| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 8, August 2024, pages 186-194
Exploring Atrial Fibrillation: Understanding the Complex Relation Between Lifestyle and Genetic Factors
Rafael Tamayo-Trujilloa, c , Elius Paz-Cruza, c
, Santiago Cadena-Ullauria, c
, Patricia Guevara-Ramireza, c
, Viviana A. Ruiz-Pozoa
, Rita Ibarra-Castillob, Jose Luis Laso-Bayasb
, Ana Karina Zambranoa, d
aCentro de Investigacion Genetica y Genomica, Facultad de Ciencias de la Salud Eugenio Espejo, Universidad UTE, Quito, Ecuador
bClinical Cardiac Electrophysiologist, Quito, Ecuador
cThese authors contributed equally to this work and share first authorship.
dCorresponding Author: Ana Karina Zambrano, Centro de Investigacion Genetica y Genomica, Facultad de Ciencias de la Salud Eugenio Espejo, Universidad UTE, Quito, Ecuador
Manuscript submitted May 15, 2024, accepted June 21, 2024, published online July 25, 2024
Short title: Lifestyle and Genetic Factors of AF
doi: https://doi.org/10.14740/jmc4250
| Abstract | ▴Top |
Cardiovascular diseases (CVDs) are the leading cause of death worldwide across diverse ethnic groups. Among these, atrial fibrillation (AF) stands as one of the most prevalent types of arrhythmias and the primary cause of stroke. Risk factors associated with AF include alcohol consumption, aging, high blood pressure, hypertension, inflammation, and genetic factors. A family history of CVD could indicate an increased risk. Consequently, genetic, and genomic testing should be performed to identify the molecular etiology of CVDs and assess at-risk patients. It is important to note that CVDs are the results of the complex interplay of genes and environmental factors, including ethnicity. In this case, the proband’s clinic story includes a history of smoking abuse for 10 years (10 cigarettes per day), obesity, hypertension, and an associated familial history. These risk factors, along with genetic variants, could trigger the early onset of AF. In recent years, genetic and genomic studies have significantly advanced our understanding of CVD etiology, given that next-generation sequencing (NGS) allows for the identification of genetic variants that could contribute to these pathologies. Furthermore, NGS facilitates early diagnosis, personalized pharmacological approaches, and identification of novel biomarkers. Thus, NGS is a valuable tool in CVD management. However, such studies are limited in Ecuador, a low- and middle-income country. Several challenges contribute to this gap, encompassing economic, infrastructural, and educational obstacles. Notably, the cost of genetic and genomic studies may also pose a barrier, restricting access to a portion of the population. In this case report, we present a 56-year-old Ecuadorian woman, who has been diagnosed with AF; however, after performing NGS no disease-associated variants were found, despite having strong clinical signs and symptoms. In summary, this case report contributes valuable insights into the complex interplay between genetic and lifestyle factors in the development and management of AF. The case report aims to underscore the potential impact of genetic variants on disease risk, even when classified as variants of uncertain significance, and the importance of an integral approach to patient care that includes genetic screening, lifestyle interventions, and tailored pharmacological treatment.
Keywords: Atrial fibrillation; Next-generation sequencing; Cardiovascular diseases; Genetics; Ecuador
| Introduction | ▴Top |
The World Health Organization (WHO) has identified cardiovascular diseases (CVDs) as the primary cause of death, accounting for more than 17.9 million deaths worldwide across diverse ethnic groups [1]. In Ecuador, the WHO reported that CVDs accounted for 21% and 20% of total male and female deaths, respectively, in 2014 [2]. Furthermore, these figures rose in 2018, with reported CVD-associated deaths reaching 24% [3].
Genetics may play a pivotal role in CVDs. A family history of CVD could indicate an increased risk, as many cardiac disorders have a hereditary component [4]. Consequently, genetic, and genomic testing should be performed to identify the molecular etiology of CVDs and assess at-risk patients, particularly in diseases like hypertrophic cardiomyopathy and arrhythmias [5]. It is important to note the complex interplay of genes and environmental factors can contribute to CVDs and their risks factors, further complicating the understanding of CVD etiology [5]. Additionally, ethnicity can influence the risk of CVDs. For instance, reports have suggested that Black adults may bear a higher burden of CVD-associated risk factors such as obesity or hypertension [6] (Fig. 1).
