| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 8, August 2024, pages 153-158
Synchronous Double Primary Lung Adenocarcinomas With EGFR L858R Point Mutation and MET Exon 14 Skipping Mutation
Seijitsu Andoa, Shinji Futamia, Koji Azumaa, Kanako Nishimatsua, Takuma Shirasakab, Seigo Minamia, c
aDepartment of Respiratory Medicine, NHO Osaka National Hospital, Osaka City, Osaka 540-0006, Japan
bAIDS Medical Center, NHO Osaka National Hospital, Osaka City, Osaka 540-0006, Japan
cCorresponding Author: Seigo Minami, Department of Respiratory Medicine, NHO Osaka National Hospital, Osaka City, Osaka 540-0006, Japan
Manuscript submitted March 30, 2024, accepted June 11, 2024, published online July 5, 2024
Short title: Double Lung Cancers With Mutant EGFR and MET
doi: https://doi.org/10.14740/jmc4210
| Abstract | ▴Top |
Various driver mutations and the corresponding molecular-targeted drugs have been detected and developed in non-small cell lung cancer. There were many cases in which surgical specimens had happened to find double primary cancers. However, to our knowledge, our case was the first report of synchronous double primary lung adenocarcinomas harboring epidermal growth factor receptor (EGFR) L858R and mesenchymal-to-epithelial transition (MET) exon 14 skipping mutations. A 75-year-old Japanese woman with chronic heart and renal failures was referred to our department because of a growing nodule in the right upper lung field on chest X-ray films. Chest computed tomography (CT) detected a nodule in the right S1 and another nodule in the left S1+2. Bronchoscopic biopsy diagnosed the right S1 nodule as moderately differentiated adenocarcinoma. Oncomine Dx Target Test Multi-CDx system of the right S1 adenocarcinoma detected EGFR L858R mutation. The 18F-fluorodeoxyglucose positron emission tomography/CT showed abnormal uptakes both in the right S1 and the left S1+2 nodules, and in the bilateral inferior paratracheal lymph nodes. We made a diagnosis of c-stage IIIA (cT1bN2M0) of adenocarcinoma in the right S1 and suspected another primary lung cancer in the left S1+2. Considering her general conditions, comorbidities and wishes, we started osimertinib. The right S1 cancer achieved partial response (PR), while the left S1+2 nodule and lymph nodes enlarged. Aspiration cytology from the left supraclavicular lymph node showed adenocarcinoma. The FoundationOne® Liquid CDx tumor profiling test detected not only EGFR L858R, but also MET exon 14 skipping mutation. We made a diagnosis of another primary adenocarcinoma from the left S1+2 nodule (cT1bN3M0, c-stage IIIB) with MET mutation, and changed osimertinib to capmatinib. Although the left S1+2 cancer achieved and maintained PR by capmatinib, the right S1 cancer increased, and several new metastases appeared. The subsequent switch from capmatinib to osimertinib could not control cancers. In this case, we tried to switch monotherapies from osimertinib to capmatinib for double primary adenocarcinomas harboring different two driver mutations, according to each cancer progression. The temporal and spatial heterogeneity reinforces the need for primary tissue biopsy if dual primaries are suspected. Temporally distinct liquid biopsies, not standard at present, may be considered.
Keywords: EGFR L858R point mutation; MET exon 14 skipping mutation; Synchronous double primary lung cancers; Multiple primary lung cancers; Adenocarcinoma; Osimertinib; Capmatinib
| Introduction | ▴Top |
Multiple primary lung cancer (MPLC) is uncommon, and the actual frequency of MPLCs remains unknown. According to the Surveillance, Epidemiology, and End Results (SEER) Program, MPLCs are classified into synchronous and metachronous by 2-month interval between the diagnoses of the first and second primary cancers [1]. The incidences of synchronous and metachronous MPLCs were 6.0% and 5.0% in a Japanese Surgical Registry [2]. In the case of multifocal lung cancers and identical histological types, it remains difficult to discriminate MPLCs from intrapulmonary metastases, decide the exact clinical stage and select the optimal treatment strategy in the clinical setting [3]. Based on the analyses of ground-glass nodules, the discrepancy rate of driver mutations in MPLCs was 80% to 92% [4, 5]. This discrepancy leads to different responses to molecular-targeted therapy and may present unique challenges to targeted drugs for MPLC patients.
Epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) has been dramatically improved in prognosis by development of EGFR tyrosine kinase inhibitors (TKIs). Overall EGFR mutation frequency was 45% (range: 21-68%) in Japanese patients with adenocarcinoma histology [6]. Common mutations of exon 19 deletion and L858R point mutation in exon 21 accounted for 44.8% and 39.8% of EGFR mutations, respectively [7]. In the phase III FLAURA trial, the median progression-free survival (PFS) and overall survival (OS) were 18.9 and 38.6 months for osimertinib, a third-generation EGFR-TKI, but 10.2 and 31.8 months for control regimen of gefitinib or erlotinib, first-generation EGFR-TKIs, respectively [8]. Compared with gefitinib and erlotinib, osimertinib tended to display milder adverse events, especially in skin rash and hepatic toxicity. Thus, osimertinib was approved by Japanese medical insurance in August 2018 as the first-line EGFR-TKI for inoperable or recurrent NSCLC.
On the other hand, mesenchymal-to-epithelial transition (MET) gene exon 14 skipping mutation accounts for 3-4% of lung adenocarcinoma [9]. As molecular-targeted antitumor drugs for MET mutation, capmatinib was approved by Japanese medical insurance in June 2020, based on the phase II trial of GEOMETRY mono-1 [10] study. For capmatinib in Japan, only FoundationOne CDx is currently approved as companion diagnostics. Capmatinib provided overall response rate of 41% and 68%, the median PFS of 5.4 and 12.4 months in pretreated and chemo-naive patients, respectively [10].
Herein, we report a rare patient with synchronous double primary lung adenocarcinomas harboring different driver mutations, EGFR L858R point mutation and MET exon 14 skipping mutation.
| Case Report | ▴Top |
Investigations
A 75-year-old Japanese woman was referred from the Cardiologic Department in our hospital in September 2020 because of a growing nodular shadow in the right upper lung field on chest X-ray films within 4 months’ interval. Chest computed tomography (CT) detected a nodule with the diameter of 2.6 cm in the right S1, another nodule in the left S1+2, which consisted of a partly solid ground-glass nodule with the maximum diameter of 1.8 cm and a solid core with the diameter 1.3 cm, and localized gland glass opacities in both upper lobes and right lower lobe.
Her serum carcinoembryonic antigen level was elevated to 12.6 ng/mL. She was an ex-smoker with an 11-pack-year smoking history. She had been treated with angiotensin-converting enzyme inhibitor (enalapril maleate), diuretic (azosemide and spironolactone) and β-blocker (carvedilol) since October 2013 for chronic heart failure after acute myocardial infarction, with reduced ejection fraction (22%), elevated H-brain natriuretic peptide (161.9 pg/mL, with a normal range < 18.4 pg/mL) and New York Heart Association functional class IV symptoms. She also had chronic kidney disease stage Kidney Disease Improving Global Outcomes (KDIGO) G3b (estimated glomerular filtration rate 41 mL/min) after acute kidney injury transiently treated with continuous hemodialysis filtration in December 2017. Her Eastern Cooperative Oncology Group Performance Status Scale (ECOG-PS) was 2.
Diagnosis
Bronchoscopic biopsy pathologically diagnosed the right S1 nodule as moderately differentiated adenocarcinoma (Fig. 1). Genetic testing of the right S1 adenocarcinoma (Oncomine Dx Target Test multi-CDx system (ODxTT); Thermo Fisher Scientific, Waltham, MA, USA) detected EGFR L858R point mutation. The tumor proportion score of a programmed cell death ligand 1 was below 1%. 18F-fluorodeoxyglucose positron emission tomography/CT showed abnormal uptakes in the nodules in the right S1 (maximum standardized uptake value (SUVmax): 7.1) and in the left S1+2 (SUVmax: 2.1), and in the bilateral inferior paratracheal lymph nodes #4R and 4L (SUVmax, 4.49) (Fig. 2). Contrast-enhanced brain magnetic resonance imaging did not find brain metastasis. From the left S1+2 nodule, we tried neither trans-bronchoscopic biopsy nor CT-guided aspiration biopsy because of technically difficult specimen collection. From the slightly swollen para-tracheal lymph nodes, we did not try endobronchial ultrasound-guided trans-bronchial needle aspiration, because the lymph nodes were apparently too small for us to obtain some tissues or cells. We suspected the para-tracheal lymphadenopathy as mediastinal metastases from the right S1 cancer, and the S1+2 nodule as another primary lung cancer. We made a diagnosis of adenocarcinoma with c-stage IIIA (cT1bN2M0) in the right S1, and suspected unconfirmed another lung cancer with c-stage IA2 (cT1bN0M0) in the left S1+2.
