| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 9, September 2024, pages 222-226
Hemophagocytic Lymphohistiocytosis Secondary to Acute Human Immunodeficiency Virus and COVID-19
Selia Chowdhurya, c, Harshavardhan Sanekommua, Paula Gonzaleza, Evgeniya Angelovab, Swapnil Patela
aDepartment of Medicine, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA
bDepartment of Pathology, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA
cCorresponding Author: Selia Chowdhury, Department of Medicine, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA
Manuscript submitted April 12, 2024, accepted July 29, 2024, published online August 10, 2024
Short title: HLH Secondary to Acute HIV and COVID-19
doi: https://doi.org/10.14740/jmc4226
| Abstract | ▴Top |
Hemophagocytic lymphohistiocytosis (HLH), characterized by acute and progressive hyperinflammation, is a rare syndrome documented in a limited number of coronavirus disease 2019 (COVID-19) and human immunodeficiency virus (HIV) cases. While severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can provoke extensive immune activation and systemic inflammation, individuals with HIV, susceptible to immune dysregulation, are at heightened risk of severe complications from SARS-CoV-2. We report a case of a 24-year-old male with no significant medical history presenting with fever, weight loss, respiratory symptoms, and acute renal failure. Initial diagnosis revealed HIV with a CD4 count < 20 and concurrent COVID-19 infection leading to development of HLH. Despite aggressive management including antiretroviral therapy (ART), dexamethasone and supportive care, the patient deteriorated rapidly, leading to multiorgan failure. Coinfection with HIV and SARS-CoV-2 presents unique challenges, especially when complicated by secondary conditions such as HLH, which remains a diagnostic and therapeutic dilemma. Prompt recognition and aggressive management are crucial, necessitating a high index of suspicion and comprehensive evaluation including bone marrow biopsy to improve diagnostic accuracy and guide therapeutic interventions in such complex scenarios.
Keywords: HLH; COVID-19; HIV; SARS-CoV-2
| Introduction | ▴Top |
Hemophagocytic lymphohistiocytosis (HLH) is an uncommon disorder characterized by excessive and unregulated activation of cytotoxic T lymphocytes, natural killer cells, and macrophages and decrease of interferon gamma production. This dysregulated immune response leads to the overproduction of inflammatory cytokines and macrophage activation, resulting in systemic inflammation and immune-mediated injury to multiple organs [1-5]. There are two forms of HLH: primary or familial and secondary or reactive. The secondary form is triggered by an infection, malignancy, or autoimmune stimulus without a genetic predisposition [2]. Here, we present a case of secondary HLH in a patient with two viral infections: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and human immunodeficiency virus (HIV). Each of these viruses alone is a rare cause of HLH [6, 7]. Our report sheds light on the potential for a synergistic effect when these viruses coexist and emphasizes the importance of considering HLH in patients with viral coinfections.
| Case Report | ▴Top |
A 24-year-old male with an unremarkable medical history presented with a progressive 1 - 2-month history of fatigue and lethargy, initially manifesting as a productive cough with hemoptysis, febrile episodes with diaphoresis and chills, and a significant unintentional weight loss of 50 pounds, accompanied by mild dyspnea. On arrival, the vitals were temperature of 36.8 °C, blood pressure of 105/67 mm Hg, heart rate of 104 beats per minute, respiratory rate of 18 with saturation of 91% on room air. His body mass index was 17.2. He was immediately kept on a 2-L nasal cannula with oxygen saturation of 98%. Physical examination revealed bitemporal wasting, scleral icterus, and nonpalpable liver and spleen. The laboratory results are shown in Table 1. Chest X-ray and computed tomography (CT) of the chest showed bilateral infiltrates. Kidney and abdominal ultrasounds were negative. Patient was started on a ceftriaxone and azithromycin for the treatment of presumed community-acquired pneumonia.