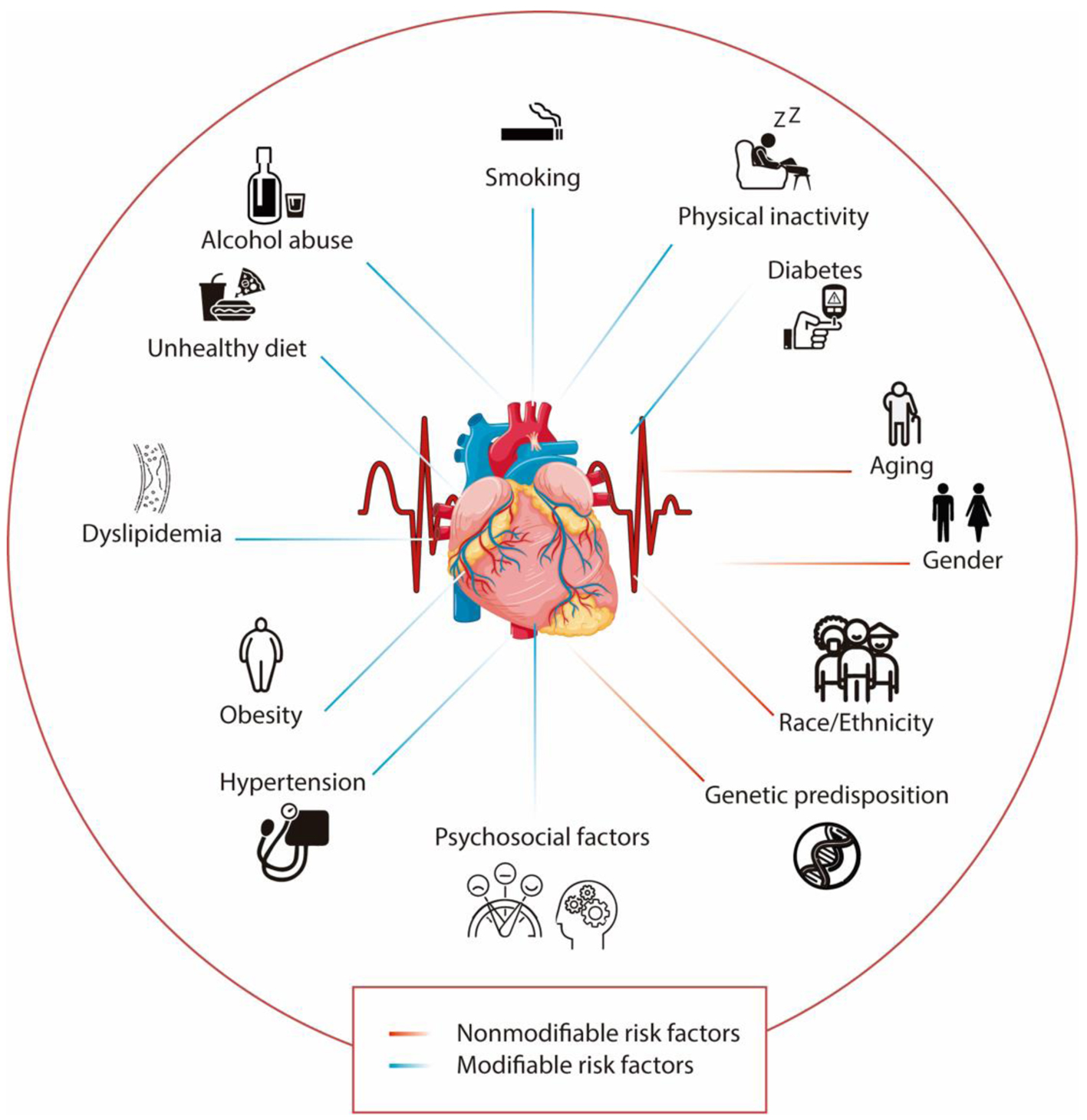 Click for large image | Figure 1. Modifiable and nonmodifiable risk factors associated with cardiovascular diseases. The lines in blue represent the modifiable risk factors, while the lines in red show the nonmodifiable risk factors. |
In recent years, genetic and genomic studies have significantly advanced our understanding of CVD etiology. However, such studies are limited in Ecuador, a low- and middle-income country. Only a few studies have been conducted, yielding interesting results related to the Ecuadorian population [7-9]. Several challenges contribute to this gap, encompassing economic, infrastructural, and educational obstacles. Notably, the cost of genetic and genomic studies may pose a barrier, restricting access to a portion of the population [10].
