| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 7, July 2024, pages 143-147
Can Hyperthermia Unveil Brugada Pattern?
Poornima Vinoda, c, Hiten Patelb
aDepartment of Internal Medicine, University of North Carolina Health at Southeastern, Lumberton, NC 28358, USA
bDivision of Interventional Cardiology, University of North Carolina Health at Southeastern, Lumberton, NC 28358, USA
cCorresponding Author: Poornima Vinod, Department of Internal Medicine, University of North Carolina Health at Southeastern, Lumberton, NC 28358, USA
Manuscript submitted May 6, 2024, accepted June 5, 2024, published online June 19, 2024
Short title: Hyperthermia and the Brugada EKG Pattern
doi: https://doi.org/10.14740/jmc4242
| Abstract | ▴Top |
Brugada syndrome (BrS) is characterized by ST segment elevations in the right precordial leads, V1 - V3, with additional findings of ventricular arrhythmias and family history (FH) of sudden cardiac death (SCD) at a young age. Here, we describe a case of hyperthermia, unveiling the Brugada electrocardiography (EKG) pattern and the resolution of EKG findings with appropriate hyperthermia management. It is important to distinguish the Brugada EKG pattern from other causes of ST elevations and treat appropriately to prevent patients from developing ventricular fibrillation and SCD. It is key to identify environmental triggers in patients presenting with Brugada EKG pattern and closely monitor for ventricular fibrillation. Educating patients on prompt fever treatment with antipyretics and avoiding medications like sodium channel blockers during the febrile event is paramount to counter patients going into ventricular fibrillation. It is also crucial for close follow-up of these patients, offering them genetic testing for BrS and screening families of patients with BrS.
Keywords: Brugada syndrome; Hyperthermia; Electrocardiography findings; Ventricular arrhythmias; Sudden cardiac death
| Introduction | ▴Top |
Brugada syndrome (BrS) is a rare but life-threatening genetic disorder responsible for ventricular fibrillation (VF) and sudden cardiac death (SCD) [1]. The BrS accounts for about 28% of cases of SCD with a structurally normal heart and 5-10% of cases of resuscitated cardiac arrest [1, 2]. There are two clinical entities, the Brugada pattern and BrS. The Brugada pattern is characterized by coved-type ST-segment elevation greater than 2 mm, followed by a descending negative T wave in at least two precordial leads (V1 - V3) [1]. The definitive diagnosis of BrS is with Brugada electrocardiography (EKG) pattern with additional clinical features of VF, polymorphic ventricular tachycardia (VT), inducible VT, family history (FH) of SCD, and unexplained syncope [1, 3]. It is important to understand that patients with the Brugada pattern have only the classic EKG findings described above but are asymptomatic and have no additional clinical features [1]. It is crucial to risk stratify patients with Brugada patterns who would benefit from close follow-up and genetic testing. An implantable cardiac defibrillator (ICD) implantation is the most effective therapeutic option for patients diagnosed with BrS [1]. However, making an accurate diagnosis and minimizing environmental triggers in patients presenting with EKG findings suggestive of BrS is important. Here, we discuss a unique case of hyperthermia unveiling the Brugada EKG pattern but without other alarming clinical signs like ventricular arrhythmias, syncope, or SCD. Conservative measures like antipyretics are all that is needed in asymptomatic cases of the Brugada pattern [1].
| Case Report | ▴Top |
Investigations
A 61-year-old male with a past medical history significant for congestive heart failure (CHF), dementia, mental retardation, and hypertension presented to the emergency department (ED) by emergency medical services (EMS) for altered mental status, shortness of breath, and EKG changes suggestive of ST-elevation myocardial infarction (STEMI). Home medications included aspirin, atorvastatin, chlorthalidone, and Lasix. His initial vitals on presentation were a rectal temperature of 40.4 °C, heart rate (HR) of 133 beats per minute (bpm), respiratory rate (RR) of 24 breaths/min, blood pressure (BP) of 116/61 mm Hg, and saturating 94% on 4 L of oxygen. Physical examination was significant for nonverbal status, lethargy, toxic-appearing, tachycardia at a regular rate and rhythm, left lower extremity circumferential erythema, and warmth.
