| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 7, July 2024, pages 148-151
Asplenia-Associated Babesiosis: A Quagmire Traversed by Exchange Transfusion
Muhammad Umer Riaz Gondala, g , Luke Rovenstineb, Fawwad Ansaric, Zainab Kiyanid, Syed Ayan Zulfiqar Bokharie, Devi Parvathy Jyothi Ramachandran Naira, Toqeer Khanf, Syed Jaleela
aDepartment of Internal Medicine, Tower Health, Reading Hospital, West Reading, PA, USA
bDepartment of Internal Medicine, Drexel University, West Reading, USA
cDepartment of Internal Medicine, Piedmont Athens Regional, Athens, GA, USA
dDepartment of Internal Medicine, Islamabad Medical and Dental College, Islamabad, Pakistan
eDepartment of Internal Medicine, Foundation University, Islamabad, Pakistan
fDepartment of Internal Medicine, Lincoln Medical Center, Bronx, NY, USA
gCorresponding Author: Muhammad Umer Riaz Gondal, Department of Internal Medicine, Tower Health, Reading Hospital, West Reading, PA, USA
Manuscript submitted May 14, 2024, accepted June 1, 2024, published online June 19, 2024
Short title: Asplenia-Associated Babesiosis
doi: https://doi.org/10.14740/jmc4247
| Abstract | ▴Top |
Babesiosis is a potentially life-threatening tick-borne parasitic infection. Severe disease in splenectomized individuals may require exchange transfusion. A 58-year-old male with a history of splenectomy presented with 2 weeks of subjective fever, weakness, and abdominal pain. He denied any rashes, tick bites, or recent travel. He had a motor vehicle accident a few years ago and had undergone an emergency splenectomy. On examination, the patient was febrile (39.3 °C), tachycardic (106/min), and jaundiced. Labs revealed anemia and thrombocytopenia. Computed tomography (CT) abdomen revealed asplenia. As it was summer, there was concern for a tick-borne illness. A peripheral smear showed schistocytes, and labs revealed hyperbilirubinemia, high lactate dehydrogenase (LDH), low haptoglobin, and reticulocytosis (13%), consistent with hemolysis. Testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Ehrlichia, Borrelia, Anaplasma, and viral hepatitis was negative. Antibody testing for Babesia microti was positive. A blood parasite smear confirmed Babesia microti with a parasitemia of 9.5%. The patient received intravenous azithromycin and atovaquone for severe babesiosis. On day 2 of hospitalization, parasitemia increased to 14.7%. Hemoglobin and platelets dropped further on day 3. His parasite load remained consistently above 10% despite medical treatment. A decision was made for a red blood cell (RBC) exchange transfusion for severe disease, which was performed on the fourth day of hospitalization. Clinical improvement was seen after one session of exchange RBC transfusion. Hemoglobin remained stable, and thrombocytopenia improved 1 day after RBC exchange transfusion. Parasitemia dropped to 1.2% after 4 days of exchange transfusion, and azithromycin was switched to oral. He received 9 days of inpatient azithromycin and atovaquone. He was discharged with a plan to continue the oral antimicrobials for 3 more weeks. Asplenia and parasitemia > 10% are associated with severe babesiosis. Asplenia, in particular, is associated with severe infection, hospitalization, and prolonged duration of therapy. Exchange transfusion in severe babesiosis can be lifesaving.
Keywords: Babesiosis; Parasitemia; Exchange transfusion; Asplenia
| Introduction | ▴Top |
Babesiosis is an intraerythrocytic infection caused by the protozoan Babesia. It is primarily spread by tick vectors, with rodents being the reservoir and humans serving as accidental, dead-end hosts. Babesia microti is the most common species.
