| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 8, August 2024, pages 201-207
Navigating Aspirin Hypersensitivity in Patients Undergoing Percutaneous Coronary Intervention
Kai Shiang Lina, Keston Rattana, Jensen Georgea, Samantha Cavusoglua, Christy Josepha, Varsha Talankia, Sabu Johnb, c
aDepartment of Internal Medicine, SUNY Downstate Health Sciences University, Brooklyn, NY 11203, USA
bDepartment of Cardiology, SUNY Downstate Health Sciences University, Brooklyn, NY 11203, USA
cCorresponding Author: Sabu John, Department of Cardiology, SUNY Downstate Health Sciences University, Brooklyn, NY 11203, USA
Manuscript submitted May 7, 2024, accepted June 19, 2024, published online July 25, 2024
Short title: Aspirin Hypersensitivity in Patients Undergoing PCI
doi: https://doi.org/10.14740/jmc4239
| Abstract | ▴Top |
Aspirin hypersensitivity continues to be a major clinical challenge in patients with coronary artery disease (CAD), particularly in those requiring percutaneous coronary intervention (PCI) in the absence of a validated alternative antiplatelet regimen. Although true aspirin allergies are uncommon, they can manifest with severe reactions such as angioedema or anaphylaxis, highlighting the critical role of diagnostic challenge tests and tolerance induction strategies. Here, a 61-year-old female with end-stage renal disease (ESRD) on hemodialysis presented with new-onset heart failure and elevated troponins in the setting of a hypertensive emergency. A subsequent left heart catheterization revealed severe multivessel disease, but PCI was deferred due to her history suggestive of aspirin-induced angioedema and the absence of a known optimal approach in this scenario. Given the feasibility of completing a desensitization protocol, aspirin desensitization was pursued, facilitating the successful placement of a drug-eluting stent. This case highlights the need for validated protocols to manage aspirin hypersensitivity, as the current treatment paradigm necessitates a highly individualized approach by the treating clinician.
Keywords: Aspirin; Allergy; Angioedema; Percutaneous coronary intervention; Heart failure; Acute coronary syndrome; Hypersensitivity; Dual antiplatelet therapy
| Introduction | ▴Top |
Aspirin hypersensitivity in patients undergoing percutaneous coronary intervention (PCI) is a relatively rare finding, with a prevalence of 2.6% among patients admitted for cardiac catheterization, according to a recent retrospective study [1]. Of these patients, 1.0% presented with respiratory manifestations including asthma or rhinitis, while the remaining 1.6% exhibited cutaneous manifestations such as urticaria or angioedema. Although infrequent, this spectrum of hypersensitivity is significant in contemporary medical practice due to aspirin’s pivotal role in treating coronary artery disease (CAD), particularly in the setting of dual antiplatelet therapy (DAPT). Comprising aspirin and a P2Y12 inhibitor, DAPT represents the cornerstone of post-PCI medical therapy and is used mainly to mitigate the risk of cardiovascular complications, such as stent thrombosis (ST). In concert with this approach are the current guidelines which recommend at least 6 months of DAPT post-PCI to achieve optimal therapeutic benefit [2]. However, because the exact prevalence of aspirin allergy is unclear, in part due to ambiguity of definition and the lack of awareness with respect to pseudoallergies and true allergies, aspirin therapy also presents a substantial challenge to this recommendation, as the safety and efficacy of alternative oral antiplatelet combinations without aspirin have yet to be established, likely due to a scarcity of high-quality evidence. This case report and literature review aims to illustrate this clinical scenario and discuss plausible alternatives as well as underscore the need for further research in the area.
| Case Report | ▴Top |
History of presentation
A 61-year-old female was brought in by emergency medical services (EMS) for sudden-onset generalized weakness and shortness of breath that began within the last 12 h. The patient had similar symptoms in the past in the context of missed hemodialysis sessions, and denied any exertional nature to her symptoms, also denying chest pain, palpitations, nausea, vomiting, lightheadedness, and epigastric pain. Her last hemodialysis session via her left upper extremity arteriovenous fistula was 3 days before presentation, and she endorsed baseline paroxysmal nocturnal dyspnea as well as orthopnea requiring the use of two pillows to sleep at night, but not dyspnea on exertion. Prior to presentation, she desaturated to an oxygen saturation of 66% on room air that failed to improve on supplemental oxygen at home, prompting her to notify EMS.
