| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 6, June 2024, pages 110-114
A Rare Case of Gas Forming Enterobacter cloacae Leading to Bleeding Mycotic Pseudoaneurysm of Transplant Renal Artery Culminating in Graft Nephrectomy
Muhammad Abdul Mabood Khalila, c, Nihal Mohammed Sadagaha, Moayad Majed Alqurashib, Ahmed Abdelahad Bashaa, Hisham Ismael Mohamed Sakrana, Ibrahim Mohammed Nasser Assiria, Ghaleb Anas Aboalsamha, Salem H. Al-Qurashia
aCentre of Renal Diseases and Transplantation, King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia
bDivision of Adult Infectious Diseases, Department of Medicine, King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia
cCorresponding Author: Muhammad Abdul Mabood Khalil, Centre of Renal Diseases and Transplantation, King Fahad Armed Forces Hospital, Jeddah 23311, Saudi Arabia
Manuscript submitted April 20, 2024, accepted May 20, 2024, published online May 25, 2024
Short title: Mycotic PA of Transplant Renal Artery by ECC
doi: https://doi.org/10.14740/jmc4231
| Abstract | ▴Top |
Enterobacter cloacae belongs to Enterobacter genus. It is a common gram-negative, facultative anaerobic, rod-shaped organism. It causes a variety of nosocomial infections including urinary tract infection, pneumonia, wound infection, osteomyelitis and endocarditis. Over time Enterobacter cloacae complex (ECC) has developed to be resistant to antibiotics including carbapenem. It has been rarely reported to cause gas gangrene and never been reported to cause pseudoaneurysm (PA) of transplant renal artery. We report and share our experience with this rare case of gas forming and muti-drug resistant ECC which led to mycotic PA of transplant renal artery, complicated by bleeding and infected hematoma and which resulted in graft nephrectomy.
Keywords: Enterobacter cloacae complex; Gas forming infection; Mycotic pseudoaneurysm; Kidney transplant; Graft nephrectomy
| Introduction | ▴Top |
Kidney transplantation is the treatment of choice for patients with end-stage renal disease. However, it is associated with serious complications. Intense immunosuppression in early transplant period can lead to serious infections. Mycotic aneurysm of the graft renal artery can lead to graft nephrectomy and even mortality [1]. Commonly fungal infections such as Candida spp. [2, 3] and Aspergillus spp. [4] along with various bacterial infections such as Pseudomonas spp. and Klebsiella spp. [5] have been reported in literature to cause mycotic aneurysm in kidney transplant patients. Gas forming organisms generally are rare to cause mycotic aneurysm or pseudoaneurysm (PA). Among gas forming organism, our literature search showed species such as Bacteroides fragilis, Clostridium spp. and Propionibacterium spp. which caused mycotic aneurysm and PA in general population [6]. We found no prior report implicating Enterobacter cloacae complex (ECC) to cause PA. Here we highlight a case of multi-drug resistant ECC causing mycotic PA of transplant renal artery and infected hematoma which despite antibiotic treatment, ended up in graft nephrectomy.
| Case Report | ▴Top |
Investigations
A 71-year-old gentle man who was known diabetic, hypertensive, with coronary artery disease and end-stage renal disease, underwent living-related kidney transplant on August 18, 2023. His past medical history included a coronary artery bypass surgery done 2 years back. He had induction with basiliximab and methyl prednisolone. He had no delayed graft function. Post-transplant ultrasound showed good perfusion. Patient was discharged on August 23, 2023 with a creatinine of 71 µmol/L. On the 13 September, he presented to emergency department with fever, vomiting and diarrhea. On examination, he was febrile with temperature of 39.2 °C, respiratory rate of 16/min, heart rate of 74/min and blood pressure of 160/90 mm Hg. His abdomen was soft with mild lower abdominal tenderness. Chest and cardiovascular examinations were normal. An initial imaging with computed tomography (CT) scan showed peri-graft hematoma with gas bubbles for which a drain was placed surgically. Despite initial management with intravenous (IV) meropenem and fluids, patient developed mild coagulopathy and graft dysfunction. On day 7, patient had sudden deterioration in his health. The drain showed active bleeding and a drop of hemoglobin from 8 to 5 g/L was observed. Re-imaging with Doppler ultrasound (US) and CT without contrast found PA of the renal artery and re-accumulation of hematoma. An urgent operation theater was arranged. He had two cardiac arrests in the operation theater and was successfully resuscitated. Surgical finding showed a collection of pus mixed with blood and necrotic tissues in the peri-renal area. An infected PA of the renal artery was found oozing blood and a graft nephrectomy was done.