Babesiosis can be asymptomatic or potentially life-threatening, especially in immunocompromised individuals. We describe a case of a male with a history of splenectomy admitted with babesiosis. The patient was at risk for severe disease, and his clinical status improved after just one session of red blood cell (RBC) exchange transfusion.
| Case Report | ▴Top |
A 58-year-old male with a history of splenectomy presented with 2 weeks of subjective fever, weakness, decreased oral intake, and abdominal pain. He denied any rashes, tick bites, or recent travel. He revealed that he slept outdoors on his farm in Pennsylvania. He denied any sick contact or eating outside food. He had a motor vehicle accident a few years ago and had undergone an emergency splenectomy. The abdominal pain was mild, intermittent, right sided, and not related to eating.
On examination, the patient was febrile (39.3 °C), tachycardic (106/min), and appeared jaundiced and in discomfort due to pain. An abdominal exam was remarkable for right upper quadrant tenderness.
Labs revealed anemia, hemoglobin 10.4 g/dL (reference range: 13.5 - 17.5 g/dL), thrombocytopenia 74 × 109/L (reference range: 150 - 400 × 109/L), and white blood count within normal limits. A computed tomography (CT) of the abdomen showed asplenia. As it was summer season, there was a concern for a tick-borne illness causing low cell counts.
A peripheral smear showed schistocytes. Labs showed hyperbilirubinemia (1.7 µmol/L, reference range: 0.3 - 1.0), high lactate dehydrogenase (LDH) (583 U/L, reference range: 140 - 280 U/L), low haptoglobin (< 30 mg/dL, reference range: 41 - 165 mg/dL), and reticulocytosis (13%, reference range: 0.5-2.5%). That was consistent with hemolysis. Testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Ehrlichia, Borrelia, Anaplasma, and viral hepatitis was negative.
Antibody testing for Babesia microti revealed positive immunoglobulin (Ig)G (> 1:16) and IgM (> 1:20). A blood parasite smear confirmed multiple rounded, paired, and multiple Babesia microti with a parasitemia of 9.5%, as seen in Figure 1. The malarial parasite was not identified on peripheral smear. The polymerase chain reaction (PCR) for babesia was thus not performed.
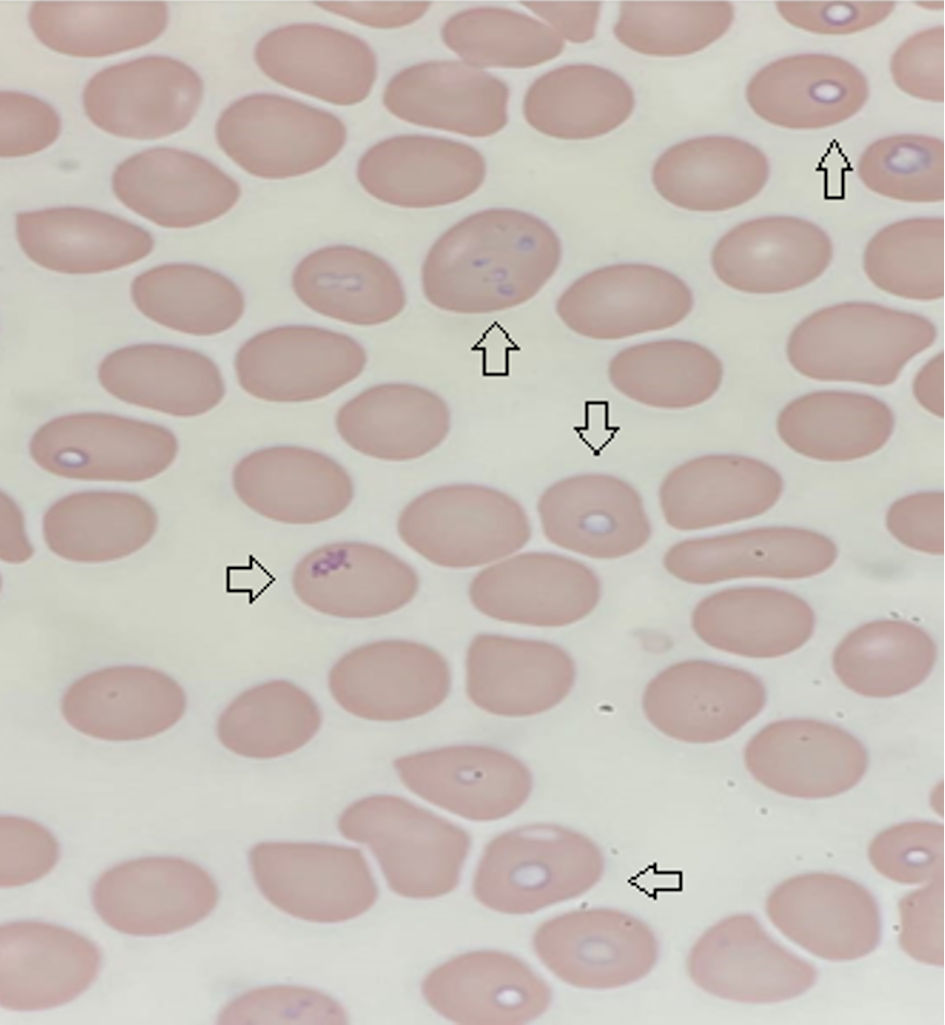 Click for large image | Figure 1. Giemsa stain thin blood smear with arrows pointing towards cells infected with Babesia microti. |