Diagnosis
Initial evaluation in the ED showed evidence of a white blood cell count (WBC) of 11.6 × 109/L, hemoglobin of 11.5 g/dL, and platelet count of 104 × 109/L. The basic metabolic workup showed normal sodium, potassium, calcium, and magnesium levels. The patient did have evidence of acute kidney injury (AKI) with a creatinine of 1.9 mg/dL with a baseline creatinine of 1.2 mg/dL. Thyroid-stimulating hormone (TSH) and free T4 levels were within normal limits. Initial high-sensitivity troponin was elevated at 126 ng/L, and subsequent levels were down-trending. The respiratory pathogen panel was positive for influenza B, and lactic acid level of 1.8 mmol/L. Initial blood cultures from one of the two bottles showed coagulase-negative Staphylococcus aureus. However, this was considered contamination, and repeat cultures showed no growth. EKG on presentation, as shown below in Figure 1, during the initial febrile episode (40.4 °C) showed evidence of a Brugada pattern, and repeated EKG in Figure 2 following antipyretics showed resolution of ST changes in the leads V1 - V2. The patient’s presentation warranted a consideration of differential diagnoses including STEMI, BrS, and Brugada EKG pattern.
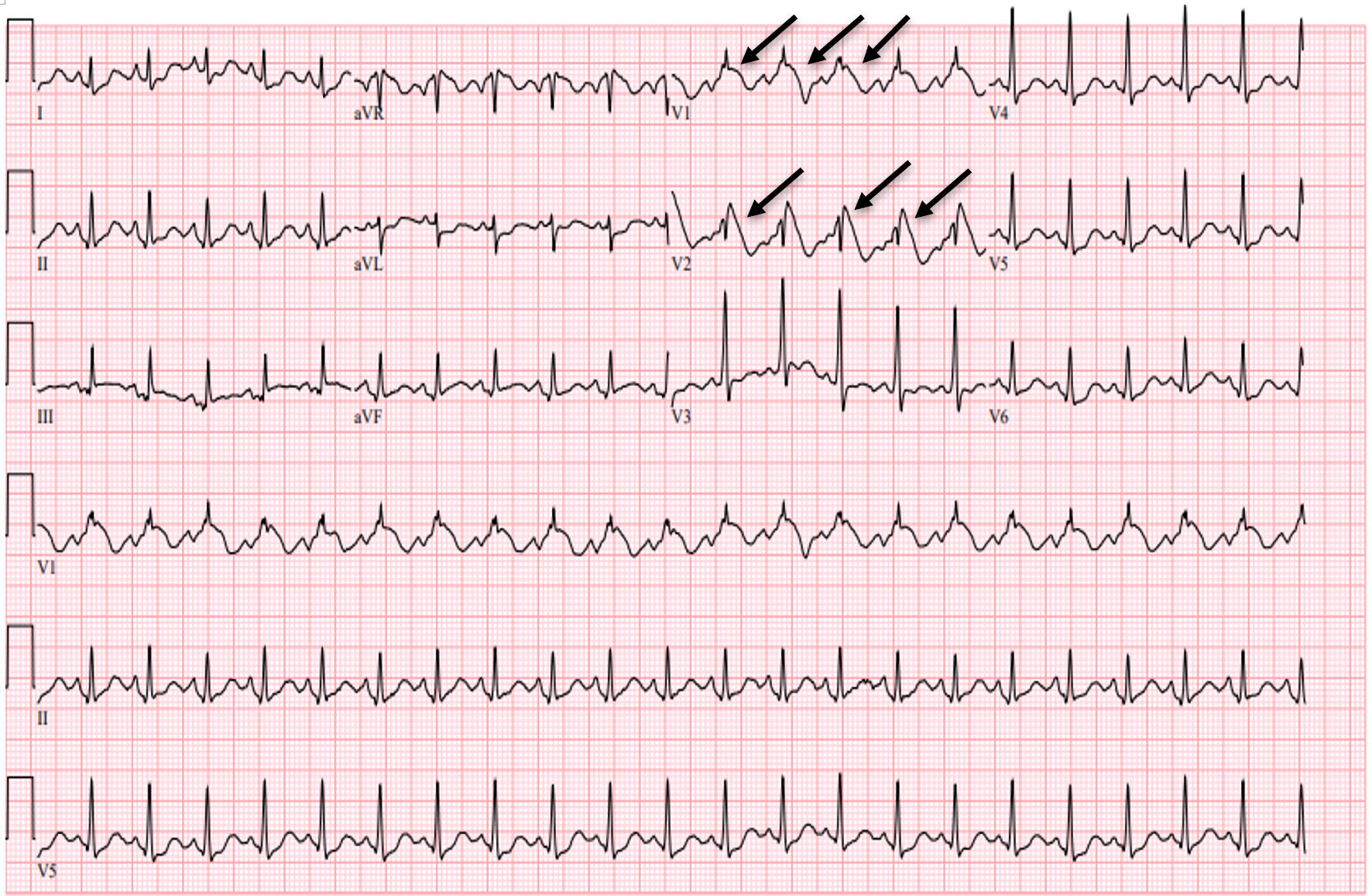 Click for large image | Figure 1. EKG showing sinus tachycardia with the Brugada EKG pattern, type 1 (arrows, coved ST elevation with inverted T waves in V1 - V2), and right bundle branch block during the febrile event on initial presentation. EKG: electrocardiogram. |
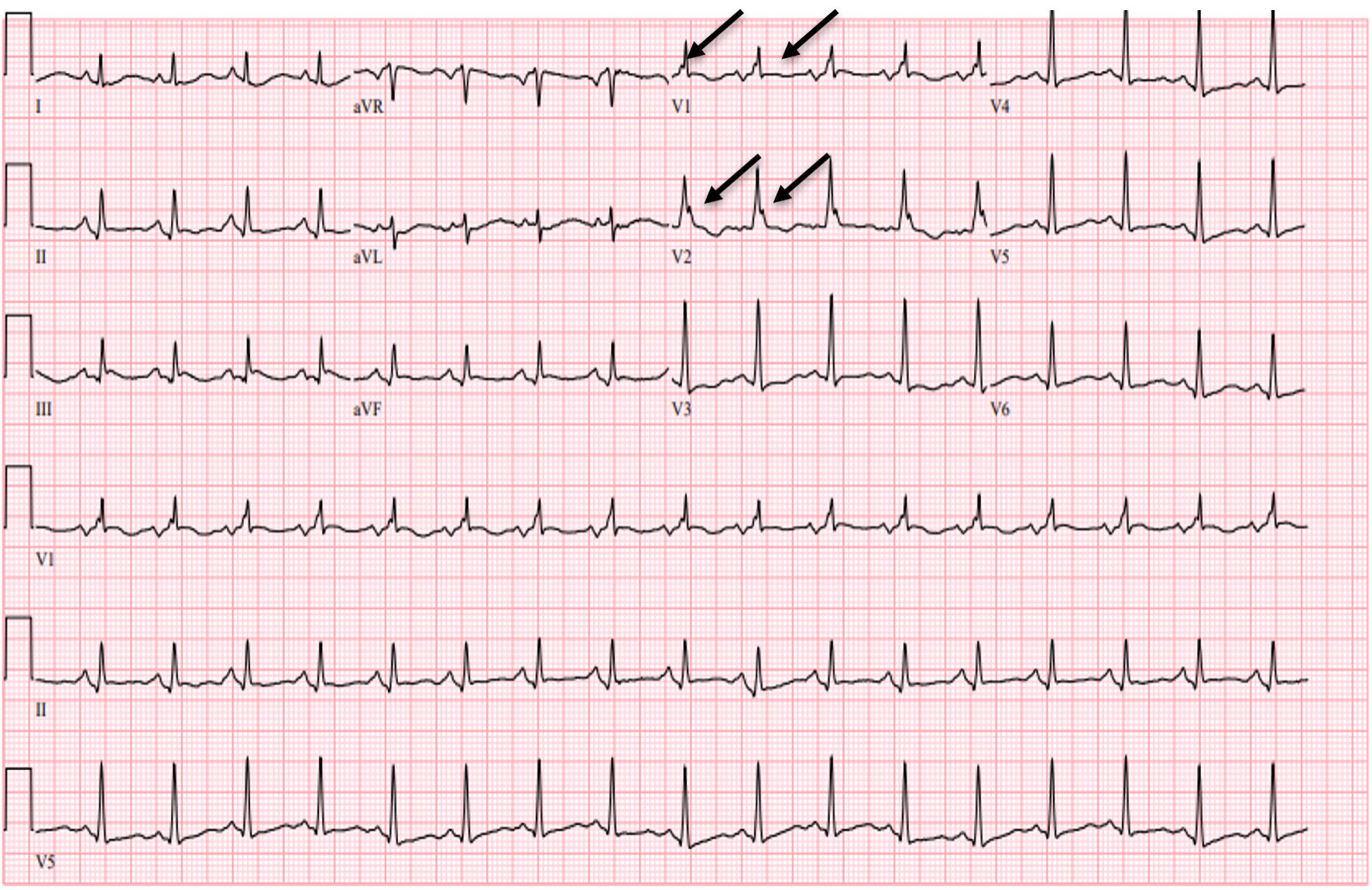 Click for large image | Figure 2. EKG showing resolution of ST elevation in leads V1 - V2 (arrows) after fever resolution with antipyretics and persistent right bundle branch block. EKG: electrocardiogram. |
Treatment