On arrival, her vital signs were notable for a blood pressure of 193/80 mm Hg (normal range: 90/60 mm Hg to 120/80 mm Hg), respiratory rate of 20 breaths per minute (normal range: 12 - 20 breaths per minute), heart rate of 70 beats per minute (normal range: 60 - 100 beats per minute), a temperature of 98.9 °F (normal range: 98.6 °F to 100.4 °F), and an oxygen saturation of 80% as detected by pulse oximetry (normal range: 95% or higher) that improved to 98% on bilevel positive airway pressure. Her exam was significant for crackles in the bilateral lower and middle lung fields as well as 1+ pitting edema in the bilateral lower extremities. Laboratory workup revealed elevated levels of B-type natriuretic peptide, troponin, and glucose, and decreased levels of hemoglobin. A chest X-ray showed bilateral pulmonary congestion, alveolar edema, and pleural effusions, and a standard 12-lead electrocardiogram (ECG) showed normal sinus rhythm, left ventricular hypertrophy, and poor R wave progression, all of which were unchanged from her baseline that was taken 3 years ago (Fig. 1). The posterior ECG was unremarkable. Nephrology and cardiology were consulted for urgent hemodialysis and elevated troponins, respectively, and the patient was admitted for acute decompensated heart failure and rule-out of acute coronary syndrome (ACS) in the setting of hypertensive emergency.
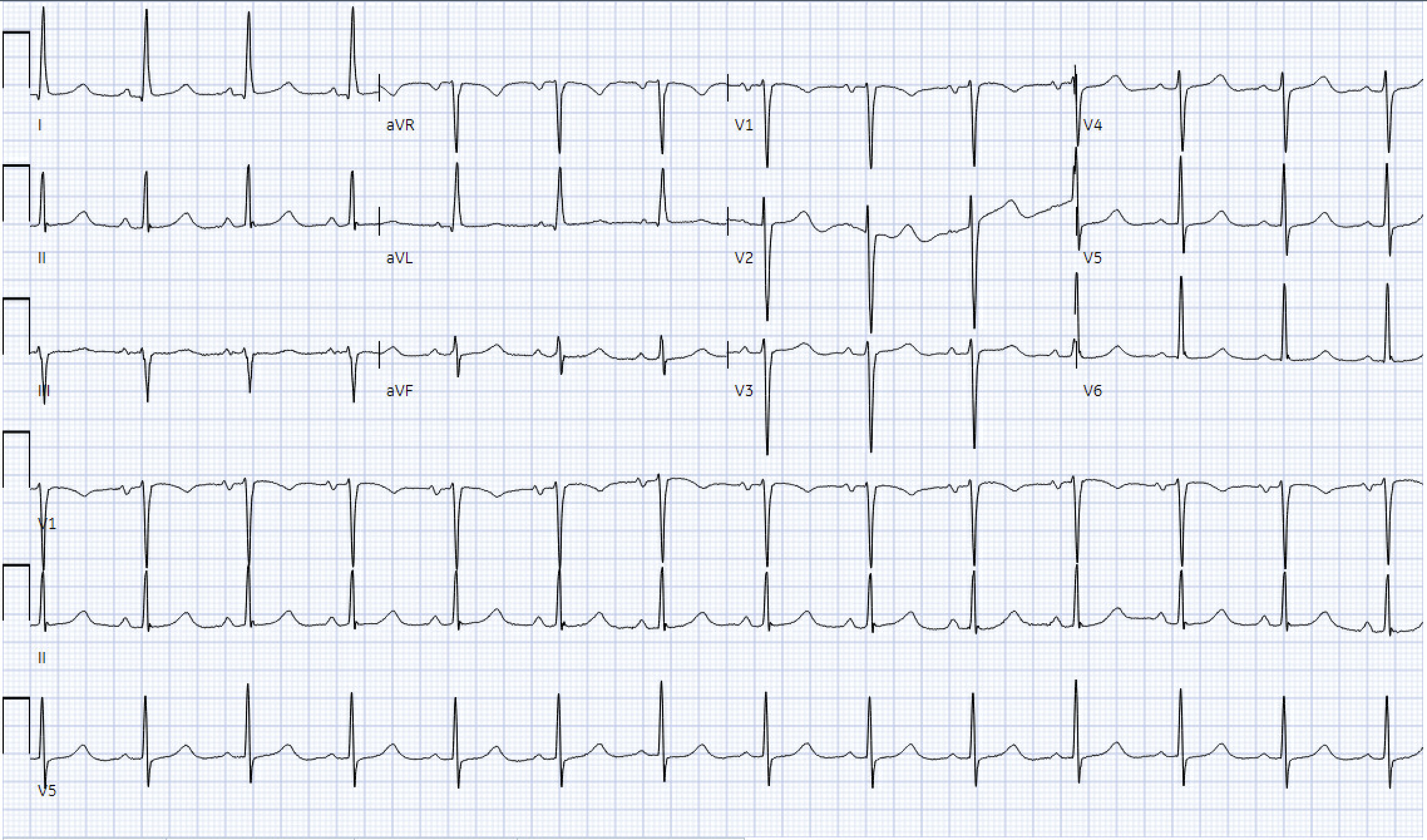 Click for large image | Figure 1. ECG on admission, showing normal sinus rhythm, left ventricular hypertrophy, poor R wave progression. ECG: electrocardiogram. |
Past medical history
The patient had a history of hypertension, insulin-dependent diabetes mellitus, ischemic cerebrovascular accident, heart failure with reduced ejection fraction (ejection fraction 35% with grade 2 diastolic dysfunction and moderate diffuse hypokinesis, and no prior ischemic workup), end-stage renal disease on hemodialysis. Home medications included detemir, aspart, furosemide, clopidogrel, sevelamer, metoprolol succinate, amlodipine, and atorvastatin.
