| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 7, July 2024, pages 130-135
Metastatic Bladder Urothelial Carcinoma Masquerading as Myositis
Christopher Dillia, William Mia, Miguel A. Guzmana, b
aDepartment of Pathology, Saint Louis University, St. Louis, MO, USA
bCorresponding Author: Miguel Guzman, Department of Pathology, Saint Louis University, SSM Health Cardinal Glennon Ch, St. Louis, MO 63104, USA
Manuscript submitted May 1, 2024, accepted June 5, 2024, published online June 19, 2024
Short title: Metastatic Bladder Urothelial Carcinoma as Myositis
doi: https://doi.org/10.14740/jmc4236
| Abstract | ▴Top |
Skeletal muscle metastases are uncommon, and metastases of urothelial carcinoma to the skeletal muscle are particularly rare. The most common presentation of skeletal muscle metastases is a focal mass, but their clinical and radiographic findings can be diverse. We present an unusual case of a 71-year-old male without prior known history of malignancy who presented with skeletal muscle pain with imaging most consistent with an inflammatory or infectious process but was ultimately determined to be metastatic urothelial carcinoma from the bladder. This case demonstrates the need to keep an expanded differential for muscular pain, particularly when initial treatments are ineffective.
Keywords: Metastatic urothelial carcinoma; Metastatic transitional cell carcinoma; Myositis; Skeletal muscle metastasis
| Introduction | ▴Top |
Urinary bladder cancers are the 10th most common cancer globally, with urothelial (transitional cell) carcinoma accounting for over 90% of all bladder cancer cases [1]. Bladder cancers are more common in men, with new cases of bladder cancer presenting in nearly a 4:1 ratio of males to females [2]. Histopathologic evaluation of urothelial carcinoma involves assessment of invasion [3, 4]. Most (approximately 70%) of urothelial carcinoma cases are non-invasive or early invasive (without muscularis propria invasion), while detrusor muscle invasion is seen in the remaining cases [5]. An estimated 10-15% of patients with muscle-invasive urothelial carcinoma will have metastasis at the diagnosis, with the most common sites including the liver, lung, and bone [6, 7]. Skeletal muscle metastases (SMMs) are extremely rare in general and are particularly uncommon for primary urothelial cell carcinomas [8]. We present an unusual case of a patient with urothelial carcinoma metastatic to skeletal muscle in a patient with no known urothelial carcinoma prior to presentation.
| Case Report | ▴Top |
Investigations
A 71-year-old male with past medical history notable for coronary artery disease and type 2 diabetes mellitus presented to the emergency department for evaluation of a 5-month history of progressively worsening right lower extremity pain and swelling. His pain localized to the right quadriceps muscle group, rated 8 out of 10 in severity and was associated with an inability to bend the knee. Treatment with non-steroidal anti-inflammatory drugs (NSAIDs) provided mild relief, while physical therapy and steroids were ineffective. The patient denied trauma to the affected limb and any additional symptoms other than pain and swelling. Review of systems was otherwise negative, including skin, neurological, sensory, and genitourinary symptoms. He had a several decade-long history of statin use, and denied alcohol, tobacco, and illicit drug use. Physical examination was notable for right quadriceps swelling and warmth without overlying skin changes. Prior to admission, the patient had undergone multiple imaging studies, including X-rays and magnetic resonance imaging (MRI) of the spine and hip, revealing no pathologic process.
Diagnosis
The initial clinical impression was that of myositis, either infectious or inflammatory. Computed tomography (CT) scans of the right thigh with contrast were performed and shown in Figure 1. The imaging shows diffuse edema and abnormal enhancement within the quadriceps musculature, consistent with myositis, as well as rhabdomyolysis and compartment syndrome. Blood cultures were drawn for concern of infectious etiology, and empiric antibiotic treatment with intravenous vancomycin and zosyn was initiated. The microbiology results showed no growth, and the antibiotics were ineffective for symptom relief. A comprehensive antibody panel for autoimmune antibodies, including anti-nuclear antibody and myositis-specific antibodies, was completely negative. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were elevated to 51 mm/h (RR 0 - 20 mm/h) and 14.10 mg/dL (RR < 0.50 mg/dL), respectively. Additional CT scans of the chest, abdomen, and pelvis were unremarkable.