The patient received intravenous azithromycin 500 mg daily and atovaquone 750 mg 12 h for severe babesiosis. On day 2 of hospitalization, parasitemia increased to 14.7%. Hemoglobin dropped to 8.7 g/dL and platelets to (52 × 109/L) on day 3. His parasite load remained consistently above 10% despite treatment for severe babesiosis. The medical team formulated a multidisciplinary plan for RBC exchange transfusion with the infectious disease and hematology teams, performed on the fourth day of hospitalization.
Clinical improvement was seen after one session of RBC exchange transfusion. Hemoglobin remained stable at 8.7 g/dL, and thrombocytopenia improved to (81 × 109/L) 1 day after RBC exchange transfusion. Parasitemia dropped to 1.2% after 4 days of exchange transfusion, and azithromycin was switched to oral. He received 9 days of inpatient azithromycin and atovaquone. He was discharged with a plan to continue the oral antimicrobials for 3 more weeks. At a follow-up 5 days after discharge, parasitemia had dropped to 0.2%, and the patient was doing clinically well.
| Discussion | ▴Top |
There are several species of Babesia, of which Babesia microti is the most common. It is endemic to North America’s Northeast and Upper Midwest and is spread by Ixodes scapularis. Other species include Babesia duncani, which is spread by the Ixodes pacificus tick and endemic to the Pacific Northwest; Babesia divergens, endemic to Europe; and Babesia venatorum and Babesia crassa, endemic to Northeast China.
Other transmission methods include tick bites, blood transfusion, organ transplantation, and transplacental transmission. Over 250 cases of transfusion-transmitted babesiosis have been reported [1, 2]. Two cases have been reported of babesiosis spreading following renal transplant, as the donor had received many transfusions on the day he expired [3]. Finally, more than 10 cases of congenital babesiosis have been reported in the USA [4].
The number of cases of babesiosis has steadily risen, necessitating the Centers for Disease Control and Prevention (CDC) to categorize it as a reportable disease in January 2011. From 2011 to 2019, annual cases increased from 1,126 to 2,418 [5]. The prevalence of Babesia microti is 1% in newly named endemic areas and 20% in well-established areas.
The spleen is a critical defense in mitigating disease as the red pulp utilizes macrophages to target and phagocytose infected erythrocytes. Patients without a spleen will have a worse presentation due to the risk of higher levels of parasites in the blood.