Allergies
The patient has a remote history of oral numbness and tingling and possibly angioedema with the use of aspirin many years before presentation. She was told by a physician at the time to never use aspirin again and has never done so since then.
Investigation and management
Following admission, serial ECGs were performed and were unremarkable. Serial troponins rose to a peak of 0.66 ng/mL before falling to undetectable levels within the next day. Euvolemia was achieved through a combination of aggressive diuresis which led to a small amount of urine production, inpatient hemodialysis for further excess volume removal, and blood pressure control, all of which resulted in the restoration to euvolemic weight and the resolution of shortness of breath, lung crackles, and pitting edema. Transthoracic echocardiography (TTE) revealed an improvement in ejection fraction from her baseline of 35% to 50-55% without any regional wall motion abnormalities as well as a grossly normal valvular function and structure. A subsequent left heart catheterization revealed severe, two-vessel disease with 70-80% occlusion and an instantaneous wave-free ratio (iFR) of 0.63 in the mid-left anterior descending artery (LAD) as well as 60-70% occlusion and iFR of 0.93 in the proximal left circumflex artery (LCX) (Fig. 2). However, PCI was deferred at that time due to a possible history of angioedema with aspirin use, conflicting with her need for DAPT after stenting. The patient subsequently underwent successful aspirin desensitization without the development of any adverse reactions, and a staged PCI with deployment of a drug-eluting stent was performed within hours of completion of the desensitization protocol (Table 1). The patient was discharged on DAPT with aspirin and clopidogrel and continued on the rest of her home medications with outpatient cardiology follow-up.