Diagnosis
On admission, his white blood cell (WBC) count was 9.86 × 109/L (3.3 - 10.8 × 109/L), hemoglobin was 9.5 g/dL (13.5 - 17.5 g/dL) and platelets were 244 × 109/L (150 - 450 × 109/L). C-reactive protein (CRP) was 165 mg/L (≤ 5 mg/L). He had normal graft function with urea of 6.5 mmol/L (2.8 - 7.3 mmol/L), creatinine of 77 µmol/L (64 - 11 µmol/L), sodium of 137 mmol/L (135-145 mmol/L), potassium of 4.1 mmol/L (3.5 - 5.1 mmol/L), and carbon dioxide in serum of 19 mmol/L (22 - 29 mmol/L). Initial Doppler US identified normal perfusion with a peri-graft collection. An urgent CT without contrast showed transplanted kidney which was edematous with significant retroperitoneal fat stranding. There was a complex multilocular perinephric collection having dimension of 11 × 3.4 × 16.4 cm. Collection showed different levels of hyper-densities indicating different ages of bleeding. The collection was inseparable from the left psoas muscle and left external iliac vessels. Tiny gas locules were noted in most inferior and medial aspect of the graft (Fig. 1). The blood culture grew ECC (multi-drug resistant organism). The organism was detected using VITEK-2 system (BioMerieux, Brussels, Belgium) which has the ability of excellent identification, but it could not differentiate between the species among the complex. It was sensitive to amikacin, meropenem, imipenem, ertapenem but was resistant to gentamicin, ceftriaxone, ampicillin, cefepime and ciprofloxacin. Urine analysis was negative for nitrite and culture did not grow any organism. Upon first CT finding, surgical evacuation was done and a drain was put. Tissue and fluid cultures which were taken also grew the same organism with similar sensitivities as that of the blood culture.
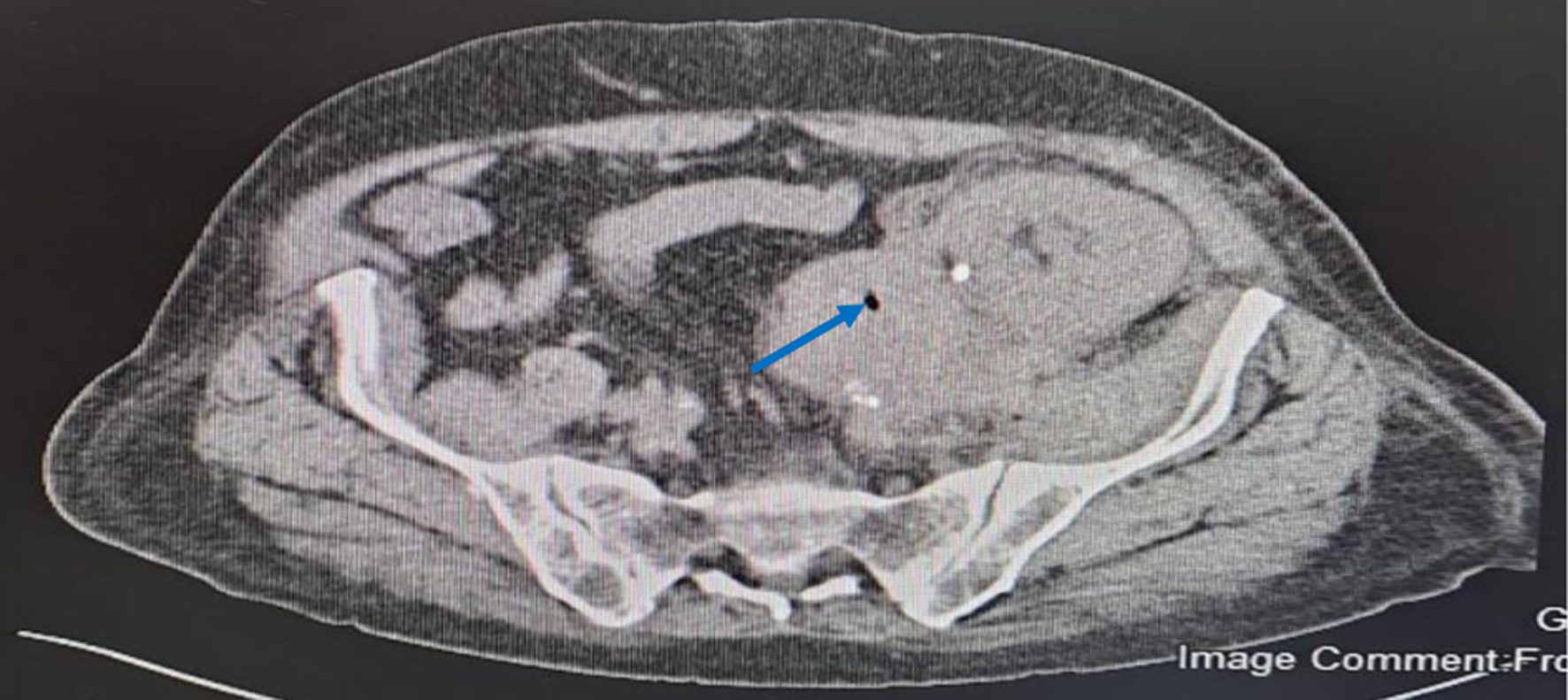 Click for large image | Figure 1. Unenhanced computed tomography showing collection with different level of hyper-densities and air locule. The blue solid arrow points to air locule. |