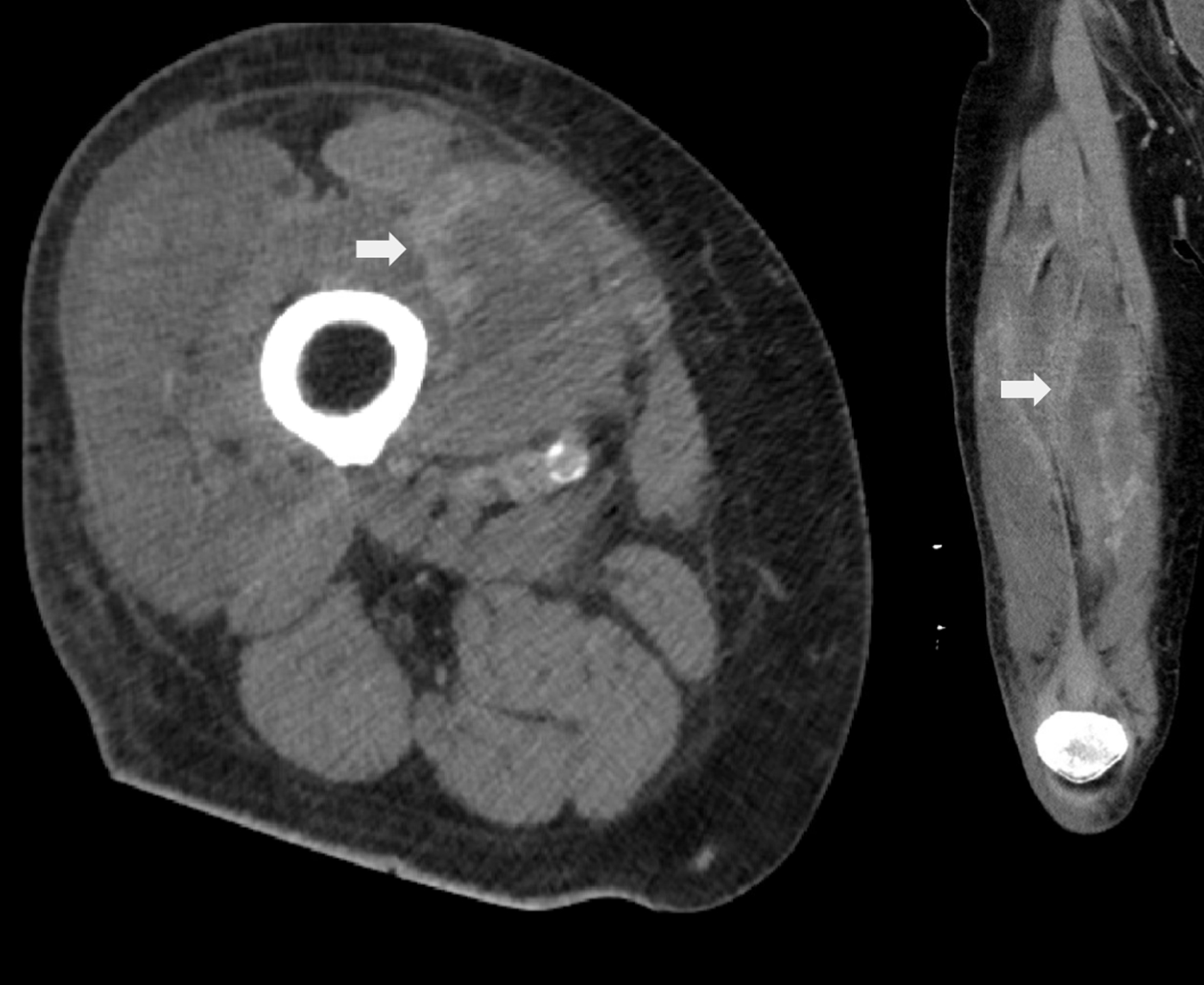 Click for large image | Figure 1. Computed tomography (CT) scans of the right thigh with contrast showing abnormal enhancement within the quadriceps musculature (arrow). Listed differentials included acute infectious/inflammatory myositis, rhabdomyolysis, and compartment syndrome. |
Given the non-response to antibiotics and negative antibody panel, a CT-guided muscle biopsy of the right vastus lateralis was performed. Figure 2 shows the hematoxylin and eosin (H&E) and select immunohistochemical stains of the muscle biopsy. Microscopic examination showed poorly differentiated carcinoma with hyperchromatic, anaplastic nuclei that infiltrated skeletal muscle fibers. Immunohistochemical staining of the neoplastic cells demonstrated positive staining for markers most consistent with urothelial carcinoma (GATA-binding protein 3, keratin 7, and keratin 20) and negative for markers of common metastatic carcinomas such as lung, kidney, prostate, thyroid, and colorectal carcinomas (transcription termination factor 1, NK2 homeobox 1, paired box 8, NK3 homeobox 1, caudal type homeobox 2). The marker of proliferation Ki-67 immunostaining showed that the neoplastic cells had a very high proliferative index, consistent with an aggressive malignancy.