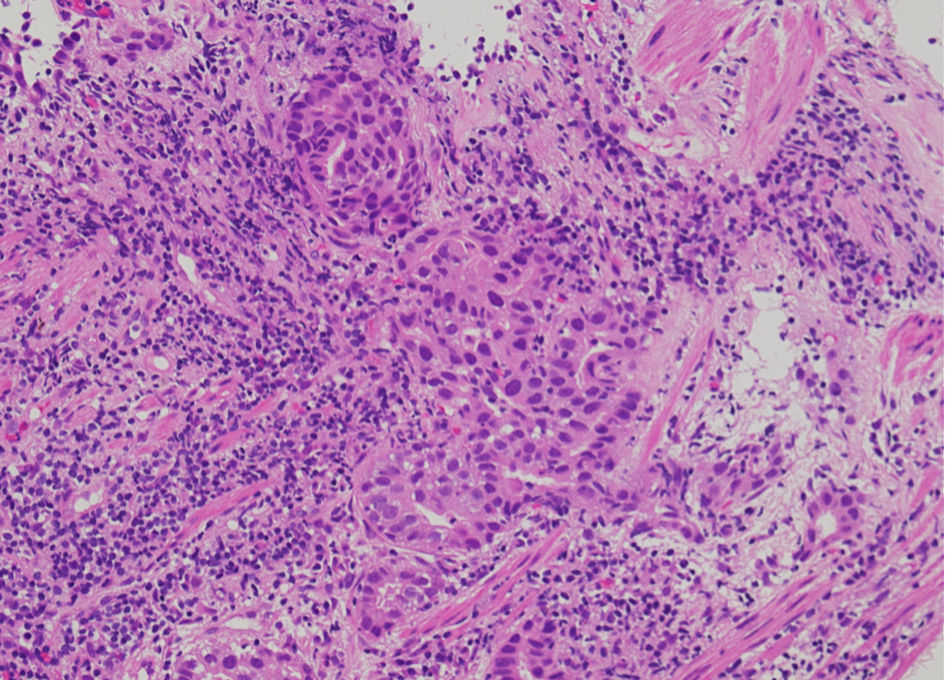 Click for large image | Figure 1. Histology of bronchoscopic specimen from the tumor in the right upper lobe (hematoxylin and eosin stain, magnification, × 200). |
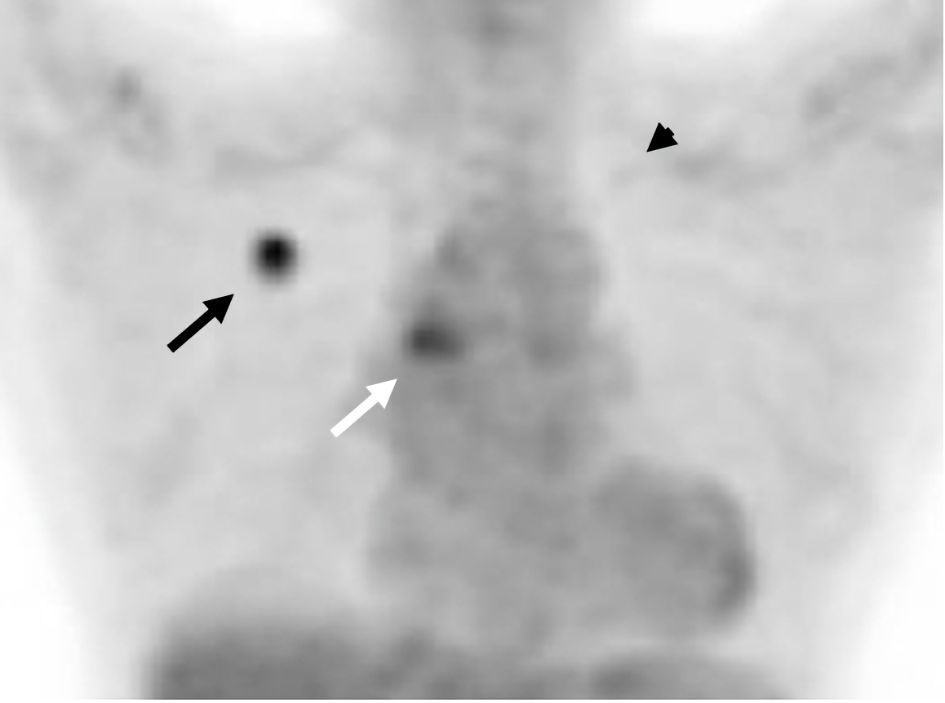 Click for large image | Figure 2. FDG-PET-CT showed the accumulation of FDG in the right S1 (SUVmax: 7.1) (shown by black arrow) and in the left S1+2 (SUVmax: 2.1) (shown by black arrowhead), and in the bilateral inferior paratracheal lymph nodes (SUVmax: 4.49) (shown by white arrow). FDG-PET-CT: 18F-fluorodeoxyglucose positron emission tomography-computed tomography. |
Treatment
Considering her general conditions, comorbidities and unwillingness to receive invasive and aggressive treatment, we decided not to provide curative-intent chemoradiotherapy and surgery. We also decided not to provide radial radiotherapy to both primary tumors and mediastinal metastases for fear of wide range of irradiation, adverse event of radiation pneumonitis and then deterioration of her heart failure. Instead, we started osimertinib (80 mg/day, once daily) in December 2020. We reduced the dose of osimertinib down to 40 mg/day due to grade 2 of diarrhea 2 weeks after the initiation of osimertinib. The right S1 cancer achieved partial response (PR) in April 2021, while the left S1+2 nodule enlarged against osimertinib (Fig. 3). The