The incubation period for Babesia microti is 1 - 6 weeks if tick-borne or 6 - 9 weeks and up to 6 months if received via blood transfusion. Presentation ranges from asymptomatic to mild/moderate to severe. The severity of presentation depends on immunocompetence and the presence of a spleen to participate in erythrocyte phagocytosis. Mild disease is seen when parasitemia is < 4%. Symptoms typically include flu-like illnesses with fever, malaise, headache, and, less commonly, arthralgias and sore throat. Fever is usually intermittent or sustained and can be as high as 40.9 °C. Physical exam may reveal splenomegaly or hepatosplenomegaly, jaundice, retinopathy, and pharyngeal erythema. A rash is typically not common in babesiosis and, if present, may indicate co-infection with Lyme disease. Labs may exhibit anemia, elevated LDH, low haptoglobin, reticulocytosis, transaminitis, elevated total/indirect bilirubin, thrombocytopenia, and elevated blood urea nitrogen (BUN)/creatinine (Cr). In severe disease, labs are similar but more pronounced [2].
Severe disease occurs in immunocompromised individuals, patients over 50, neonates, or those who are without a spleen. Presentation is similar to mild/moderate disease but with more acute symptoms. The presence of nausea, vomiting, and diarrhea predicts severe infection. Complications of severe disease include acute respiratory distress syndrome, congestive heart failure, renal failure, splenic rupture, disseminated intravascular hemolysis, hepatitis, coma, and death. Half of all hospitalized babesiosis patients will develop complications [1]. The most common complication is acute respiratory distress syndrome. A study of 22 patients found mortality in a patient’s hospital course to be higher if they got acute respiratory distress syndrome than if not (37% vs. 14%) [6]. Splenic rupture is rare but occurs in healthier, younger patients. Rupture is spontaneous and secondary to excess erythrophagocytosis in the red pulp. Before rupture, most of these patients have splenomegaly, low parasitemia, and no other complications. Other complications of this disease include warm autoimmune hemolytic anemia (2 - 4 weeks following resolution of treatment), relapsing disease (fatal in 20% of cases), and fatality (3-9% of hospitalized patients and 20% for those who acquired it through blood transfusion) [5].
Coinfection is common in babesiosis as the tick vector, Ixodes scapularis, carries Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia muris. Half of patients with babesiosis have concurrent Lyme disease. Co-infection may present with a more significant number of acute symptoms and a longer duration of Lyme disease. Studies have suggested that co-infection with Lyme disease does not affect the severity of babesiosis [7].
Investigation for babesiosis should begin when clinical signs are apparent, tick bites have been reported, one lives or has traveled to an endemic area, or when other explanations have been exhausted. The first diagnostic test is a thin peripheral blood smear using Giemsa or Wright staining. This test allows for rapid turn-around time and adequately quantifies parasitemia. In early infection, parasitemia may be low; therefore, diagnosis requires multiple smears over several days [1]. The stain shows trophozoites as round, oval, and pear-shaped with purple chromatin dots. Various infections may be within each cell, and rings may resemble Plasmodium falciparum. Babesia infection can be distinguished from malaria with a pathognomonic merozoite tetrad confirmation (Maltese cross), extracellular forms, absence of hemozoin deposit in ring forms, and absence of gametocytes [2]. Real-time PCR testing is sensitive and can detect the 18s RNA gene of Babesia with 100% specificity. It is typically used if no microscopy expert is available, the species of Babesia must be distinguished, or parasitemia is very low. Serology studies are used as confirmatory tests, using indirect fluorescent antibody testing. A single serology study is not specific as it will not distinguish between acute versus past infection; therefore, acute and convalescent studies are necessary. Finally, in severe disease, radiographic imaging may reveal pulmonary edema on the chest radiograph and splenic infarct and splenic rupture on the abdominal CT scan.
The differential for babesiosis is broad and includes tick-borne and arbor-borne illnesses. Patients with rickettsial infection typically present with fever, headache, thrombocytopenia, leukopenia, and transaminase elevation. Rickettsia, however, presents with petechial rash or eschar several days into the disease course. Malaria has clinical manifestations that mirror babesiosis. Diagnosis of malaria should be suspected in the setting of epidemiologic exposure and is verified by visualizing parasites on peripheral smears or using a rapid diagnostic test. As stated above, babesiosis may be distinguished from malaria on a thin blood smear. The diagnosis of these other diseases is established by microscopy, PCR, or serology.