One of the most prevalent types of arrhythmias and the primary cause of stroke is atrial fibrillation (AF). AF is caused by an abnormal electrical activity in the atria of the heart, resulting in an irregular heart rhythm that can disrupt blood flow, thereby increasing the risk of thrombus formation [11]. Risk factors associated with AF include alcohol consumption, aging, high blood pressure, genetic factors, hypertension, and inflammation [12]. Moreover, mutations in the genes ABCC9, KCNH2, KCNJ2, KCNQ1, LMNA, PRKAG2, RYR2, and SCN5A have been linked to AF [13].
In this case report, we present a 56-year-old Ecuadorian woman, who has been diagnosed with AF; however, after performing next-generation sequencing (NGS) no disease-associated variants were found, despite having strong clinical signs and symptoms. The present article aims to highlight the potential effect of genetic variants and CVDs, even when these variants are classified as variants of uncertain significance. Furthermore, the study underscores the challenges in the management of CVD in Ecuador, a low- and middle-income country with limited resources.
| Case Report | ▴Top |
In 2012, a 44-year-old woman with a family history of CVD and diabetes reported frequent palpitations. These palpitations, which began following a tooth extraction, persisted continuously for 3 days and intermittently (lasting 12 h each) over the subsequent 5 days. During the final 48 h, she also experienced occasional shortness of breath. Three years prior, in 2009, she was diagnosed with an arrhythmia, presenting with irregular palpitations three times weekly, ranging from 30 min to 4 h, in stress or at rest. That year, she was also diagnosed with hypothyroidism, receiving 6 months of treatment before discontinuation by a physician. Furthermore, her anthropometric measurements revealed a height of 1.69 m, a weight of 97 kg, and a body mass index (BMI) of 37.89, indicating obesity. She reported undergoing a 2-month dyslipidemia treatment, no alcohol consumption, and a history of smoking from age 26 to 33 (10 cigarettes per day).
Following the consultation, the patient underwent a Holter test and an electrocardiogram (ECG). The Holter test showed diurnal systolic arterial hypertension, while the ECG exhibited periods of premature ventricular contractions (PVCs), most of which were not being perceived by the patient. A Doppler echocardiogram was also performed, which was within normal limits. Based on these tests, the diagnosis was primary hypertension and an unspecified cardiac arrhythmia. Treatment consisted of a daily dose of carvedilol (12.5 mg) for 30 days. Two months later, in addition to carvedilol, she was prescribed acetylsalicylic acid (100 mg) once daily. Follow-up assessments were performed 3 and 6 months after consultation and the patient presented a good general condition and no signs of heart failure.
In 2019, the patient was diagnosed with chronic AF and began a daily regimen of 200 mg amiodarone, following an ECG that showed normal limits and various Holter tests that indicated paroxysmal fibrillation and flutter. Concurrently, she was diagnosed with pre-diabetes and initiated treatment with 0.075 mg of levothyroxine for hypothyroidism.
Three years later, in 2022, she attended medical consultation, in which she reported persistent nocturnal palpitations leading to sleep disturbances, lipothymic episodes, and anxiety. Her anthropometric data showed a height of 1.69 m, weight of 93 kg, and a BMI of 33, maintaining the obesity previously diagnosed. Subsequent evaluations confirmed the continued need for the existing amiodarone dosage and frequency, adjusted the levothyroxine dose to 0.150 mg daily, and ceased her hypertension treatment.
The episodes of care are shown as a timeline in Figure 2.