Despite IV antibiotics and drainage of hematoma, patient remained sick. His WBC went up to 19.3 × 109/L. CRP remained high at 179 mg/L. Procalcitonin was 15.4 ng/mL. His graft function deteriorated and creatinine went up to 203 µmol/L. On day 7 of admission, patient had sudden deterioration in his clinical status with the drain showing active bleeding. He had mild coagulation derangement with prothrombin time of 18.1 s, international normalized ratio of 1.35 and activated partial thromboplastin time (APTT) of 33.3 s. The patient’s hemoglobin dropped from 8 to 5 g/L. An urgent CT scan showed a large left iliac fossa peripherally enhancing lobulated heterogenous hypodense collection measuring 10.7 × 7 × 8.15 cm. Its content had a mottled appearance of air locules and heterogenous hyper-densities in the lower aspect suggestive of blood component. It was associated with surrounding fat stranding and was inseparable from psoas muscle. It was engulfing left external iliac artery causing irregularity of its wall. The radiologist suspected liquified hematoma with superadded infection leading to abscess formation. The Doppler US done identified a possible PA in the graft renal artery (Fig. 2) which was reconfirmed intraoperatively. Renal biopsy taken during surgery for graft nephrectomy showed mildly congested renal glomeruli with no gross abnormality in cortical and medullary parenchyma. The renal pelvis showed hemorrhage, neutrophilic infiltration, fat necrosis and foreign body giant cell reaction. No granuloma or fungal organism was seen. Special stains (Grocott or Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS)) for fungal organism were also negative.
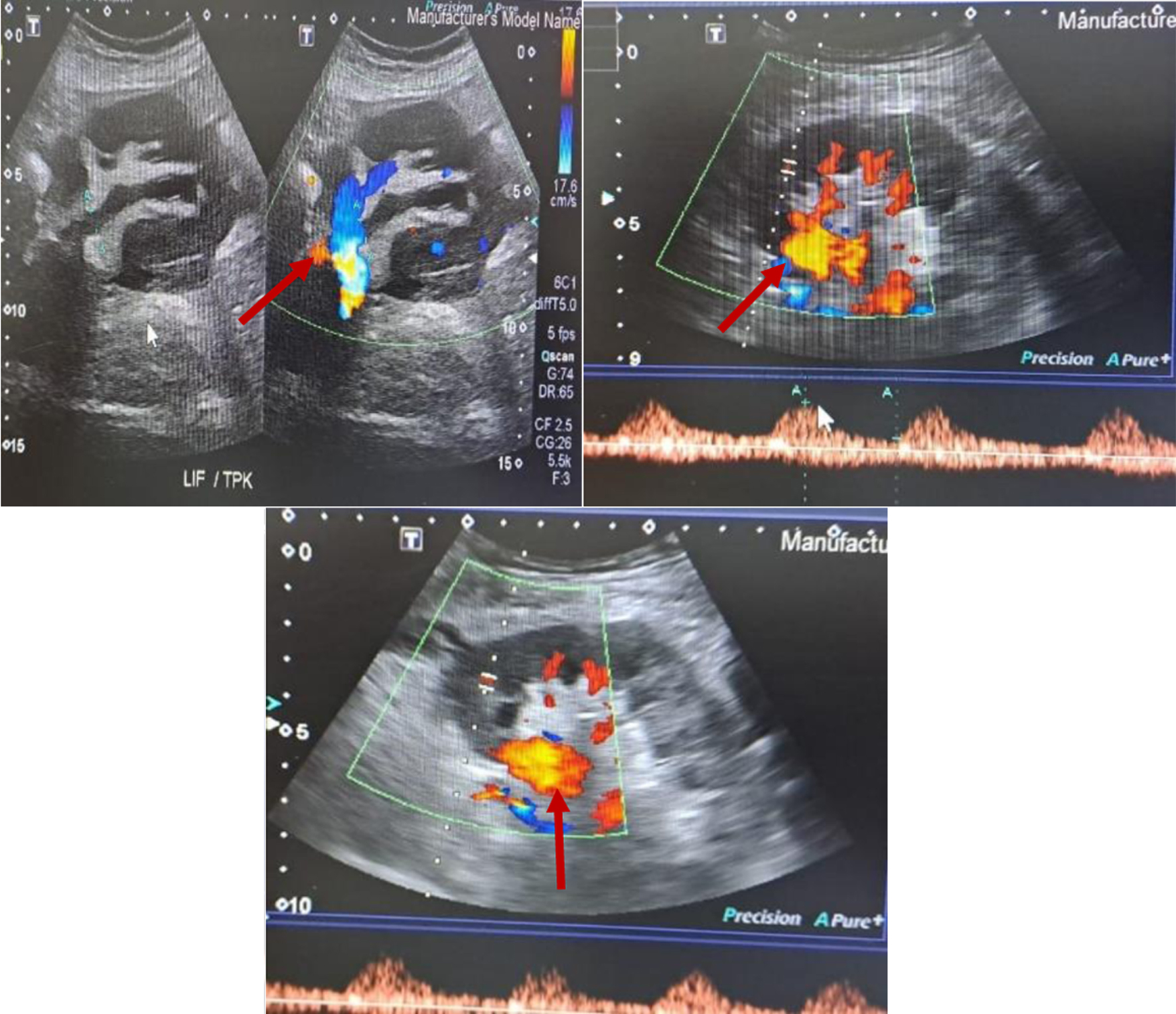 Click for large image | Figure 2. Doppler ultrasound showing pseudoaneurysm. The red solid arrows point to pseudoaneurysm. |
Treatment
Upon admission, patient was started on IV meropenem 1 g every 8 h along with IV normal saline. After the first CT scan, patient was taken into operation theater for evacuation. An infected hematoma was found which was drained. No active bleeding source was identified. A drain was placed and patient IV meropenem was continued. Despite antibiotics, the patient developed mild coagulopathy and progressive worsening of the graft function. On day 7, the patient suffered a sudden deterioration in his clinical status. Active bleeding was noticed in the drain tube and a significant drop of hemoglobin of 3 g/L was observed. Patient was resuscitated with blood transfusion. Re-imaging with Doppler US and CT showed mycotic PA of renal artery and re-accumulation of hematoma as described in diagnosis. Patient was taken