 Click to view | Table 1. Laboratory Results |
However, on day 2, he was transferred to the intensive care unit due to acute renal failure necessitating dialysis and pancytopenia of uncertain etiology. Concurrently, he tested positive for Streptococcus pneumoniae antigen in urine, prompting continuation of targeted antibiotic therapy. Workup for pancytopenia revealed a new diagnosis of HIV test with a CD4 count of < 20 (normal range: 500 - 1,600 cells/mm3) and viral load of 762,000 (normal range: < 20 - 75 copies/mL). He was started on bictegravir/emtricitabine/tenofovir alafenamide. Pneumocystis jiroveci pneumonia (PCP) prophylaxis was also started. Additionally, he was diagnosed with coronavirus disease 2019 (COVID-19) using the polymerase chain reaction (PCR), requiring escalating oxygen support. He finished 3 days of remdesivir before it was stopped due to worsening liver function and continued dexamethasone 6 mg daily for 10 days. On day 4, acute liver failure worsened with aspartate aminotransferase (AST) > 6,000, alanine aminotransferase (ALT) 1,829, and total bilirubin 3.9. He received N-acetyl cysteine for a total of 3 days with no improvement in the AST, ALT and total bilirubin. The workup for acute liver failure included: viral and autoimmune hepatitis panel, antimitochondrial antibody, ceruloplasmin, alpha-1 antitrypsin, and hemostatic iron regulator gene. All workup was negative. Despite aggressive supportive measures, including vasopressor therapy and blood product transfusions, multiorgan dysfunction ensued. Transthoracic echocardiogram (TTE) revealed a reduced ejection fraction of 21-25% and severe global hypokinesis. On day 5, the method of dialysis was changed to continuous venovenous hemodialysis (CVVHD). He also developed severe hepatic encephalopathy and acute respiratory distress syndrome (ARDS) requiring intubation. His antibiotics were escalated to meropenem. Bronchoscopy was done with lavage and was negative for Streptococcus pneumoniae. Quantitative PCR for PCP was also negative. The patient was paralyzed and kept in a prone position. Despite the proning, he had refractory hypoxemia.
On day 5, after the patient developed severe ARDS, idiopathic liver failure and heart failure, HLH was strongly considered. Patient was evaluated according to the HLH-2004 protocol, and he initially met four of the eight criteria, with a H-score of 185 points indicating a 70-80% probability of HLH. While waiting for bone marrow biopsy results, considering the patient’s unstable condition, he was started on dexamethasone 40 mg daily and etoposide was deferred. Bone marrow biopsy showed increased histiocytes with hemophagocytosis (Fig. 1), and this result met the fifth criterion for diagnosing HLH (Table 2). His pressor requirement escalated, and the patient developed severe acidosis despite the CVVHD. After 4 days of receiving dexamethasone, on day 9, the patient had a cardiac arrest and passed away.
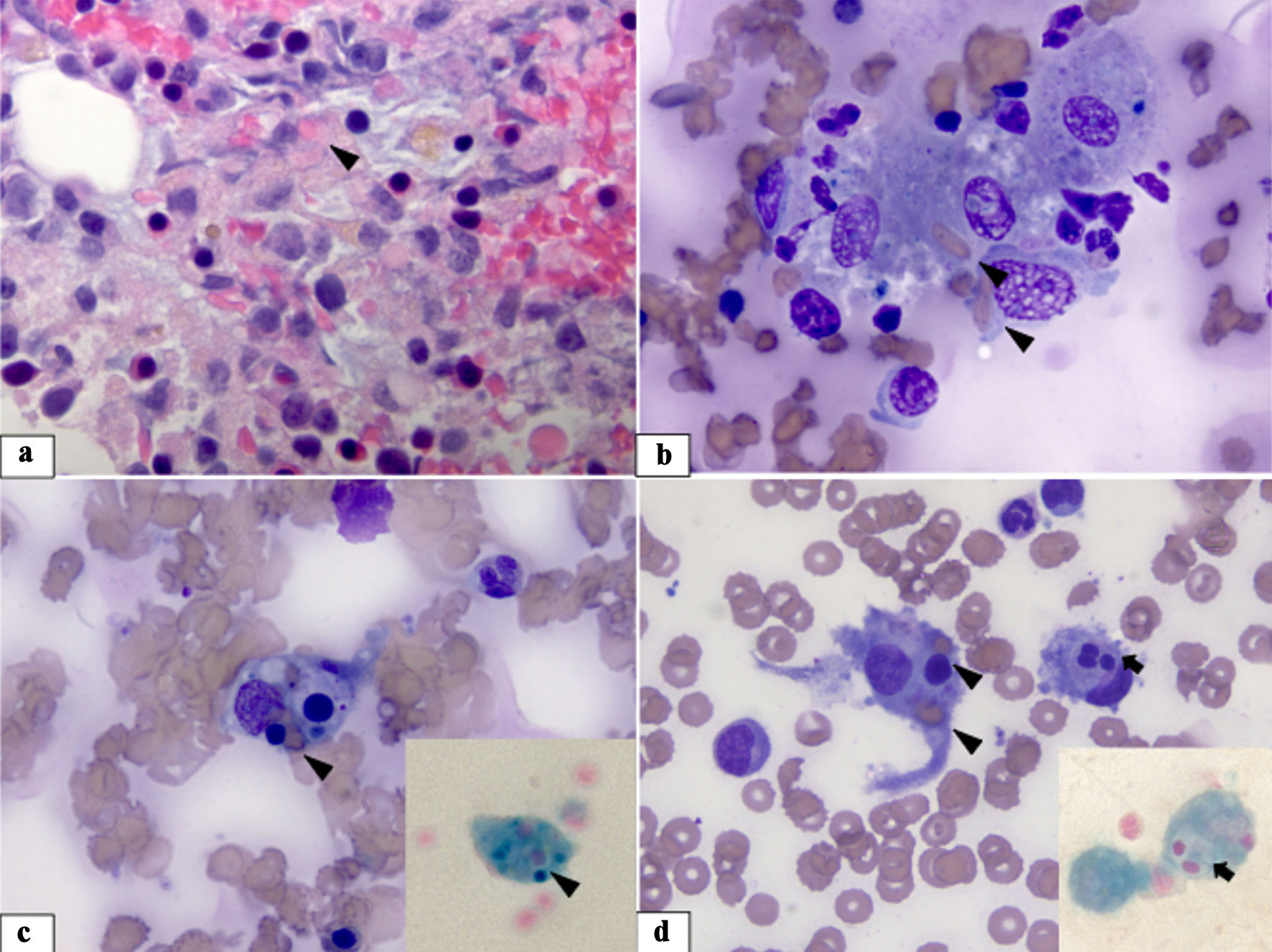 Click for large image | Figure 1. Bone marrow specimen. (a) Bone marrow biopsy showing an increased number of macrophages mixed with normal bone marrow elements. Some of the macrophages have engulfed red cells (arrowhead) in their cytoplasm (H&E stain, × 1,000). (b-d) Bone marrow aspirate smears revealing increased macrophages with increased phagocytic activity - phagocytosed red cells and platelets (arrowhead) and neutrophils (arrow) (Wright-Giemsa stain, × 1,000). The insets show iron-stained smears highlighting iron-laden macrophages with ingested hematopoietic cells (iron stain, × 1,000). H&E: hematoxylin and eosin. |
 Click to view | Table 2. HLH Criteria |
| Discussion | ▴Top |