lymphadenopathies appeared in the left hilum in July 2021, and then in the left mediastinum and the left supraclavicular fossa in October 2021. Aspiration cytology from the left supraclavicular lymph nodes showed adenocarcinoma (Fig. 4). As a result of consultation to our otorhinolaryngologists, we gave up surgical biopsy from the supraclavicular lymph nodes at the risk of the surrounding large vessels. Thus, we suspected progression of another adenocarcinoma in the left S1+2 (cT1bN3M0, c-stage IIIB). She refused cytotoxic chemotherapy at that time. The treatment choice became molecular target therapy or immune checkpoint inhibitor or best supportive care. Because we could not obtain enough tissue specimen for ODxTT genetic test and AmoyDx Pan Lung Cancer PCR Panel, we requested FoundationOne® Liquid CDx tumor profiling test (Foundation Medicine, Inc., USA). This genetic test detected not only EGFR L858R point mutation (allele frequency (AF); 0.0014), but also MET exon 14 skipping mutation (AF; 0.13) (Table 1). Following the recommendation from the expert panel, we started capmatinib (800 mg/day, twice daily) in February 2022. The left S1+2 nodule and multiple lymph metastases remarkably reduced and achieved PR 2 weeks after the initiation of capmatinib. This drug was gradually reduced to 400 mg/day due to repeated grade 2 of nausea and fatigue.