In the ED, the patient received 650 mg of rectal Tylenol and 3 L of normal saline and was initiated on broad-spectrum antibiotics with intravenous (IV) vancomycin and piperacillin-tazobactam. An interventional cardiologist on call was consulted for the evaluation of EKG changes concerning for STEMI. STEMI team was not activated as the EKG changes were thought to be consistent with the Brugada type 1 EKG pattern in the setting of hyperthermia and sepsis. An urgent two-dimensional (2D) echocardiogram in the ED showed a hyperdynamic left ventricle with EF of 65-70%, normal diastolic function, and no regional wall motion abnormalities. A repeat EKG was obtained within 2 h of the above management, showing the resolution of ST changes (Fig. 2). The patient was admitted to a telemetry-monitored floor and continued on IV antibiotics and oseltamivir. His mental status improved, and he was alert and following instructions compared to his lethargic status on presentation. He was discharged on day 5 of the hospitalization.
Follow-up and outcomes
The patient was recommended to follow up with cardiology in 2 weeks.
| Discussion | ▴Top |
BrS is an inherited cardiac arrhythmia syndrome that increases the risk of ventricular tachyarrhythmias and SCD [1, 4]. About 18 genes have been associated with the BrS [5, 6]. Thus far, nine sodium channels (Nav 1.1 - 1.9) have been isolated in humans, and the predominant sodium channel in the human heart is isoform Nav 1.5 [1]. The loss of function mutation in the cardiac voltage-gated sodium channel gene SCN5A is responsible for 15-30% of cases of BrS, which encodes for the alpha subunit of the Nav 1.5 sodium channel [5-7]. The mutations in the calcium and potassium channel currents are also described in other cases of BrS [8]. Recent literature supports a polygenic mode of inheritance