The case described in this paper is of a 24-year-old who developed HLH secondary to both HIV and SARS-CoV-2 leading to multiorgan failure and ultimately, death. HLH is a hyperinflammatory syndrome that can be either congenital or acquired. Among various infectious triggers, Epstein-Barr virus and herpes virus are commonly associated with HLH. A study by Soy et al found herpes virus to be responsible for HLH in nearly 70% of diagnosed cases [8]. HLH secondary to respiratory viruses, especially SARS-CoV-2, is rare; approximately 100 cases have been reported in literature [6]. Even rarer is HLH triggered by HIV, with around 50 cases reported [7]. In our literature search, a combination of HIV and SARS-CoV-2 induced HLH has only been reported once.
Despite challenges posed by the inflammatory milieu of acute HIV and COVID-19, suspicion of HLH in our case was initially raised based on the clinical presentation and an elevated H-score of 220, indicating a high probability (93-96%) of the syndrome. However, diagnosing HLH alongside HIV and COVID-19 can pose challenges due to substantial overlap in inflammatory manifestations and organ damage between these conditions, compounded by variations in clinical presentations. Relying solely on the H-score may lack sensitivity, necessitating additional investigations such as bone marrow biopsy to enhance diagnostic accuracy, particularly in the context of COVID-19 [9, 10].
Treatment approaches for HLH vary depending on the underlying cause. However, corticosteroids are commonly utilized to mitigate immune system hyperactivity, while in some cases, etoposide is employed for its cytolytic effects on dividing T cells, thereby suppressing cytokine activity [11, 12]. Notably, some immunomodulatory agents recommended for COVID-19 treatment are also utilized in HLH management [13]. The initiation of antiretroviral therapy (ART) in individuals with advanced HIV and concurrent COVID-19 raises questions regarding its potential impact on exacerbating the hyperinflammatory state. The initiation of ART triggers a shift in cytokine production from a Th-2 to a Th-1 profile, inducing increases in interleukin (IL)-2, interferon-γ, as well as stimulating monocytes and macrophages, which may result in the production of tumor necrosis factor (TNF)α and IL-6, both of which are implicated in HLH and severe COVID-19 [13, 14]. In our case, ART initiation likely further stimulated the immune system, potentially exacerbating the already dysregulated inflammatory response characteristic of both conditions. This could have contributed to the development of multiorgan dysfunction and the rapid progression of the patient’s illness. The grim prognosis of HLH is compounded by the fact that the ultimate treatment (bone marrow transplantation) is often infeasible due to patients’ deteriorating health status. Consequently, mortality rates among adult HLH patients range from 42% to 88% [15].
In the medical literature, only one case report documents a treatment-naive patient with newly diagnosed HIV and SARS-CoV-2 coinfection who developed secondary HLH following ART initiation [13]. This patient also presented with biopsy-proven Kaposi sarcoma, along with detectable peripheral blood Epstein Barr virus (EBV) DNA PCR and cytomegalovirus (CMV) DNA PCR, and suspected PCP. Despite receiving ART, steroids, and appropriate antimicrobial therapy, the patient’s condition failed to improve, ultimately resulting in death [13]. With the addition of this second case, the mortality rate for this combination of infections along with HLH underscores its deadly nature. Clinicians should be aware of the grim prognosis and act quickly to implement appropriate management strategies.