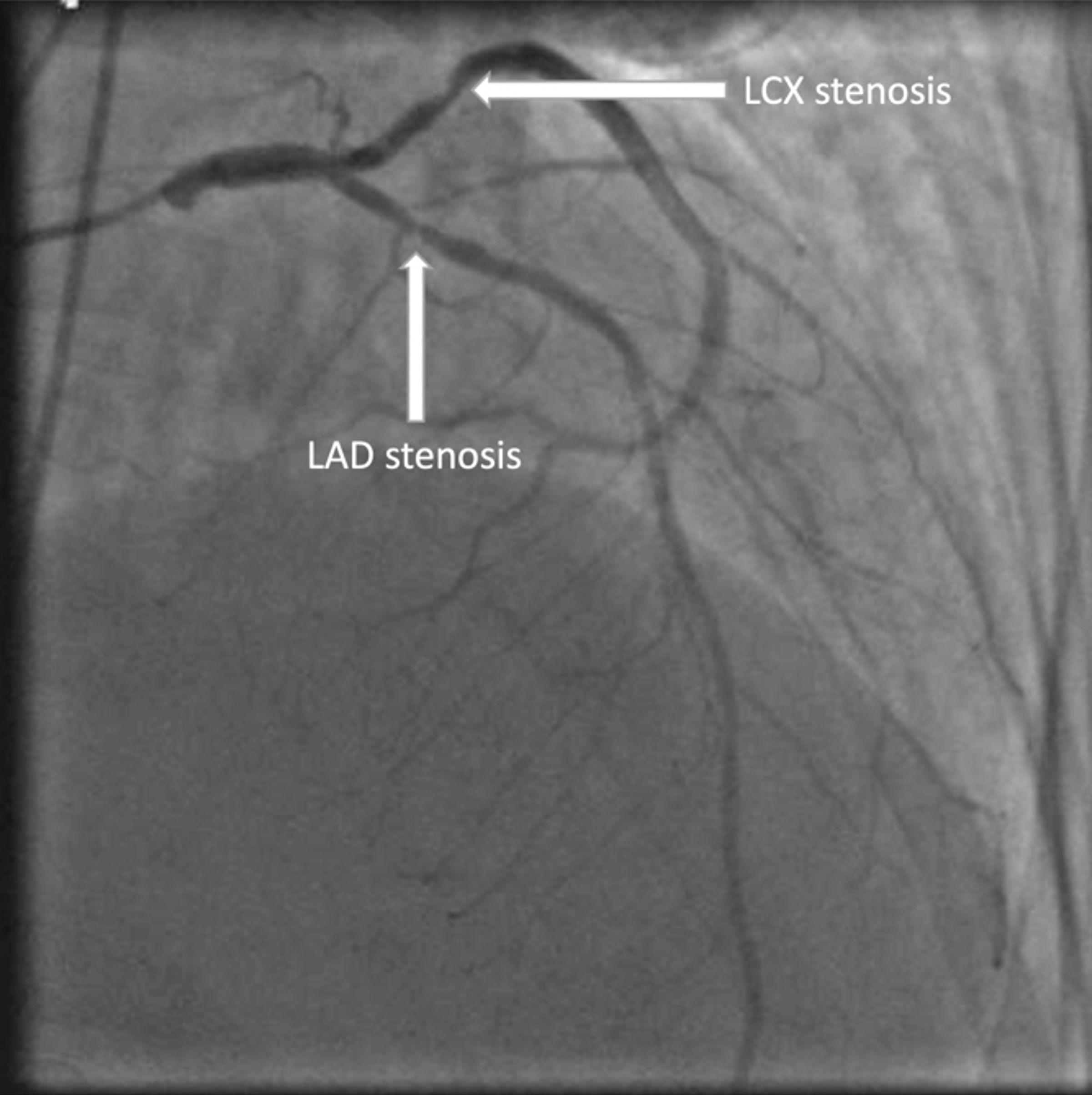 Click for large image | Figure 2. Coronary angiogram showing severe two-vessel disease with 70-80% occlusion of the mid-LAD and 60-70% occlusion of the proximal LCX. The labeled arrows point to the respective stenotic regions. LAD: left anterior descending artery; LCX: left circumflex artery. |
 Click to view | Table 1. Aspirin Desensitization Protocol |
| Discussion | ▴Top |
This case illustrates a rare clinical scenario where a patient with traditional CAD risk factors who developed ischemic symptoms underwent two coronary catheterizations due to the need for aspirin desensitization prior to stent deployment, which is a current standard of care for coronary ischemia. The delay in revascularization is particularly noteworthy, highlighting the need for improved strategies to address this crucial gap in medical literature. To explore this clinical question, the following discussion begins with a brief plunge into the evolution of PCI.