with common and rare genetic variants to exhibit the BrS phenotype [4]. Premature inactivation/blockade of the Nav 1.5 sodium channel at higher temperatures is associated with a high risk of ST-segment elevation and an increased risk of VF and SCD [9, 10]. In patients with BrS, cardiac structural changes involving the right ventricular outflow tract (RVOT) are also noted [1].
According to the old literature, BrS was characterized by three different EKG patterns: type 1(as described earlier), type 2 is characterized by > 2 mm of “saddle-back” ST elevation, and type 3 can have the morphology of either type 1 or type 2 but with < 2 mm ST elevation and is nonspecific [5]. However, according to the current literature, only the type 1 EKG pattern is described [1]. The guidelines for the diagnosis of BrS have evolved. The recently developed Shanghai score incorporates additional information, including clinical history, FH, and/or genetic testing in addition to the EKG changes and aids in the definitive diagnosis of BrS [1]. The clinical presentation of BrS includes syncope and cardiac arrest or SCD resulting from VF [1]. Monomorphic VT is seen rarely and can be seen in patients with SCN5A carriers. Males account for about 80-90% of cases of BrS [1]. The usual age for patients presenting with an arrhythmogenic event (AE) is 30 - 50 years [1]. However, females have a bimodal distribution of events [1]. According to Michowitz et al, a multicenter study was conducted on 678 patients with BrS; the mean age at the time of the AE was 29 ± 24 years [11]. About 80% of the patients presented with aborted cardiac arrest and 17% with an arrhythmic storm [11]. History of syncope, FH of SCD, and spontaneous type 1 Brugada pattern were noted in 40%, 17%, and 71% of the patients, respectively [11].