to operation theater where he has twice cardiac arrest due to asystole. He was resuscitated successfully. After identification of bleeding PA, graft nephrectomy was performed and hemostasis was secured. A drain was placed in. Thereafter, patient was put on three-time per week hemodialysis. IV antibiotics were continued. Subsequently patient remained stable during his stay.
Follow-up and outcomes
A total of 6 weeks of IV meropenem was completed. The patient remained stable and was discharged on a three-time per week hemodialysis as in outpatient setting.
| Discussion | ▴Top |
ECC is a group of closely related gram-negative, facultative anaerobic, rod-shaped, non-spore-forming bacteria [7]. They are considered to cause opportunistic infections. The presented patient being elderly, diabetic and immunocompromised could have made him more prone to develop sepsis and its sequalae. ECC organisms have the ability to acquire resistance to antibiotics by producing extended spectrum beta-lactamase enzymes, cephalosporinase enzymes and carbapenemase enzymes too [7]. Moreover, efflux and impermeability of the cell wall can complicate the mechanism of resistance and make this group of bacteria one of the resistant bacteria that is difficult to treat [7]. Studies on pathogenesis of ECC identified various virulence factors. Barnes et al reported that Enterobacter ssp. after adhesion with epithelium produces enterotoxin, α-hemolysin and Shiga-like toxin II which result in cellular damage [8]. The type III secretion system is another virulent factor. The type III secretion system consists of several proteins that deliver toxins directly into the host cells [9]. Krzyminska et al showed that TTSS genes were present in 27% of the ECC isolated from clinical specimens [10]. Another important ability of ECC is that it may induce apoptosis of human intestinal epithelial cells [11]. Curli fimbriae in various organisms help to mediate adhesion and invasion of host cells along with ability to bind host protein, which help in biofilm formation and colonization [12]. Kim et al have shown a good correlation between the phenotypic detection of curli fimbriae and the genotypic detection of the curli gene in ECC [13]. Finally, ECC is able to produce lipopolysaccharide, which is well known to cause host cell damage [14].