Conclusions
This case report illustrates the daunting challenge posed by secondary HLH in the context of concurrent HIV and COVID-19 infections. Despite aggressive therapeutic measures, the patient experienced rapid deterioration, highlighting the aggressive nature of HLH and its poor prognosis. This underscores the critical importance of considering HLH in patients with HIV and COVID-19 coinfection who present with fever, cytopenias, and signs of organ failure. While serum triglyceride, fibrinogen, and ferritin levels aid in diagnosis, confirmation typically requires a bone marrow biopsy. Ultimately, if feasible, definitive treatment through allogeneic hematopoietic stem cell transplantation should be pursued. Additional investigation is needed to comprehend immune dysfunction, including HLH, in individuals experiencing concurrent HIV and SARS-CoV-2 infection.
Learning points
HLH may coexist with viral infections such as HIV and COVID-19, necessitating careful identification of its symptoms amidst overlapping manifestations of the underlying illnesses.
Confirmation of HLH often demands a bone marrow biopsy, underscoring the necessity for comprehensive diagnostics in intricate cases.
Precision in treatment planning is crucial, accounting for potential interactions among therapies for HIV, COVID-19, and HLH to ensure the best possible outcomes for patients
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Consent has been obtained from the patient.
Author Contributions
SC contributed to conception and design, manuscript writing and editing. HS contributed to conception and manuscript writing and editing. PG and SP contributed to final review and editing of manuscript. EA contributed to histopathological analysis. Manuscript has been read and approved by all the authors.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- La Rosee P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477.
doi pubmed - Bhatt NS, Oshrine B, An Talano J. Hemophagocytic lymphohistiocytosis in adults. Leuk Lymphoma. 2019;60(1):19-28.
doi pubmed - Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, Yamamoto K, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86(1):58-65.
doi pubmed - Imashuku S, Morimoto A, Ishii E. Virus-triggered secondary hemophagocytic lymphohistiocytosis. Acta Paediatr. 2021;110(10):2729-2736.
doi pubmed - Retamozo S, Brito-Zeron P, Siso-Almirall A, Flores-Chavez A, Soto-Cardenas MJ, Ramos-Casals M. Haemophagocytic syndrome and COVID-19. Clin Rheumatol. 2021;40(4):1233-1244.
doi pubmed pmc - Kayaaslan BU, Asilturk D, Eser F, Korkmaz M, Kucuksahin O, Pamukcuoglu M, Guner R. A case of Hemophagocytic lymphohistiocytosis induced by COVID-19, and review of all cases reported in the literature. J Infect Dev Ctries. 2021;15(11):1607-1614.
doi pubmed - Tabaja H, Kanj A, El Zein S, Comba IY, Chehab O, Mahmood M. A review of hemophagocytic lymphohistiocytosis in patients with HIV. Open Forum Infect Dis. 2022;9(4):ofac071.
doi pubmed pmc - Soy M, Atagunduz P, Atagunduz I, Sucak GT. Hemophagocytic lymphohistiocytosis: a review inspired by the COVID-19 pandemic. Rheumatol Int. 2021;41(1):7-18.
doi pubmed pmc - Nunez-Torron C, Ferrer-Gomez A, Moreno Moreno E, Perez-Mies B, Villarrubia J, Chamorro S, Lopez-Jimenez J, et al. Secondary haemophagocytic lymphohistiocytosis in COVID-19: correlation of the autopsy findings of bone marrow haemophagocytosis with HScore. J Clin Pathol. 2022;75(6):383-389.
doi pubmed - Leverenz DL, Tarrant TK. Is the HScore useful in COVID-19? Lancet. 2020;395(10236):e83.
doi pubmed pmc - Kumar B, Aleem S, Saleh H, Petts J, Ballas ZK. A personalized diagnostic and treatment approach for macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in adults. J Clin Immunol. 2017;37(7):638-643.
doi pubmed - Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125(19):2908-2914.
doi pubmed - Davari S, Taunk PS, Madaline T. COVID-19 complications in a newly diagnosed HIV Patient: a case of multiple herpesvirus reactivation and HLH post-ART initiation. Am J Case Rep. 2023;24:e939847.
doi pubmed pmc - Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762-768.
doi pubmed pmc - Premec H, Zivko M, Mijic M, Jelic-Puskaric B, Lalovac M, Filipec Kanizaj T, Sobocan N. Acute liver failure caused by secondary hemophagocytic lymphohistiocytosis after COVID-19 vaccination - case report and literature review. Int Med Case Rep J. 2023;16:449-455.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