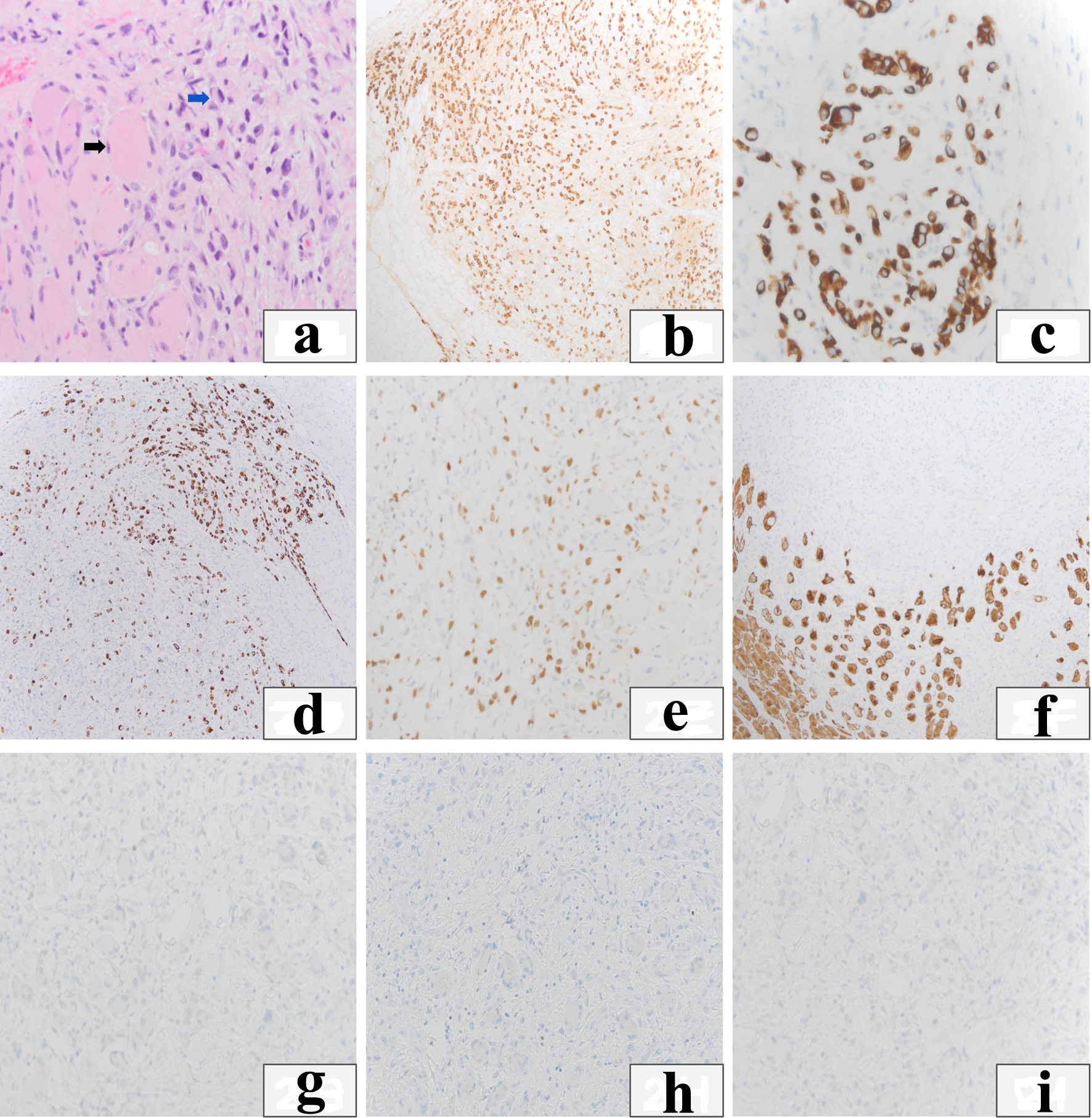 Click for large image | Figure 2. Immunohistochemical stains. (a) Hematoxylin and eosin (H&E) depicts infiltrative neoplastic cells into skeletal muscle (black arrow: skeletal myocytes are large and eosinophilic, blue arrow: infiltrating neoplastic cells). (b) Pan-keratin shows the neoplastic cells are carcinoma cells which also stain positive for cytokeratin 7 (c) and cytokeratin 20 (d). (e) GATA-binding protein 3 (GATA3) shows nuclear staining of carcinoma cells. (f) Desmin highlights the degeneration of muscle bundles secondary to the infiltrative carcinoma. (g-i) Carcinoma cells stain negative for transcription termination factor 1 (TTF-1), paired box 8 (PAX-8) and NK3 homeobox 1 (NKX 3.1). |
Treatment
The immunohistochemical profile of the invasive carcinoma was highly suspicious for urothelial origin, leading to follow-up cystoscopy. Cystoscopy identified a 2 - 3 cm right-sided bladder tumor with papillary features and a broad base. Transurethral resection of the bladder tumor was scheduled as an outpatient procedure, and the patient was discharged to a skilled nursing facility.
Follow-up and outcome
Prior to planned resection of the bladder tumor, the patient was brought to the hospital by ambulance for altered mental status and fatigue. This hospital course was complicated by acute kidney injury, urosepsis, and hematemesis. Two weeks after the muscle biopsy identified metastatic bladder carcinoma, the patient died in the hospital. An autopsy was not requested.
| Discussion | ▴Top |
Skeletal muscle is a rarely reported site of metastasis, despite constituting a large portion of body mass and enjoying ample blood supply [9]. The rarity of reported SMMs is likely multifactorial. It is postulated that skeletal muscles offer an unfavorable environment for cancer growth, due to mechanical destruction with muscle movement, a highly acidic environment, and an ability to clear tumor lactic acid that would otherwise drive angiogenesis [9]. SMMs are also thought to be underreported in the literature, as they may be asymptomatic, non-specific, and missed during clinical assessment, or simply not written up due to lack of biopsy [10]. Autopsy studies have found rates of SMM as high as 17.5% [11], though by nature of the study population they may disproportionately