin-4 (IL-4) with dupilumab [12]. Other therapies targeting IL-5, such as mepolizumab, reslizumab, and benralizumab, are currently being studied for use in EoN [13]. Randomized controlled trials and comparative studies between drug type and efficacy need to be done in the future.
Conclusion
In conclusion, our case report aims to educate and highlight a variable presentation of eosinophilic jejunitis, such as persistent leukocytosis. Per protocol, physicians must do the infectious and eosinophilia workup to rule out other causes. As reflected in our case, enteroscopy/endoscopic evaluation is insufficient as it may miss serosal layers for biopsy; therefore, a full-thickness biopsy is warranted. Our case also highlights that worsening clinical condition in EoN warrants early intravenous steroids as treatment with a favorable prognosis and considers psychosocial with the financial aspect of the disease on the patient’s health. Misdiagnosis is possible as these overlapping symptoms may be attributed to irritable bowel syndrome, inflammatory bowel disease, and (viral/bacterial/parasitic) gastroenteritis, and timely diagnosis may avoid legal action.
Acknowledgments
None to declare.
Financial Disclosure
We have no funding sources to report.
Conflict of Interest
We have no conflict of interest to declare.
Informed Consent
Informed consent was taken from the patient.
Author Contributions
DT: patient care, writing the case report, revisions and submission process; SG: patient care, writing the case report, revisions; EI: patient care, writing the case report, revisions. All authors reviewed the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31(1):54-58.
doi pubmed pmc - Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a national administrative database. J Pediatr Gastroenterol Nutr. 2016;62(1):36-42.
doi pubmed pmc - Abassa KK, Lin XY, Xuan JY, Zhou HX, Guo YW. Diagnosis of eosinophilic gastroenteritis is easily missed. World J Gastroenterol. 2017;23(19):3556-3564.
doi pubmed pmc - Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004;113(1):11-28.
doi pubmed - Avram RI, Trifan AV, Girleanu I, Juncu SS, Muzica CM, Singeap AM, Huiban L. Eosinophilic enteritis: the great mimic. Arch Clin Cases. 2023;10(4):183-186.
doi pubmed pmc - MacCarty RL, Talley NJ. Barium studies in diffuse eosinophilic gastroenteritis. Gastrointest Radiol. 1990;15(3):183-187.
doi pubmed - Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH. Eosinophilic gastroenteritis. Medicine (Baltimore). 1970;49(4):299-319.
doi pubmed - Mungan Z, Attila T, Kapran Y, Tokatli IP, Unal Z. Eosinophilic jejunitis presenting as intractable abdominal pain. Case Rep Gastroenterol. 2014;8(3):377-380.
doi pubmed pmc - Chen MJ, Chu CH, Lin SC, Shih SC, Wang TE. Eosinophilic gastroenteritis: clinical experience with 15 patients. World J Gastroenterol. 2003;9(12):2813-2816.
doi pubmed pmc - Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43(2):257-268.
doi pubmed - de Rooij WE, Bennebroek Evertsz F, Lei A, Bredenoord AJ. Mental distress among adult patients with eosinophilic esophagitis. Neurogastroenterol Motil. 2021;33(7):e14069.
doi pubmed pmc - Freeman HJ. Longstanding eosinophilic gastroenteritis of more than 20 years. Can J Gastroenterol. 2009;23(9):632-634.
doi pubmed pmc - Meek PD, Hemstreet B. Emerging therapies for eosinophilic esophagitis. Pharmacotherapy. 2023;43(4):338-348.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