Treatment is initiated in individuals who are symptomatic or asymptomatic with positive blood smears or PCR results for more than 3 months after the initial positive result. Two standard regimens: 7 - 10 days of atovaquone plus azithromycin or 7 - 10 days of quinine plus clindamycin. However, the latter regimen has significantly higher rates of adverse drug reactions. In those with severe disease, the preferred regimen is clindamycin plus quinine. Intravenous (IV) quinidine can be used but necessitates cardiac monitoring for QT prolongation. Extended treatment of 6 weeks plus 2 weeks following negative blood smears for severe conditions or those with immunocompromised states is necessary as persistent or relapsing disease is common [7]. Exchange transfusion therapy is recommended if parasitemia is > 10% and hemoglobin is < 10 g/dL and for those with severe Babesia divergens infection, pulmonary, renal, or hepatic dysfunction, regardless of parasitemia levels. Exchange transfusion reduces parasitemia and cytokines and corrects anemia [5, 8]. Finally, empiric doxycycline should be initiated where Lyme disease is endemic, pending definitive diagnostic tests. In those who do not improve within 48 h following the babesiosis regimen, empiric doxycycline should be started pending further diagnostic data.
Conclusions
Severe infection in babesiosis is more common in patients with asplenia and parasitemia > 10 %, such as ours. Asplenia is associated with severe illness, hospitalization, and prolonged duration of therapy. Exchange transfusion, in addition to antimicrobials, can reduce morbidity and mortality.
Learning points
Babesiosis is a tick-born illness endemic to the Northeastern USA, with peak season in the spring and summer. Severe babesiosis occurs in individuals with asplenia, immunocompromised individuals, and those above 50, and can be life-threatening with multiorgan failure. Exchange transfusion therapy is recommended if parasitemia is 10% and hemoglobin is < 10 g/dL.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Patient consent has been obtained.
Author Contributions
Muhammad Umer Riaz Gondal: writing - original draft, and writing - review and editing, conceptualization, data curation, formal analysis, and investigation. Luke Rovenstine, Fawwad Ansari, Zainab Kiyani, Devi Parvathy Jyothi Ramachandran Nair, and Syed Ayan Zulfiqar Bokhari: writing - review and editing, conceptualization. Toqeer Khan: project administration, supervision, validation, and visualization. Syed Jaleel MD: supervision, validation, and visualization.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am. 2015;29(2):357-370.
doi pubmed pmc - Gubernot DM, Lucey CT, Lee KC, Conley GB, Holness LG, Wise RP. Babesia infection through blood transfusions: reports received by the US Food and Drug Administration, 1997-2007. Clin Infect Dis. 2009;48(1):25-30.
doi pubmed - Brennan MB, Herwaldt BL, Kazmierczak JJ, Weiss JW, Klein CL, Leith CP, He R, et al. Transmission of babesia microti parasites by solid organ transplantation. Emerg Infect Dis. 2016;22(11):1869-1876.
doi pubmed pmc - Joseph JT, Purtill K, Wong SJ, Munoz J, Teal A, Madison-Antenucci S, Horowitz HW, et al. Vertical transmission of Babesia microti, United States. Emerg Infect Dis. 2012;18(8):1318-1321.
doi pubmed pmc - Gray EB, Herwaldt BL. Babesiosis surveillance - United States, 2011-2015. MMWR Surveill Summ. 2019;68(6):1-11.
doi pubmed - Centers for Disease Control and Prevention (CDC). Babesiosis surveillance - 18 States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(27):505-509.
pubmed - Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315(16):1767-1777.
doi pubmed pmc - Krause PJ, Auwaerter PG, Bannuru RR, Branda JA, Falck-Ytter YT, Lantos PM, Lavergne V, et al. Clinical practice guidelines by the infectious diseases society of America (IDSA): 2020 guideline on diagnosis and management of babesiosis. Clin Infect Dis. 2021;72(2):185-189.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