Enterobacter spp. have been implicated in various nosocomial infections including urinary tract infections, respiratory infections, soft tissue infections, osteomyelitis, and endocarditis [15]. Yet, ECC has been rarely reported to cause gas formation in infected tissues. At the same time, it has never been reported to cause PA of transplant renal artery. Traditionally Clostridium spp. have been thought to produce gas. The CT scan of our patient showed air locules in the peri-graft hematoma pointing to bacteria which can make gas. Culture from all the three sources including peripheral blood, drain fluid and tissue culture from the surgery grew ECC. Being a facultative anaerobe, under stress ECC may become completely dependent on anaerobic respiration leading to gas formation. An analysis of gas sample collected from infected tissues by gas forming organism showed 5.9% hydrogen, 3.4% carbon dioxide, 74.5% nitrogen and 16.1% oxygen leading to speculation that this could be due to glucose fermentation [16]. Our literature search showed gas formation by ECC in a patient with leukemia [17] and a liver transplant recipient [18]. We found none in kidney transplant recipients causing gas formation and PA of renal artery of the graft.
Small aneurysms/PA are asymptomatic and may be an incidental finding picked up on imaging done for other reason. On the other hand, large aneurysm may cause pressure symptoms. Mycotic aneurysm and PA are one of the rare complications in recipients of kidney transplant with an incidence of less than 1% [5]. Mycotic PA and aneurysm present with fever, pain, graft dysfunction, fatal hemorrhage and even graft loss [1]. Conservative managements along with active surveillance, endovascular intervention such as thrombin injection, endovascular stenting and coiling, surgical repair and graft nephrectomy are the various treatment options [1]. PA is a false aneurysm secondary to arterial damage complicated by hematoma which over time develops a wall due to cross linkage between fibrin and platelets. It does not contain any true vessel wall [19]. Management of mycotic PA includes observation, endovascular intervention such as thrombin injection, endovascular stenting and coiling, surgical repair and graft nephrectomy [20]. Small PA < 2 cm should be observed and may close spontaneously [21]. Larger aneurysm and PA may need intervention. Bindi and his colleagues in their literature review of renal allograft renal artery aneurysm found approximately 16% (9/56) mortality and 62.5% (35/56) of patients ended with graft nephrectomy [1]. Infected aneurysm and PA are managed with surgical removal of PA and antibiotics should be given for a total of 6 weeks [22]. Bleeding mycotic aneurysm/PA is an emergency and can result in life-threatening situation. The presented patient despite being on meropenem, remained sick and later on developed active bleeding and hemodynamic instability prompting transplant team to go for graft nephrectomy. Graft nephrectomy could be demoralizing for the transplant team and the patient but is lifesaving. Our patient was successfully resuscitated and post-procedure, he was started on three-time per week hemodialysis.
Learning points
Mycotic PA of transplanted renal artery is a rare but life-threatening complication which may lead to graft nephrectomy. Mycotic PA due to gas forming ECC though rare but should be considered in etiology of mycotic PA of transplant renal artery. Appropriate and prolonged course of antibiotics along with graft nephrectomy may be lifesaving in cases of PA caused by gas forming ECC.
We conclude that ECC is a rare gas forming organism which can lead to bleeding mycotic PA of transplant renal vessel. Kidney transplant recipients are prone to a variety of viral and bacterial infections in the first 6 months due to intense immunosuppression. ECC should be considered in differential diagnosis of early transplant mycotic PA.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Written informed consent was obtained from the patient.
Author Contributions
MAMK, SHA, NMS and GAA conceived the idea of the study. MAMK drafted the initial draft and all authors (NMS, MMA, AAB, HIMS, IMNA, GAA, SHA) critically reviewed the draft. MAMK revised and all authors (NMS, MMA, AAB, HIMS, IMNA, GAA, SHA) approved the final manuscript.