represent advanced-stage disease, while one series of full body CT/positron emission tomography (PET) of 500 patients with cancer found a 1.8% rate of soft tissue metastasis [8]. The most common primary sites of malignancy in SMM were noted to be genital tumors (24.6%), gastrointestinal tumors (21.3%), urological tumors (16.4%) and malignant melanoma (13.1%) [12].
The most common clinical presentation of intramuscular metastases is as a focal mass. Similarly, Surov et al identified that the most common radiologic presentation of SMM on CT scans is that of a focal mass exhibiting homogenous contrast enhancement (type I), accounting for approximately 50% of the cases in their large series of 461 patients with varying primary tumors [12]. In descending order of prevalence, the other described radiologic presentations are abscess-like lesions with rim enhancement (type II), diffuse infiltration with muscle swelling and inhomogeneous enhancement (type III), multiple intramuscular calcifications (type IV), and intramuscular bleeding (type V) [12].
The rarity of investigated cases of skeletal muscle prognosis makes it difficult to accurately determine the prognosis for SMMs, but as metastatic disease they present in the setting of advanced-stage malignancy and tend to carry a poor prognosis. Though there is no clear consensus on treatment for SMM, options include surgical resection, chemotherapy, and radiotherapy, with successful reduction in symptoms and tumor burden [13].
Urothelial carcinomas are the 10th most common cancer globally, have a male preponderance with a 4:1 ratio of males to females [2], and are histologically urothelial (transitional cell) carcinoma in 90% of cases. The most substantial risk factor for bladder cancer is cigarette smoking, with current cigarette use corresponding to a three-fold higher risk of bladder cancer than in non-smokers [14]. An estimated 7% of patients with muscle-invasive urothelial carcinoma will have metastasis at the diagnosis [15]. Patients with untreated urothelial carcinoma have a 3 - 6 months median survival time [15]. Bladder cancer characteristically spreads through the lymphatic system to lymph nodes, lungs, liver, bones, and adrenal glands [7]. Metastasis of bladder cancer to skeletal muscle is a rare presentation, with very few cases reported in the literature.