The introduction of PCI by Andreas Gruntzig in 1977 revolutionized the treatment of CAD [3]. No longer was cardiothoracic surgery the only available treatment modality when medical therapy proved inadequate, and PCI has since become the subject of intense research and development. Significant milestones, such as the introduction of bare-metal stents (BMS), drug-eluting stents (DES), and biodegradable polymer-based DES, have successively enhanced treatment capabilities and established PCI as a crucial component of standard care. Despite these advancements, PCI is not without limitations; ST remains a significant concern, and minimizing its occurrence has been a key therapeutic objective for several decades. Although rare, ST is a catastrophic event characterized by the sudden thrombotic occlusion of a previously patent stent, leading to sudden death or a large myocardial infarction (MI) in most cases. It typically presents subacutely, defined as the development of acute thrombosis within the first 30 days after PCI, but can also occur acutely, late, or very late, which correspond to acute thrombotic events within 24 h, 1 year, or more than 1 year post-PCI, respectively [4]. The 30-day incidence of nonfatal MI in patients with DES who had angiographically confirmed ST is approximately 60%, whereas the 30-day mortality rates for angiographically confirmed ST and clinically identified ST, with BMS and DES, are 7%, 19%, and 15%, respectively [5, 6].
Major risk factors for the development of ST include intraprocedural trauma to the coronary endothelium and the introduction of metal to the coronary vasculature. The former exposes subendothelial collagen and tissue factor to blood, triggering the coagulation cascade, while metal itself is inherently thrombogenic [7]. To mitigate the risk of ST, the primary therapeutic measure is antithrombotic therapy. Notably, the specific antithrombotic regimen depends on the timing of its administration relative to the timing of PCI. For instance, combined antiplatelet and anticoagulant therapy, such as aspirin and unfractionated heparin, is typically indicated prior to and during PCI, whereas antiplatelet therapy alone is usually indicated post-PCI, particularly in the absence of an alternate indication for anticoagulation. Yet regardless of the timing of administration, the antiplatelet regimen almost invariably includes aspirin and a P2Y12 receptor blocker, collectively known as DAPT, thanks to the significant reduction in ST when compared to antiplatelet monotherapy, particularly in the early period following PCI [8]. In fact, DAPT is so important that the absence of a P2Y12 receptor blocker is the single most important predictor for ST at the time of the event [5, 6, 8]. With robust evidence from randomized clinical trials and the absence of another nonsteroidal anti-inflammatory drug (NSAID) with equally selective and irreversible blockade of platelet cyclooxygenase-1 (COX-1), aspirin’s role as a unique and indispensable antiplatelet agent for stented patients is firmly established [9-11]. Interestingly, the strict adherence to aspirin in current DAPT regimens is in stark contrast to the more lenient approach to the choice of P2Y12 receptor blocker, which can vary depending on the specific indication [12, 13].
While DAPT is effective in reducing PCI-related complications, it presents its own set of challenges as well. In addition to the risk of bleeding, which is often a reason for DAPT discontinuation, the concurrent presence of aspirin hypersensitivity can be problematic and potentially preclude the use of DAPT. Because current DAPT regimens unanimously advocate for aspirin use, and that the safety and efficacy of oral antiplatelet combinations excluding aspirin remain unestablished, the optimal approach in this clinical scenario remains unclear and is compounded by additional considerations including the type and severity of the aspirin allergy as well as the urgency of PCI.