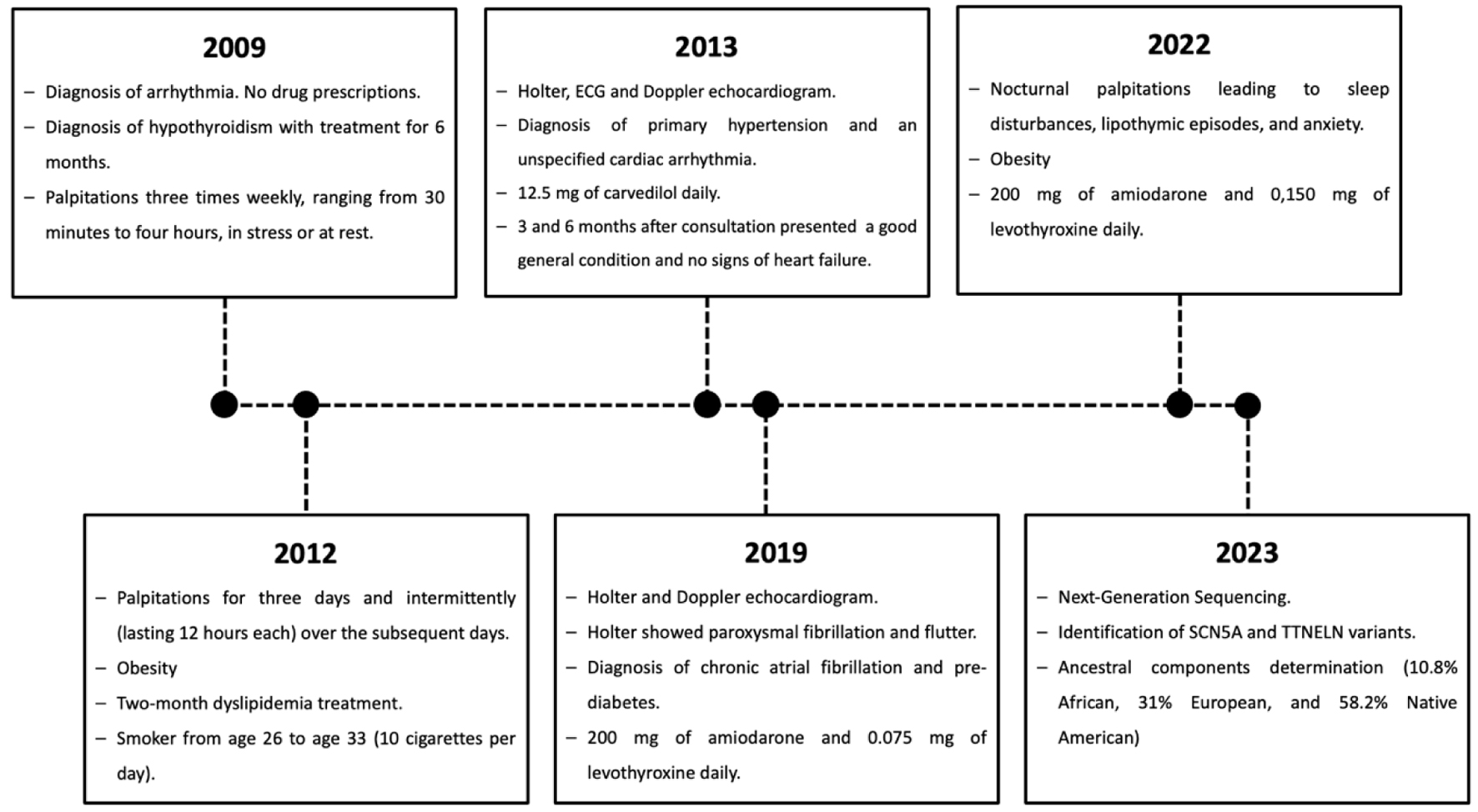 Click for large image | Figure 2. Timeline highlighting the relevant episodes of care of the subject. |
A peripheral blood sample was taken, and DNA was extracted using the PureLinkTM Genomic DNA Mini Kit. DNA concentrations were quantified using the 1× dsDNA high sensitivity (HS) and broad range (BR) assay kits on the Qubit™ 4 fluorometer. NGS was performed at the Centro de Investigacion Genetica y Genomica (CIGG) using the TruSight™ Cardio (TSC) Sequencing Panel on the Illumina MiSeq platform. The TSC Sequencing Panel includes 174 genes with known associations with 17 inherited cardiovascular conditions. For the bioinformatics analyses, DRAGEN Enrichment v3.9.5, Annotation Engine v3.15, PolyPhen, Sift, and Variant Interpreter v2.16.1.300 platforms were used.