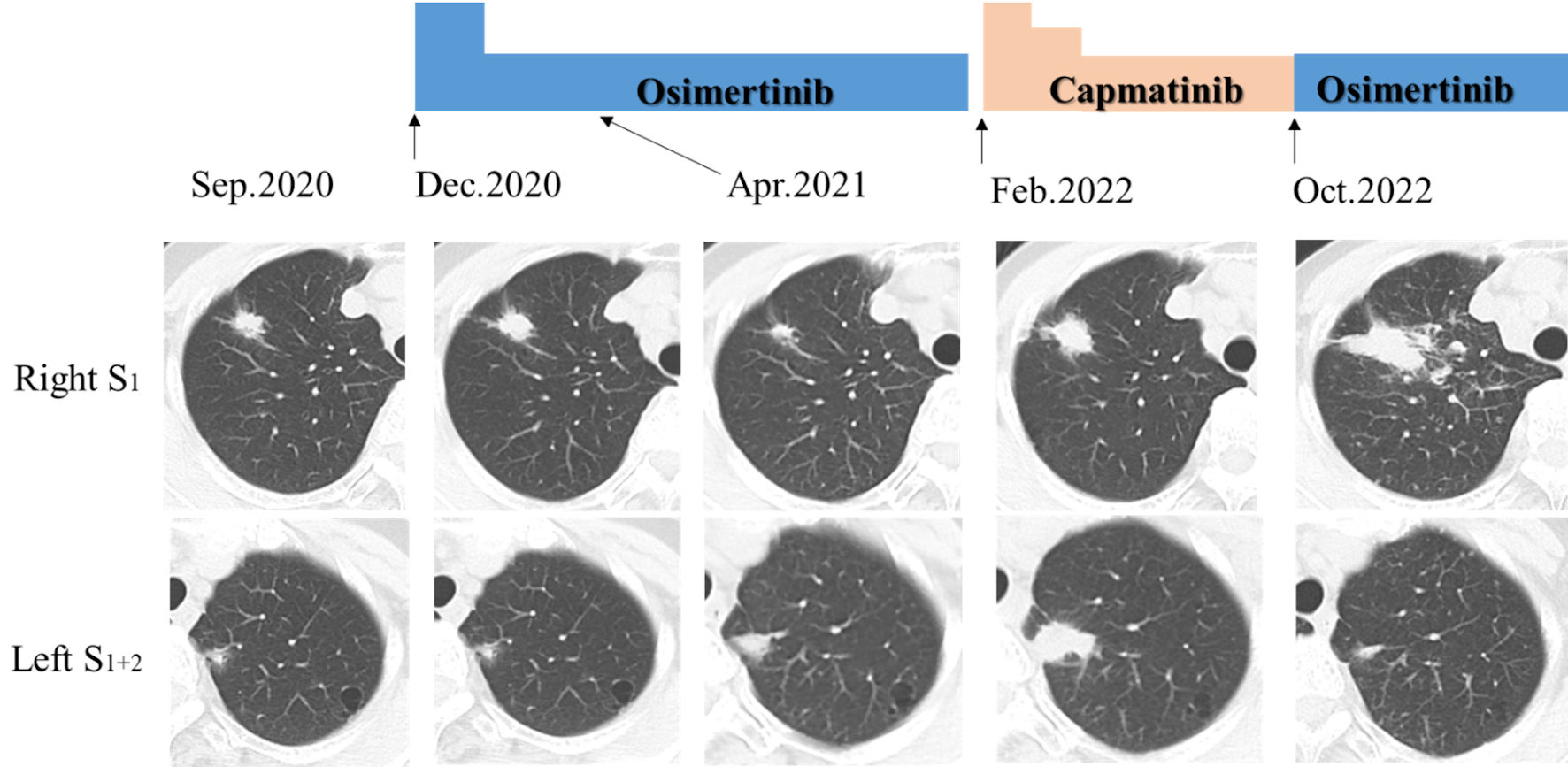 Click for large image | Figure 3. Chest computed tomography (CT) demonstrated nodular shadows in the right S1 and the left S1+2 at the diagnosis (in September 2020). These nodules changed before and 4 months after osimertinib administration (partial response in the right S1), and before and 8 months after capmatinib administration (partial response in the left S1+2 and progression disease in the right S1). |
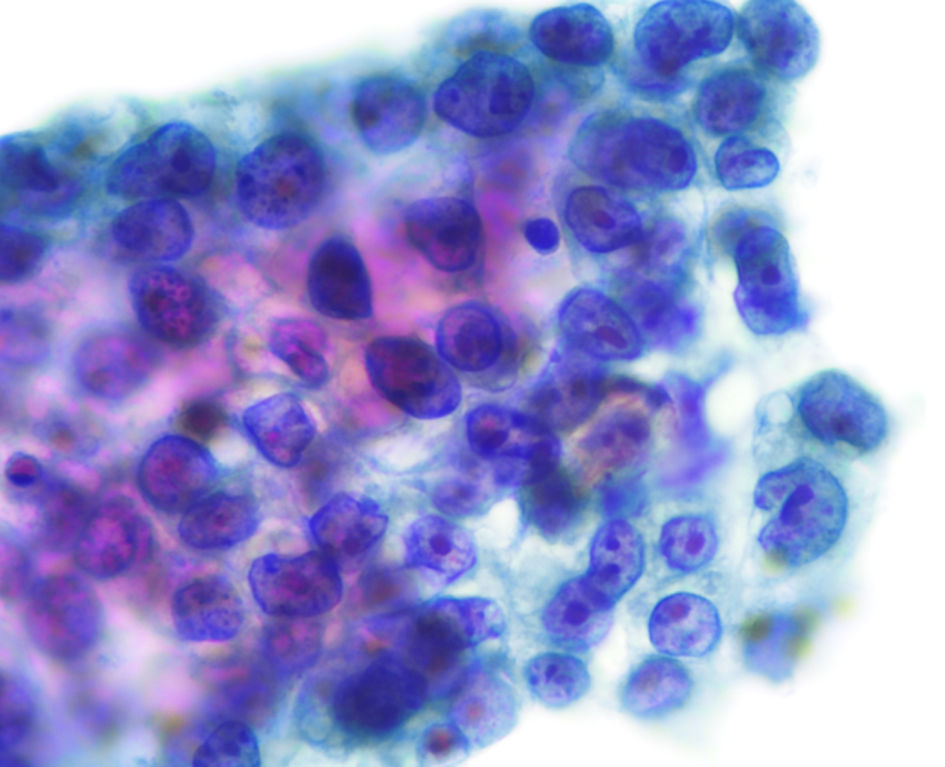 Click for large image | Figure 4. Cytology of fine-needle aspiration from the left supraclavicular lymph nodes (papanicolaou stain, magnification, × 400). |
 Click to view | Table 1. List of Positive Mutations Detected by FoundationOne® Liquid CDx Tumor Profiling Test |
Follow-up and outcomes
Although the left S1+2 primary nodule and lymph node metastases in hilum, mediastinum, supraclavicular fossa and left axilla maintained reduction, the chest CT in May 2022 detected new lesions of diffuse intrapulmonary metastases and right pleural dissemination. She still refused cytotoxic chemotherapy and immune checkpoint inhibitor. We were also afraid of rapid growth of the tumor after discontinuation of capmatinib. Thus, we dared to continue capmatinib in spite of progression disease. While the tumor in the left S1+2 did not grow, the tumor in the right S1 enlarged and then multiple liver metastases also appeared (Fig. 3). However, despite of switch from capmatinib to osimertinib in October 2022, new metastases appeared in the 9 - 12 thoracic vertebra, which was then irradiated for palliative pain control. After discontinuation of osimertinib and discharge from our hospital, she chose home palliative care service and died in January 2023.
| Discussion | ▴Top |
This case was the first report of synchronous double primary lung adenocarcinomas harboring different driver mutations, EGFR L858R point mutation and MET exon 14 skipping mutation.