Within cardiac pathologies, there are three main indications for urgent aspirin therapy: patients with suspected ACS for whom revascularization is not planned, patients with suspected ACS with plans for revascularization but delayed aspirin initiation due to concerns for hypersensitivity, and patients requiring nonurgent coronary revascularization. Similarly, aspirin or NSAID allergies can be broadly categorized into pseudoallergies and true allergies, with the former thought to be nonimmunologic reactions secondary to NSAID-induced inhibition of COX-1 and other related biochemical pathways, and the latter being presumed, immunoglobulin E (IgE)-mediated immunologic reactions. Regardless of the type or category of allergies, identifying key manifestations of aspirin sensitivity such as anaphylaxis, urticaria or angioedema, and aspirin-exacerbated respiratory disease (AERD), is essential to guide further management [14].
A relatively common condition affecting 5-7% of all asthmatics, AERD has an overall prevalence of approximately 10% whereas the prevalence for aspirin-induced cutaneous sensitivity ranges from 0.07% to 0.2%; the prevalence for aspirin-related anaphylaxis remains unknown as it has never been conclusively documented to date [15-17]. The main reason for identifying these key hypersensitivity manifestations is to determine the need for a premedication regimen prior to the administration of aspirin, with the goal of preventing or reducing the severity of a potential reaction. Prompt administration of aspirin is often preferred, especially in patients requiring urgent PCI despite the potential for inducing hypersensitivity, and premedication is usually reserved for those suspected of having AERD, which include those with a history of aspirin-induced chest tightness or wheezing, or those with confirmed or suspected prior anaphylactic reactions suggestive of AERD. In these populations, premedication is often done with leukotriene-modifying agents such as montelukast or oral glucocorticoids due to their efficacy in alleviating or even preventing asthma exacerbations. Conversely, patients who develop aspirin-related cutaneous reactions or suspected anaphylactic reactions without symptoms indicative of AERD generally do not require premedication, owing to the more benign nature of these reactions and that the majority of aspirin-related anaphylactic reactions are actually severe respiratory reactions in patients with underlying AERD.
In addition to the type and severity of aspirin allergies, the urgency of PCI is another crucial consideration in determining the optimal approach in this clinical dilemma. Patients requiring PCI can be categorized into those for whom PCI is elective, those for whom PCI can be postponed several hours, and those for whom PCI is urgent without sufficient time to introduce aspirin. Whereas PCI in the first two scenarios can be performed after aspirin desensitization or postponed for several hours to allow for the introduction of low-dose aspirin, respectively, the optimal approach in the third scenario, where PCI is urgent, remains less clear. The result is a handful of postulated alternatives, including oral P2Y12 receptor blocker monotherapy, perioperative intravenous glycoprotein (GP) IIb/IIIa antagonist therapy with subsequent aspirin desensitization, and low-dose rivaroxaban in conjunction with a P2Y12 receptor blocker. These options all appear to be viable alternatives, but in fact share a common flaw - the lack of formal validation in the clinical setting [18-20]. With respect to the introduction of aspirin, which can be performed in the second scenario, a simple protocol involving low-dose aspirin, usually 100 mg or less daily, can be attempted (Table 2) [21]. This protocol can be completed within several hours and has been demonstrated to be safe in patients with aspirin-induced cutaneous manifestations such as urticaria or angioedema [20]. Should patients with suspected or confirmed AERD develop respiratory symptoms during the protocol, bronchodilator therapy and antihistamines are available treatment options. Notably, our patient was started on a lower dose, 20.25 mg, than that outlined in this protocol, 40.5 mg, due to our patient being high-risk and her personal desire to start at the lowest dose possible. Remarkably, the development of anaphylaxis with this protocol is usually not considered even in patients with true aspirin allergies, given the aforementioned absence of conclusive evidence for aspirin-specific anaphylaxis and the low likelihood of symptom induction with the low dosages of aspirin used in this protocol. In patients who may require a single, higher loading dose of aspirin, which is typically a one-time dose of 325 mg, a second protocol extending an additional day can be added to the initial protocol (Table 3) [22]. It should be noted that the likelihood of triggering respiratory symptoms is higher during this second protocol, likely due to the higher dosages of aspirin used, although the same bronchodilator and antihistamine therapies can be used in the event of symptom development.