Data Availability
All data in our report were obtained from the patient’s hospitalization. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
CT: computed tomography; ECC: Enterobacter cloacae complex; IV: intravenous; PA: pseudoaneurysm
| References | ▴Top |
- Bindi M, Ferraresso M, De Simeis ML, Raison N, Clementoni L, Delbue S, Perego M, et al. Allograft artery mycotic aneurysm after kidney transplantation: a case report and review of literature. World J Clin Cases. 2020;8(5):912-921.
doi pubmed pmc - Madhav D, Kumar P, Mohan C, Vijay, Mahesh U, Anusha Suneetha, et al. Candida-associated pseudo-aneurysm of the transplant renal artery presenting as malignant hypertension and managed successfully without nephrectomy. Saudi J Kidney Dis Transpl. 2015;26(5):1000-1005.
doi pubmed - Laouad I, Buchler M, Noel C, Sadek T, Maazouz H, Westeel PF, Lebranchu Y. Renal artery aneurysm secondary to Candida albicans in four kidney allograft recipients. Transplant Proc. 2005;37(6):2834-2836.
doi pubmed - Asif S, Bennett J, Pauly RR. A Rare case of an infectious pseudoaneurysm due to aspergillus flavus in the setting of renal transplant. Cureus. 2019;11(3):e4208.
doi pubmed pmc - Leonardou P, Gioldasi S, Zavos G, Pappas P. Mycotic pseudoaneurysms complicating renal transplantation: a case series and review of literature. J Med Case Rep. 2012;6:59.
doi pubmed pmc - Brook I. Anaerobic bacteria as a cause of mycotic aneurysm of the aorta: microbiology and antimicrobial therapy. Curr Cardiol Rev. 2009;5(1):36-39.
doi pubmed pmc - Davin-Regli A, Pages JM. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:392.
doi pubmed pmc - Barnes AI, Ortiz C, Paraje MG, Balanzino LE, Albesa I. Purification and characterization of a cytotoxin from Enterobacter cloacae. Can J Microbiol. 1997;43(8):729-733.
doi pubmed - Stuber K, Frey J, Burnens AP, Kuhnert P. Detection of type III secretion genes as a general indicator of bacterial virulence. Mol Cell Probes. 2003;17(1):25-32.
doi pubmed - Krzyminska S, Mokracka J, Koczura R, Kaznowski A. Cytotoxic activity of Enterobacter cloacae human isolates. FEMS Immunol Med Microbiol. 2009;56(3):248-252.
doi pubmed - Krzyminska S, Koczura R, Mokracka J, Puton T, Kaznowski A. Isolates of the Enterobacter cloacae complex induce apoptosis of human intestinal epithelial cells. Microb Pathog. 2010;49(3):83-89.
doi pubmed - Zogaj X, Bokranz W, Nimtz M, Romling U. Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infect Immun. 2003;71(7):4151-4158.
doi pubmed pmc - Kim SM, Lee HW, Choi YW, Kim SH, Lee JC, Lee YC, Seol SY, et al. Involvement of curli fimbriae in the biofilm formation of Enterobacter cloacae. J Microbiol. 2012;50(1):175-178.
doi pubmed - White PJ, Gaston MA, Wilkinson SG. Composition of O-antigenic lipopolysaccharides from Enterobacter cloacae. Microbiol Immunol. 1984;28(11):1169-1179.
doi pubmed - Ramirez D, Giron M. Enterobacter infection. Treasure Island, FL: StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK559296/ (accessed on April 20, 2024).
- Chi CH, Chen KW, Huang JJ, Chuang YC, Wu MH. Gas composition in Clostridium septicum gas gangrene. J Formos Med Assoc. 1995;94(12):757-759.
pubmed - Fata F, Chittivelu S, Tessler S, Kupfer Y. Gas gangrene of the arm due to Enterobacter cloacae in a neutropenic patient. South Med J. 1996;89(11):1095-1096.
doi pubmed - Pariente D. Gas gangrene in a pediatric liver transplant due to infection by Enterobacter cloacae. Pediatr Radiol. 1993;23(4):331.
pubmed - Rivera PA, Dattilo JB. Pseudoaneurysm. Treasure Island, FL: StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542244/.
- Anders L, Stephens R, Laub M, Amarath-Madav R, Mirza A, Saeed MI. Management of transplant renal artery pseudoaneurysm and literature review. Case Rep Transplant. 2022;2022:6232586.
doi pubmed pmc - D'Souza J, Bedi VS, Indrajit IK, Pant R. Non surgical management of pseudoaneurysms. Med J Armed Forces India. 2007;63(2):115-119.
doi pubmed pmc - Kim YW. Infected aneurysm: current management. Ann Vasc Dis. 2010;3(1):7-15.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