Certain environmental factors that can influence the type 1 EKG phenotype include temperature, medications (sodium channel blockers (SCB)), electrolyte disturbances (hypokalemia, hyperkalemia, and metabolic acidosis), infections, and illicit drug use like cocaine [7, 9, 12]. In particular, hyperthermia can trigger VF and cause cardiac arrest in patients with a diagnosis of BrS; it can also induce a Brugada type 1 EKG pattern in patients without a prior diagnosis of BrS [13]. A pooled meta-analysis by Roterberg et al included 53 patients with type 1 BrS induced by fever, including 14 case reports. The incidence of AE during fever was very high (38%), and the incidence of life-threatening arrhythmia with another episode of fever was 40% [8]. About 75% of patients had no symptoms before the fever event [8]. The symptoms of fever included life-threatening arrhythmias, VF or VT in 17%, syncope in 13%, and aborted SCD in 13%, and one (1.8%) patient developed electrical storm, which led to not aborted SCD [8].
The diagnosis of BrS can be challenging. Provocation testing with SCB, procainamide, or disopyramide is considered an important adjunct in assessing patients with possible BrS due to its arrhythmogenic properties [1]. Quinidine, an antiarrhythmic drug, is used in the pharmacologic treatment of BrS [1]. An ICD implantation is the most effective therapeutic option compared to medications like quinidine in patients diagnosed with BrS [1, 4]. Histopathological studies have demonstrated excess fibrosis and collagen within the anterior RVOT in addition to inflammatory infiltrates and reduced connexin-43 [1]. Targeted radiofrequency ablation of the anterior part of the RVOT is an emerging therapy with encouraging results in patients with BrS [1, 4, 14]. However, in asymptomatic patients with only Brugada type 1 EKG pattern, there is no indication of ICD placement [15]. Minimizing environmental triggers in patients diagnosed with BrS or Brugada pattern is crucial [1]. It is key for patients to be educated on avoiding illicit drug use, including alcohol, cannabis, and cocaine, and avoiding medications like antihistamines and antidepressants [1]. Conservative measures like immediate treatment of fever with antipyretics and avoidance of drugs that can provoke Brugada EKG pattern are considered key in managing these patients [1].
According to Tsai et al, a 10-year follow-up study evaluated long-term prognosis in febrile patients with type 1 Brugada pattern on the EKG. A total of 21 individuals were studied; 86% (18 individuals) were asymptomatic, and 14% (three individuals) were symptomatic during the initial fever event [16]. The asymptomatic patients had no FH of SCD, syncope, or spontaneous type 1 Brugada pattern EKG. None of the asymptomatic patients had a ventricular AE during the 116 ± 19 months of follow-up [16]. Therefore, in patients with fever and type 1 Brugada pattern EKG, clinical presentation, history of syncope, and FH of SCD are the most important parameters in the risk stratification [16]. Based on the expert consensus recommendations, comprehensive or SCN5A-targeted genetic testing can be useful for any patient in whom a cardiologist has established a clinical index of suspicion for BrS [1, 17]. All first-degree relatives of patients with BrS or unexplained SCD should be offered screening [1]. The screening should include standard and high lead EKG, and SCB provocation testing can also be considered [1]. It is also important to perform baseline echocardiography and advanced cardiac imaging with cardiac magnetic resonance imaging to assess RVOT structure and function in complex cases [1].
Our patient did not have any prior history of ventricular arrhythmias, unexplained syncope, or FH of SCD. Also, after correcting hyperthermia, our patient’s EKG showed normalization of ST elevation, and throughout the hospitalization, our patient did not have any evidence of ventricular arrhythmia. Therefore, our patient’s presentation suggests a Brugada pattern; a definitive diagnosis of BrS cannot be made yet. It is crucial to distinguish these two entities to determine further management, and close outpatient follow-up is highly recommended. The patient was also offered genetic testing to establish a possible diagnosis of BrS.
Conclusions
BrS is a complex clinical entity with classic EKG findings and other clinical factors. Hyperthermia is one of the known triggers for the induction of the Brugada pattern. It can provoke VF and cause cardiac arrest in patients diagnosed with both BrS and Brugada pattern. It is important to distinguish between BrS and Brugada pattern, and genetic testing should be offered in the latter group if there is a high index of clinical suspicion. Aggressive management of hyperthermia plays a key role in preventing VF and SCD in these patients.
Learning points
The Brugada pattern is characterized by coved-type ST-segment elevation greater than 2 mm followed by a descending negative T wave in at least two precordial leads (V1 - V3) without other clinical features like VF, syncope, or FH of SCD.
The differential diagnosis of ST elevations in the right precordial leads include STEMI, BrS, and Brugada pattern secondary to environmental triggers like electrolyte disturbances, infection, hyperthermia, and cocaine use.