 Click to view | Table 2. Protocol for Introducing Low-Dose Aspirin (81 mg) in a Patient With Suspected or Confirmed Aspirin Allergy |
 Click to view | Table 3. Protocol for Increasing the Dosage of Aspirin From 81 mg Daily to 325 mg Daily for Loading Purposes in Patients With Suspected or Confirmed Aspirin Allergy |
In contrast to introducing low-dose aspirin to aid in DAPT initiation in more urgent clinical scenarios, aspirin desensitization is an alternative approach in which aspirin is slowly introduced into a patient with suspected or documented aspirin allergy. The main advantage of this approach is its safety and hence, popularity, although the low prevalence of true aspirin allergies suggests that this approach may be less needed than commonly believed [23]. Interestingly, the majority of patients with presumed aspirin allergies actually have drug intolerance secondary to a direct consequence of aspirin’s mechanisms of action rather than true hypersensitivity [24]. However, in the setting of ample time without the need for urgent PCI, aspirin desensitization is still commonly performed when the safest approach is preferred. Thus, in the case of our patient, aspirin desensitization was the approach pursued given the relative lack of urgency for PCI and the desire for the safest approach in the setting of a potentially severe history of angioedema. The relative clinical stability and the ease with which euvolemia was achieved, in conjunction with the absence of chest pain, shortness of breath, or other significant symptoms, suggested that there was, indeed, ample time for aspirin desensitization prior to PCI. The etiology of our patient’s acute pulmonary edema and elevated troponins was likely multifactorial, with the most likely causes being inadequate hemodialysis and coronary ischemia; importantly, the coronary angiogram was negative for ACS.
Finally, while few clinical scenarios appear to preclude the use of aspirin with either the low-dose protocol or desensitization, it is important to be cognizant of certain absolute contraindications. These include patients who have had Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome, among others. In these particular scenarios, the general recommendation is to avoid all NSAIDs, including aspirin, without attempting a rapid, low-dose introduction or slow desensitization.
Conclusions
Safe strategies to maneuver the clinical dilemma of initiating DAPT in a patient with aspirin allergy include a low-dose protocol and complete desensitization. Given that the optimal approach remains undefined, further research with randomized trials will likely be crucial in navigating this unique clinical scenario in the near future.
Learning points
Learning points from this case report and literature review include recognition of this clinical conundrum and acknowledgement of the absence of proven optimal approaches to overcome it. Despite the multiple approaches highlighted above, there remains an urgent need for further research and clinical trials to establish a standard of care for this scenario, given the high prevalence of patients with ischemic heart disease.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
The case was identified by supervising author Sabu John, MD. The manuscript was written by Kai Shiang Lin, MD, and Keston Rattan, MD. Manuscript editing was performed by Kai Shiang Lin, MD, Jensen George, MD, Samantha Cavusoglu, Christy Joseph, and Varsha Talanki. The ECG and coronary angiogram image were identified by Sabu John, MD. The final manuscript was reviewed and supervised by Sabu John, MD.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACS: acute coronary syndrome; AERD: aspirin-exacerbated respiratory disease; BMS: bare-metal stents; CAD: coronary artery disease; DAPT: dual antiplatelet therapy; DES: drug-eluting stents; ECG: electrocardiogram; EMS: emergency medical services; ESRD: end-stage renal disease; iFR: instantaneous wave-free ratio; LAD: left anterior descending artery; LCX: left circumflex artery; MI: myocardial infarction; NSAIDs: nonsteroidal anti-inflammatory drugs; PCI: percutaneous coronary intervention; ST: stent thrombosis; TTE: transthoracic echocardiography
| References | ▴Top |
- Rossini R, Angiolillo DJ, Musumeci G, Scuri P, Invernizzi P, Bass TA, Mihalcsik L, et al. Aspirin desensitization in patients undergoing percutaneous coronary interventions with stent implantation. Am J Cardiol. 2008;101(6):786-789.