We reviewed published cases of SMMs from primary bladder urothelial carcinomas, identifying 18 cases from 17 publications from 1996 to 2022, as summarized in Table 1 [16-32]. The patients were male in all 18 cases, with an average age of 62.6 years. The most common site of involvement was the lower extremity, noted in 14 of 18 cases. In 15 of the 18 cases, SMM was identified in patients with a known history of urothelial carcinoma. In one of the remaining cases (Nabi) [19], the patient presented with hematuria, and the primary and metastasis were found simultaneously, and in another (Ying-Yue) [22], both primary and SMMs were identified in the workup of a rectal mass. In the last case (Zargooshi) [18], the primary bladder cancer was identified after metastatic urothelial carcinoma was identified in a psoas muscle abscess. To our knowledge, we present the first case report of metastatic urothelial carcinoma presenting as painful myositis in a patient with no known history of urothelial carcinoma at the time of diagnosis.
 Click to view | Table 1. Summary of Published Cases of Metastatic Bladder Urothelial Carcinoma to Skeletal Muscles |
Interestingly, the patient was male in our present case and in all 18 previously reported cases of metastatic urothelial carcinoma to skeletal muscle, even though approximately 20% of patients with urothelial carcinoma are women. In large studies of SMMs, gender ratios of patients with SMMs were as high as 2.8:1 [11]. Further investigation may help determine if a physiologic cause or sampling bias is behind all 18 reported cases of urothelial carcinoma metastases being identified in men, but it may be explained by the compounding effect of both urothelial carcinoma and SMMs being significantly more common in men.
Conclusion
SMMs of urothelial carcinoma are rarely reported in the literature and are even more rarely identified in patients without a known cancer diagnosis. They are characteristically found in the lower extremities in older male patients. Though the most common presentation of SMM is a focal mass clinically and radiographically, about half of SMM cases present differently on radiology. Metastasis should be on the differential for patients with unilateral muscle pain, particularly if refractory to steroid, anti-inflammatory, and antibiotic therapies. Interesting, we note that in our review of the literature, all identified cases of urothelial carcinoma metastatic to skeletal muscle were reported in men.
Learning points
The differential diagnosis of muscle pain should be broad during investigation, especially for a subset of patients with similar presentations with chronic unilateral muscle pain unresponsive to empiric treatment of physical therapy, steroids, and antibiotics. Though the classic presentation of SMM involves a focal mass, metastases to skeletal muscles can present with various clinical and radiologic features. Definitive diagnoses with biopsy can identify the primary malignancy if not previously known.
Acknowledgments
None to declare.
Financial Disclosure
No financial disclosures to report.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was waived as a retrospective case report with no patient identifiers included.
Author Contributions
CD performed literature search, wrote discussion and introduction sections, aided in pictures of pathology slides, compiled images into figure, edited final draft. WM wrote case presentation sections, aided in pictures of pathology slide, provided caption for pathology, edited final draft. MAG provided case and initial diagnosis, created outline, edited final draft.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - American Cancer Society. Cancer Facts & Figures 2022. Atlanta, Ga: American Cancer Society; 2022.
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-Part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106-119.
doi pubmed - Clark KR. Bladder cancer: types, risk factors, diagnosis, and treatment. Radiol Technol. 2022;94(1):46-50.
pubmed - Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, Lotan Y, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224-1233.
doi pubmed - Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, Kaufman DS, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32(34):3801-3809.
doi pubmed pmc - Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol. 2011;196(1):117-122.
doi pubmed - Nguyen NC, Chaar BT, Osman MM. Prevalence and patterns of soft tissue metastasis: detection with true whole-body F-18 FDG PET/CT. BMC Med Imaging. 2007;7:8.
doi pubmed pmc - Seely S. Possible reasons for the high resistance of muscle to cancer. Med Hypotheses. 1980;6(2):133-137.
doi pubmed - Haygood TM, Wong J, Lin JC, Li S, Matamoros A, Costelloe CM, Yeung H, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
doi pubmed - Acinas Garcia O, Fernandez FA, Satue EG, Buelta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
pubmed - Surov A, Hainz M, Holzhausen HJ, Arnold D, Katzer M, Schmidt J, Spielmann RP, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
doi pubmed - Tuoheti Y, Okada K, Osanai T, Nishida J, Ehara S, Hashimoto M, Itoi E. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
doi pubmed - Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306(7):737-745.