Making accurate diagnoses and eliminating environmental triggers before implanting an ICD in patients with EKG findings suggestive of BrS is important.
Acknowledgments
None to declare.
Financial Disclosure
No grants, contracts, or other forms of financial support are involved in the work.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
Poornima Vinod: writing the original draft, including the literature search, review, and editing. Hiten Patel: supervision, review, and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
BrS: Brugada syndrome; FH: family history; EKG: electrocardiogram; SCD: sudden cardiac death; STEMI: ST-elevation myocardial infarction; VT: ventricular tachycardia; VF: ventricular fibrillation; ED: emergency department; EMS: Emergency Medical Services; ICD: implantable cardiac defibrillator; RVOT: right ventricular outflow tract; SCD: sodium channel blockers; AE: arrhythmogenic event
| References | ▴Top |
- Krahn AD, Behr ER, Hamilton R, Probst V, Laksman Z, Han HC. Brugada syndrome. JACC Clin Electrophysiol. 2022;8(3):386-405.
doi pubmed - Papadakis M, Papatheodorou E, Mellor G, Raju H, Bastiaenen R, Wijeyeratne Y, Wasim S, et al. The diagnostic yield of brugada syndrome after sudden death with normal autopsy. J Am Coll Cardiol. 2018;71(11):1204-1214.
doi pubmed - Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20(6):1391-1396.
doi pubmed - Korlipara H, Korlipara G, Pentyala S. Brugada syndrome. Acta Cardiol. 2021;76(8):805-824.
doi pubmed - Sarquella-Brugada G, Campuzano O, Arbelo E, Brugada J, Brugada R. Brugada syndrome: clinical and genetic findings. Genet Med. 2016;18(1):3-12.
doi pubmed - Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7(1):33-46.
doi pubmed pmc - Li Y, Lang S, Akin I, Zhou X, El-Battrawy I. Brugada syndrome: different experimental models and the role of human cardiomyocytes from induced pluripotent stem cells. J Am Heart Assoc. 2022;11(7):e024410.
doi pubmed pmc - Roterberg G, El-Battrawy I, Veith M, Liebe V, Ansari U, Lang S, Zhou X, et al. Arrhythmic events in Brugada syndrome patients induced by fever. Ann Noninvasive Electrocardiol. 2020;25(3):e12723.
doi pubmed pmc - Li Y, Dinkel H, Pakalniskyte D, Busley AV, Cyganek L, Zhong R, Zhang F, et al. Novel insights in the pathomechanism of Brugada syndrome and fever-related type 1 ECG changes in a preclinical study using human-induced pluripotent stem cell-derived cardiomyocytes. Clin Transl Med. 2023;13(3):e1130.
doi pubmed pmc - Amin AS, Meregalli PG, Bardai A, Wilde AA, Tan HL. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med. 2008;149(3):216-218.
doi pubmed - Michowitz Y, Milman A, Sarquella-Brugada G, Andorin A, Champagne J, Postema PG, Casado-Arroyo R, et al. Fever-related arrhythmic events in the multicenter Survey on Arrhythmic Events in Brugada Syndrome. Heart Rhythm. 2018;15(9):1394-1401.
doi pubmed - Santoro F, Crea P, Pellegrino PL, Cetera R, Gianfrancesco D, Abumayyaleh M, Giuseppe D, et al. Fever following Covid-19 vaccination in subjects with Brugada syndrome: Incidence and management. J Cardiovasc Electrophysiol. 2022;33(8):1874-1879.
doi pubmed pmc - Morita H, Zipes DP, Morita ST, Wu J. Temperature modulation of ventricular arrhythmogenicity in a canine tissue model of Brugada syndrome. Heart Rhythm. 2007;4(2):188-197.
doi pubmed - Grossi S, Bianchi F, Pintor C, Musumeci G, Gaita F. Transcatheter ablation in patients with Brugada syndrome. Eur Heart J Suppl. 2023;25(Suppl C):C38-C43.
doi pubmed pmc - Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932-1963.
doi pubmed - Tsai CF, Chuang YT, Huang JY, Ueng KC. Long-term prognosis of febrile individuals with right precordial coved-type ST-segment elevation brugada pattern: a 10-year prospective follow-up study. J Clin Med. 2021;10(21):4997.
doi pubmed pmc - Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8(8):1308-1339.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