doi pubmed - Writing Committee Members, Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2023;82(9):833-955.
doi pubmed - Canfield J, Totary-Jain H. 40 years of percutaneous coronary intervention: history and future directions. J Pers Med. 2018;8(4):33.
doi pubmed pmc - Palmerini T, Kirtane AJ, Serruys PW, Smits PC, Kedhi E, Kereiakes D, Sangiorgi D, et al. Stent thrombosis with everolimus-eluting stents: meta-analysis of comparative randomized controlled trials. Circ Cardiovasc Interv. 2012;5(3):357-364.
doi pubmed - Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, Carrozza JP, Jr., et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103(15):1967-1971.
doi pubmed - Ong AT, Hoye A, Aoki J, van Mieghem CA, Rodriguez Granillo GA, Sonnenschein K, Regar E, et al. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol. 2005;45(6):947-953.
doi pubmed - Anderson DEJ, Le HH, Vu H, Johnson J, Aslan JE, Goldman J, Hinds MT. Thrombogenicity of biodegradable metals. Bioact Mater. 2024;38:411-421.
doi pubmed pmc - Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527-533.
doi pubmed - Lembo NJ, Black AJ, Roubin GS, Wilentz JR, Mufson LH, Douglas JS, Jr., King SB, 3rd. Effect of pretreatment with aspirin versus aspirin plus dipyridamole on frequency and type of acute complications of percutaneous transluminal coronary angioplasty. Am J Cardiol. 1990;65(7):422-426.
doi pubmed - van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, Koolen JJ, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53(16):1399-1409.
doi pubmed - Hirsh J. The optimal antithrombotic dose of aspirin. Arch Intern Med. 1985;145(9):1582-1583.
pubmed - Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, et al. 2016 ACC/AHA Guideline Focused Update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123-e155.
doi pubmed - Authors/Task Force Members, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541-2619.
doi pubmed - Stevenson DD. Aspirin and NSAID sensitivity. Immunol Allergy Clin North Am. 2004;24(3):491-505.vii.
doi pubmed - Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ. 2004;328(7437):434.
doi pubmed pmc - McDonald JR, Mathison DA, Stevenson DD. Aspirin intolerance in asthma. Detection by oral challenge. J Allergy Clin Immunol. 1972;50(4):198-207.
doi pubmed - Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. 2015;135(3):676-681.e671.
doi pubmed - Schiano P, Steg PG, Barbou F, Monsegu J. A strategy for addressing aspirin hypersensitivity in patients requiring urgent PCI. Eur Heart J Acute Cardiovasc Care. 2012;1(1):75-78.
doi pubmed pmc - Verheugt FWA, Damman P, Damen SAJ, Wykrzykowska JJ, Woelders ECI, van Geuns RM. P2Y12 blocker monotherapy after percutaneous coronary intervention. Neth Heart J. 2021;29(11):566-576.
doi pubmed pmc - Wong JT, Nagy CS, Krinzman SJ, Maclean JA, Bloch KJ. Rapid oral challenge-desensitization for patients with aspirin-related urticaria-angioedema. J Allergy Clin Immunol. 2000;105(5):997-1001.
doi pubmed - White AA, Stevenson DD, Woessner KM, Simon RA. Approach to patients with aspirin hypersensitivity and acute cardiovascular emergencies. Allergy Asthma Proc. 2013;34(2):138-142.
doi pubmed - Stevenson DD, Simon RA. Selection of patients for aspirin desensitization treatment. J Allergy Clin Immunol. 2006;118(4):801-804.
doi pubmed - Lambrakis P, Rushworth GF, Adamson J, Leslie SJ. Aspirin hypersensitivity and desensitization protocols: implications for cardiac patients. Ther Adv Drug Saf. 2011;2(6):263-270.
doi pubmed pmc - Orgeron GM, Havistin R, Hahn LS, Wang J, Crichlow C, Mugmon M, Mahajan A, et al. Prevalence and management of aspirin hypersensitivity in a cardiology practice. Allergy Asthma Proc. 2020;41(2):120-125.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