Forty-six (46) ancestry-informative INDEL markers (AIMs) were amplified in a multiplex PCR reaction, according to Zambrano et al (2019). Fragment detection was performed on the 3500 Genetic Analyzer. The results were collected and analyzed on the Data Collection v3.3 and Gene Mapper v.5 platforms. The ancestral analysis was performed using STRUCTURE v.2.3.4 [14].
The coverage was ≥ 20× on 97.2% of the target regions of the TSC Sequencing Panel. Variants were classified into five categories (benign, likely benign, variants of uncertain significance (VUS), likely pathogenic, and pathogenic) following the 2015 American College of Medical Genetics and Genomics-Association for Molecular Pathology guidelines [15]. All pathogenic, likely pathogenic, and VUS variants were considered in the analysis (Table 1).
 Click to view | Table 1. Variants Identified in the Participant |
Moreover, an ancestral composition analysis was performed, and the results showed 10.8% African, 31% European, and 58.2% Native American.
Furthermore, the STRING database was used to determine the protein-protein interactions between the proteins associated with AF [16], with a minimum required interaction score of 0.7 (high confidence level). The network was generated using 19 genes related to the phenotype of the individual according to MedLine Plus, an official website of the United States government, and of these genes 17 are not included in the panel (Supplementary Material 1, www.journalmc.org). Additionally, the TTN gene was included due to the presence of a mutation in the proband. Notably, out of the 19 genes associated with AF, 17 are included in the sequencing panel (Fig. 3).
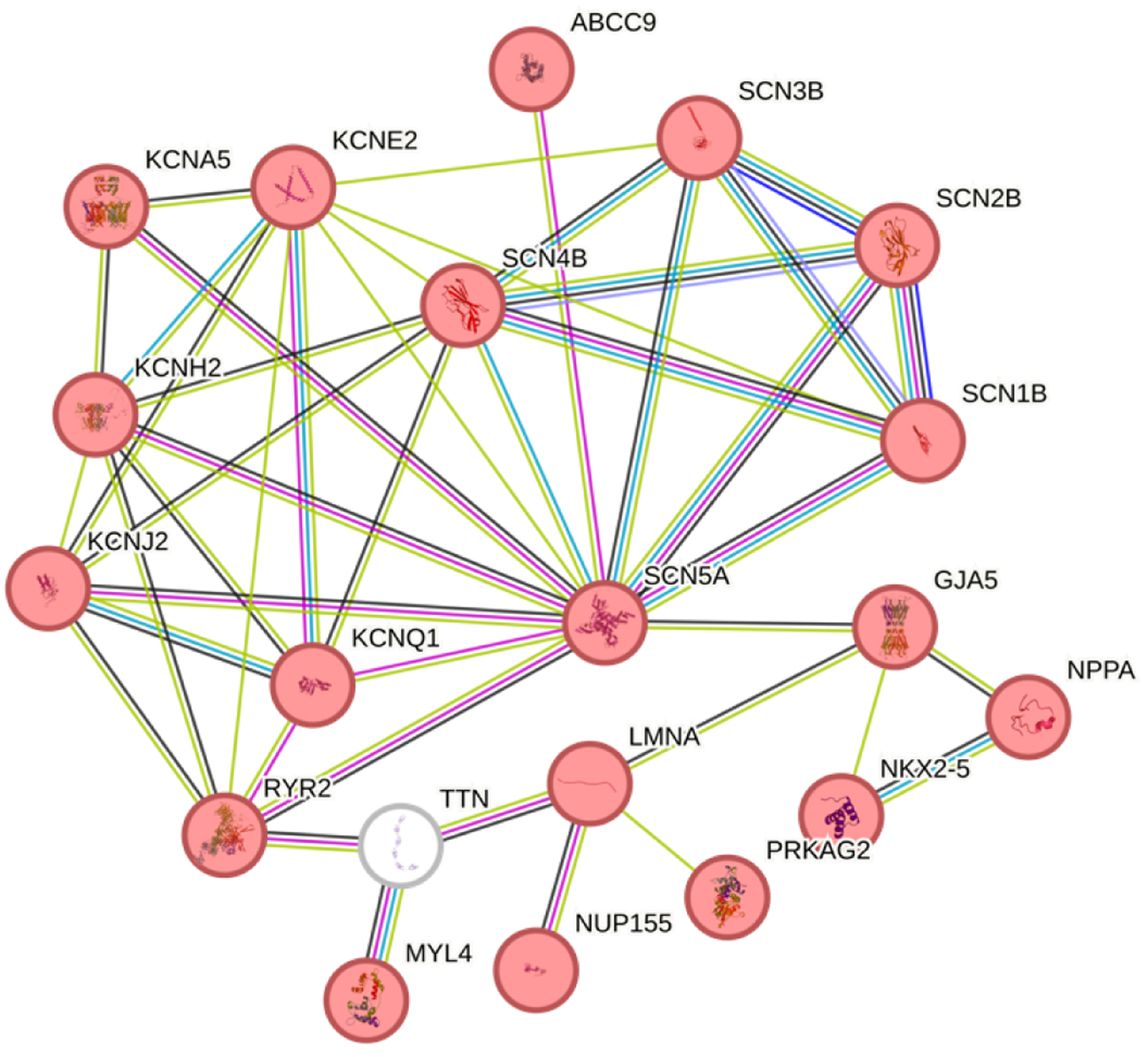 Click for large image | Figure 3. STRING protein-protein interactions. The globes in red have been associated with atrial fibrillation, and the TTN gene is shown in white. The line colors indicate the type of interaction evidence with a minimum required interaction score of 0.700 (high confidence). |
| Discussion | ▴Top |
AF is a prevalent cardiac arrhythmia associated with significant morbidity and mortality rates. Current estimates suggest that approximately 60 million cases exist globally [17]. AF incidence is increasing worldwide due to aging population, the presence of comorbidities and genetic factors [18, 19]. In Ecuador, research on AF is limited. For instance, a study in the Ecuadorian Amerindian rural population reported a low prevalence of this cardiac arrhythmia [20]; however, there are no data of AF prevalence in Ecuadorian urban areas, which could be higher due to lifestyle similarities with high-income countries. Notably, the prevalence of AF is higher in high-income countries than in developing countries, although the underutilization of molecular diagnostic tools at the hospital level in low-income countries [21], could contribute to an underestimation of AF in countries like Ecuador.