doi pubmed pmc - Shou J, Zhang Q, Zhang D. The prognostic effect of metastasis patterns on overall survival in patients with distant metastatic bladder cancer: a SEER population-based analysis. World J Urol. 2021;39(11):4151-4158.
doi pubmed - Masters JG, Cumming JA, Jennings P. Psoas abscess secondary to metastases from transitional cell carcinoma of the bladder. Br J Urol. 1996;77(1):155-156.
doi pubmed - Ekici S, Ozen H, Gedikoglu G, Aygun C. Skeletal muscle metastasis from carcinoma of the bladder. Scand J Urol Nephrol. 1999;33(5):336-337.
doi pubmed - Zargooshi J. Psoas abscess as the initial presentation of bladder cancer. Scand J Urol Nephrol. 2002;36(2):154-155.
doi pubmed - Nabi G, Gupta NP, Gandhi D. Skeletal muscle metastasis from transitional cell carcinoma of the urinary bladder: clinicoradiological features. Clin Radiol. 2003;58(11):883-885.
doi pubmed - Nagao E, Nishie A, Yoshimitsu K, Irie H, Shioyama Y, Naito S, Matsuura S, et al. Gluteal muscular and sciatic nerve metastases in advanced urinary bladder carcinoma: case report. Abdom Imaging. 2004;29(5):619-622.
doi pubmed - Efesoy O, Akbay E, Cayan S, Bozlu M, Polat A. Transitional cell carcinoma of the urinary bladder with skeletal muscle metastasis: A case report and review of the literature. Turk Uroloji Dergisi. 2009;35(4):378-383-383.
- Ying-Yue J, Shen SH, Wang JH. Unusual presentation of urothelial carcinoma of the bladder with noncontiguous rectal and diffuse muscular skeletal metastases. J Urol. 2010;184(3):1163-1164.
doi pubmed - Kashyap R, Mittal BR, Chakraborty D, Bhattacharya A, Singh B. Multiple skeletal muscle metastases in a case of transitional cell carcinoma of bladder detected by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2010;44(4):297-299.
doi pubmed pmc - Doo SW, Kim WB, Kim BK, Yang WJ, Yoon JH, Song YS, Choi IH. Skeletal muscle metastases from urothelial cell carcinoma. Korean J Urol. 2012;53(1):63-66.
doi pubmed pmc - Journeay WS, Omar A, Carette S. Skeletal muscle metastasis mimicking a shoulder effusion. J Rheumatol. 2013;40(9):1616.
doi pubmed - Koca I, Ucar M, Bozdag Z, Alkan S. Adductor longus muscle metastasis of transitional cell carcinoma of the urinary bladder. BMJ Case Rep. 2014;2014:bcr2014203768.
doi pubmed pmc - Katafigiotis I, Athanasiou A, Levis PK, Fragkiadis E, Sfoungaristos S, Ploumidis A, Michalinos A, et al. Metastasis to sartorius muscle from a muscle invasive bladder cancer. Case Rep Med. 2014;2014:524757.
doi pubmed pmc - Dell'Atti L. A rare metastatic myositis ossificans of obturator muscle secondary to urothelial carcinoma. Rare Tumors. 2015;7(3):5870.
doi pubmed pmc - Guidi M, Fusetti C, Lucchina S. Skeletal muscle metastases to the flexor digitorum superficialis and profundus from urothelial cell carcinoma and review of the literature. Case Rep Urol. 2016;2016:2387501.
doi pubmed pmc - Spinelli M, Gillibrand R. Metastasis to gluteal muscle from high grade transitional cell carcinoma of bladder. Report of a case and review of literature. Pathologica. 2018;110(1):78-81.
pubmed - Mainwaring AM, Wells H, Banks T, Ellul T, Bose P. Skeletal muscle metastasis to vastus lateralis from a urothelial carcinoma: a case report and review of its diagnosis and management. Case Rep Urol. 2019;2019:8923780.
doi pubmed pmc - Dang S, Pereira M, Singh N, Shivdasani D, Roy D, Rungta R. An unusual case of urothelial carcinoma of bladder with metastasis to brain and skeletal muscles evaluated on 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Indian J Nucl Med. 2022;37(4):394-395.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