Double mutations of EGFR and MET can be categorized into the following three patterns: 1) EGFR-mutant NSCLC acquired MET mutation during EGFR-TKIs; 2) a NSCLC harboring both EGFR and MET mutations; 3) double primary NSCLCs harboring different driver mutations. For pattern 1, MET amplification has been well known as an acquired resistance mechanism to EGFR-TKIs for EGFR-mutant NSCLC [11]. Whereas, there were only five cases of acquired MET exon 14 skipping mutation as a resistance mechanism to EGFR-TKIs for EGFR-mutant NSCLC (Table 2) [12-16]. Some of these cases had been successfully treated by addition of capmatinib [16] or crizotinib [13] to osimertinib or icotinib, respectively, or by switch from osimertinib to tepotinib [15] or crizotinib [14]. Thus, in the case of acquired MET mutation, addition of or switch to a MET-TKI may be an optimal option. For pattern 2, on the other hand, there were only four cases of NSCLCs initially and synchronously harboring this genetic combination of these two driver mutations (Table 3) [17-20]. Only one case had both MET exon 14 skipping mutation and amplification concomitantly [17], while EGFR mutation types varied among four cases. Two cases were successfully treated by combining afatinib and crizotinib [18, 20], one transiently responded to crizotinib monotherapy [17], while the other suddenly died 17 days after addition of crizotinib to osimertinib for unknown causes [19]. Treatment regimens and responses were various. Therefore, in the case of synchronous EGFR and MET mutations in one advanced tumor, combining two types of molecular-targeted drugs may be reasonable, but still be controversial in efficacy, adverse effects and costs. In the near future, a bispecific antibody targeting both EGFR and MET receptor alone or combined with an EGFR-TKI may be a potential candidate in this setting. For pattern 3, unlike these cases, our case was characterized by simultaneously coexisting two primary adenocarcinomas harboring different driver mutations. If our patient had been younger, better ECOG-PS and less comorbidities, the following two-stage strategy of sequential and curative-intent treatments might have been optimal: 1) in the initial stage for the right S1 advanced cancer, chemoradiotherapy alone or optionally followed by consolidation of an immune-checkpoint inhibitor, or surgical resection followed by adjuvant chemotherapy; and then 2) in the second stage for the left S1+2 early cancer, stereotactic body radio therapy or surgical resection. However, for frail cancer patients who seem intolerable for aggressive treatments, palliative chemotherapy may be optional. Thus, in the case of double primary cancers with different mutations, treatment strategy depends on various factors such as histology, stage, patient’s conditions, etc.
 Click to view | Table 2. Summary of the Previous Case Reports of Acquired MET Exon 14 Skipping Mutation as a Resistance Mechanism to EGFR-TKIs |
 Click to view | Table 3. Summary of the Previous Case Reports of Lung Cancers Harboring Coexistence of EGFR Mutation and MET Exon 14 Skipping Mutations |
In conclusion, we switched monotherapies from osimertinib to capmatinib for synchronous double primary adenocarcinomas harboring EGFR mutation and MET exon 14 skipping mutation, according to each cancer progression. The temporal and spatial heterogeneity in this case reinforces the need for primary tissue biopsy if dual primaries are suspected. Temporally distinct liquid biopsies, not standard at present, may be considered.
Learning points
The main take-away point is that treatment strategy for double primary cancers with different driver mutations depends on various factors such as histology, stage, patient’s conditions, etc. We experienced a rare case of synchronous double primary lung adenocarcinomas harboring different driver mutations, EGFR L858R point mutation and MET exon 14 skipping mutation. We should take various factors into consideration of treatment choice.
Acknowledgments
We thank Dr. Atsuo Inoue (Department of Diagnostic Radiology, NHO Osaka National Hospital) for his radiological diagnosis, and Dr. Kiyoshi Mori (Department of Central Laboratory and Surgical Pathology, NHO Osaka National Hospital) for his pathological diagnosis.
Financial Disclosure
None to declare.
Conflict of Interest
All authors have no conflict of interest to declare.
Informed Consent
Not applicable because the manuscript has been sufficiently deidentified to protect the patient.
Author Contributions
All authors were involved in diagnosis, treatment and management of the patient. S. Ando drafted the report. All authors read and critically reviewed the manuscript, and then approved the final submitted version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CT: computed tomography; ECOG-PS: Eastern Cooperative Oncology Group Performance Status Scale; EGFR: epidermal growth factor receptor; KDIGO: Kidney Disease Improving Global Outcomes; MET: mesenchymal-to-epithelial transition; MPLC: multiple primary lung cancer; NSCLC: non-small cell lung cancer; ODxTT: Oncomine Dx Target Test multi-CDx system; OS: overall survival; FDG-PET: 18F-fluorodeoxyglucose positron emission tomography; PFS: progression-free survival; PR: partial response; TKI: tyrosine kinase inhibitor
| References | ▴Top |
- Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res. 2014;6:119-134.
doi pubmed pmc - Shintani Y, Okami J, Ito H, Ohtsuka T, Toyooka S, Mori T, Watanabe SI, et al. Clinical features and outcomes of patients with stage I multiple primary lung cancers. Cancer Sci. 2021;112(5):1924-1935.