AF has a complex genetic etiology, characterized by numerous mutations in various genes implicated in the development of this cardiac arrhythmia. Mutations in genes associated with ion channel currents are the most frequently found in individuals with AF. However, non-ion channel gene variants have also been identified in subjects with this pathology, including the natriuretic peptide precursor A gene (NPPA) [22], atrial-specific myosin light chain gene (MYL4) [23], and titin gene (TTN) [24].
Genomic analyses were conducted using NGS on a MiSeq platform, using the TruSight Cardio Sequencing panel by Illumina. This panel comprises 174 genes associated with various CVDs. Supplementary Material 1 (www.journalmc.org) provides details on the genes correlated with AF according to MedLine Plus that are included in the TruSight Cardio Sequencing Panel. Notably, out of the 19 genes associated with AF, only two were not sequenced. This underscores the depth of the genomic analyses, suggesting that if there is a genetic component of the phenotype, NGS would likely have captured it.
Mutations in SCN5A gene have been implicated in several cardiac conditions like long QT syndrome type 3, Brugada syndrome, cardiac conduction defect, dilated cardiomyopathy, sick sinus syndrome, and AF. These pathologies display specific electrophysiological and structural cardiac characteristics, which are useful in their differentiation by analyzing the individual clinical presentation. Recognizing these distinctions is crucial for the development of targeted treatments [25].
In this case report, a Ser1103Tyr variant in the SCN5A gene is presented. This variant is a loss-of-function mutation located in the intracellular linker between domain II and III of the pore alpha subunit of cardiac sodium channel Nav1.5 [26]. Loss-of-function mutations in this gene have been linked with intra-atrial conduction slowing, which can lead to AF. Furthermore, SCN5A mutations have been associated with both AF and Brugada syndrome due to the altered gating of the sodium channel and the resultant decrease of the inward sodium current (INa). Nonetheless, these correlations are still under debate [25, 26].
The Ser1103Tyr variant in the SCN5A gene is predominantly classified as benign or likely benign, according to ClinVar, an online open database that correlates genomic variation and human health [27]. Despite this classification, the subject’s clinical story presented in this case report shows a marked AF phenotype characterized by an abnormal ECG and structurally normal heart. Notably, the Ser1103 amino acid is located on one of the extracellular parts of the SCN5A protein channel (Fig. 4) [28]. The substitution of serine to tyrosine, which has a hydrophobic side chain, in comparison with, is an amino acid with polar uncharged side chain. Thus, the tyrosine change could alter protein function and protein-protein interactions.
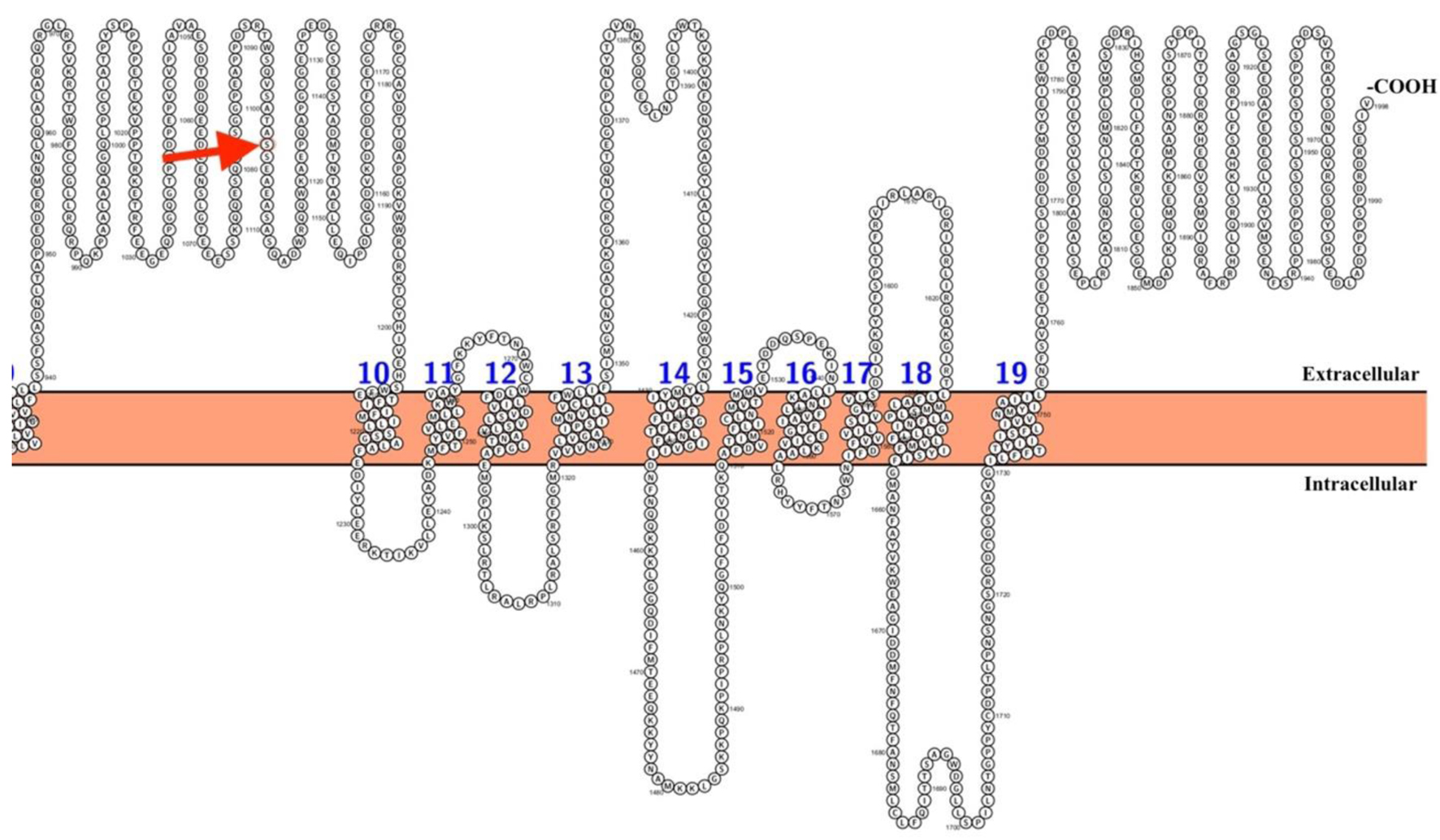 Click for large image | Figure 4. Location of the Ser1103Tyr variant in the SCN5A channel protein. Shown in red is the amino acid corresponding to position 1103. The first part of the protein was not included to specifically pinpoint the location of the variant. The Figure was created using Protter [28]. |
Moreover, the Ser1103Tyr variant has primarily been reported in populations of African descent [29-31]. Clinical outcomes in this population range from sudden unexplained death [29] to ventricular arrhythmias and heart failure [30], along with action potential alterations [31]. These reports show the high variability in the phenotype of individuals carrying the Ser1103Tyr variant. The severity of the disease appears to be associated with the co-occurrence of other genetic variants or heart failure resulting from structural abnormalities.