doi pubmed pmc - Zhao L, Liu C, Xie G, Wu F, Hu C. Multiple primary lung cancers: a new challenge in the era of precision medicine. Cancer Manag Res. 2020;12:10361-10374.
doi pubmed pmc - Liu M, He WX, Song N, Yang Y, Zhang P, Jiang GN. Discrepancy of epidermal growth factor receptor mutation in lung adenocarcinoma presenting as multiple ground-glass opacities. Eur J Cardiothorac Surg. 2016;50(5):909-913.
doi pubmed - Wu C, Zhao C, Yang Y, He Y, Hou L, Li X, Gao G, et al. High discrepancy of driver mutations in patients with NSCLC and synchronous multiple lung ground-glass nodules. J Thorac Oncol. 2015;10(5):778-783.
doi pubmed - Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892-2911.
pubmed pmc - Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci. 2016;107(9):1179-1186.
doi pubmed pmc - Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41-50.
doi pubmed - Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, Heng JC, et al. MET Exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34(7):721-730.
doi pubmed - Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, Tan DSW, et al. Capmatinib in MET Exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944-957.
doi pubmed - Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039-1043.
doi pubmed - Jiao Y, Fang C, Yang Y, Shao L, Huang Y, Sun Q, Dong Y. Rapid disease progression in a patient with advanced NSCLC harboring a germline MET Exon 14 skipping mutation: a case report. Onco Targets Ther. 2021;14:2417-2421.
doi pubmed pmc - Ou L, Tang Y, Deng Y, Guo L, He Q, He T, Feng W. Case Report: Durable partial response to icotinib plus crizotinib in a lung adenocarcinoma patient with double uncommon EGFR G719D/L861Q mutations and an acquired novel CUX1-MET fusion. Front Oncol. 2022;12:911362.
doi pubmed pmc - Pinquie F, Cortot AB, Chevalier LM, Morel A, Sandrini J, Guguen C, Morvan B, et al. A case report of successful treatment with crizotinib to overcome resistance to osimertinib in an EGFR mutated non-small-cell lung cancer patient harboring an acquired MET Exon 14 mutation. Clin Lung Cancer. 2022;23(2):e131-e134.
doi pubmed - Takamori S, Seto T, Yamaguchi M, Kinoshita F, Fujishita T, Ito K, Toyozawa R, et al. Case report: Success of tepotinib therapy in overcoming resistance to osimertinib in a patient with EGFR-mutant lung adenocarcinoma with a potential acquired MET exon 14 skipping mutation. Front Oncol. 2022;12:965741.
doi pubmed pmc - Xiang S, Zeng L, Xiang M, Zhang Y. Presence of MET exon 14 skipping and fusion as mechanism of osimertinb resistance in a lung adenocarcinoma with an EGFR exon 19 deletion that responds to combination of capmatinib and osimertinb: a case report. Heliyon. 2023;9(11):e22515.
doi pubmed pmc - Chen Y, Jiang B, He Y, Zhang C, Zhou W, Fang C, Gu D, et al. A lung adenocarcinoma patient with co-mutations of MET and EGFR exon20 insertion responded to crizotinib. BMC Med Genomics. 2022;15(1):141.
doi pubmed pmc - Kauffmann-Guerrero D, Kahnert K, Kumbrink J, Syunyaeva Z, Tufman A, Huber RM. Successful treatment of a patient with NSCLC harboring an EGFR mutation and a concomitant MET Exon 14 skipping mutation combining afatinib and crizotinib. Clin Lung Cancer. 2019;20(1):59-62.
doi pubmed - Liu Z, Song P, Zhou L, Ji D, Shen H, Dong H, Feng X. Osimertinib for an advanced NSCLC patient with two common EGFR mutations and a concomitant MET Exon 14 skipping mutation: a case report. Cancer Manag Res. 2023;15:645-650.
doi pubmed pmc - Zeng L, Xia C, Zhang Y, Yang N. Identification of a novel MET Exon 14 skipping variant coexistent with EGFR mutation in lung adenocarcinoma sensitive to combined treatment with afatinib and crizotinib. J Thorac Oncol. 2019;14(4):e70-e72.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