Although the proband has no documented history of heart failure, a mutation in the TTN gene (Thr3039Ile) was also identified. This TTN variant is currently classified as a variant of uncertain significance in the ClinVar database and has not been directly associated with AF [32]. However, rare TTN mutations have been associated with an early onset of AF in individuals without substance abuse habits or heart failure [24]. Therefore, the co-occurrence of two genetic variants (Ser1103Tyr; Thr3039Ile) may play a pivotal role in the onset of AF in this proband. A STRING analysis (Fig. 3) revealed a high confidence interaction score among the genes involved in AF, including TTN, and it can be observed that all the proteins encoded by these genes have either functional or physical associations, including TTN. These proteins participate in various GO Biological processes, such as Purkinje myocyte to ventricular cardiac muscle cell communication, regulation of atrioventricular (AV) node cell action potential, atrial cardiac muscle cell to AV node cell communication, among other processes associated with proper cardiac function [33].
The identification of high-risk variants associated with cardiac conditions has facilitated the development of “genetic risk scores”. The application of these scores holds potential for improving diagnosis and therapeutic management of cardiac arrythmias [19]. Furthermore, these risk scores, identified through genome-wide association studies, could serve as predictors of cardiac pathologies, considering population ancestry [18]. The implementation of NGS enables the execution of large-scale genome association studies, offering the opportunity to uncover ancestry-specific genetic variants implicated in the emergence of cardiac pathologies.
Populations and ethnicities exhibit diverse genetic backgrounds, and these differences can impact the risk and development of diseases, including CVDs [5]. These variants associated with an increased risk may show geographic specificity, potentially attributable to the “founder effect” [5]. Consequently, specific populations in particular locations may carry variants that correlate with various diseases.
In the present case report, the subject exhibits a predominant Native American genetic component, followed by European and African components. This ancestral composition could be associated with CVD predisposition. For instance, Gomez et al (2022) highlighted that Hispanic individuals exhibit high rates of CVD risk factors, including obesity, hypertension, diabetes, and psychological stress [34]. These risk factors may stem from environmental factors like diet and physical activity, but there may also be a genetic predisposition contributing to the increased risk. Furthermore, Guevara-Ramirez et al (2022) identified several genetic variants present in the Latin American population associated with an increased predisposition to obesity, a main risk factor for CVDs [35]. However, further research is required to fully understand the impact that Native American ethnicity has on CVD and CVD-associated risks.
The prevalence of AF is higher in North America and Western Europe, while the family predisposition and white ethnicity are risk factors for AF incident [36]. The proband has a high European ancestry (31%) considering that this proband showed an unspecified arrhythmia at the age of 32. The combination of a high European ancestry proportion along with obesity, chronic smoking, sedentary lifestyle, and SCN5A/TTN variants may have contributed to the AF phenotype in this case. Moreover, the Ser1103Tyr SNC5A variant showed a frequency of 0.32% in Latin American population [27], suggesting its potential role in the pathogenicity observed in this proband [37]. However, this interpretation must be assessed more rigorously due to the lack of direct implication of this variant with the AF emergence. Moreover, the penetrance of this variant must be evaluated in a Mestizo population due to the implications of environmental factors, lifestyle and genetic modification that could influence the variant expression [37], as observed in this case.
It has been reported that AF may occur in individuals under 45 years old who harbor various mutations, including variants in the TTN and SCN5A genes [24, 38]. Moreover, several risk factors contribute to the AF predisposition, such as smoking, obesity, hypertension, diabetes mellitus, high sodium diet, alcohol abuse, hyperthyroidism, or chronic obstructive pulmonary disease. These factors increase the susceptibility to heart failure, stroke, and sudden death [24, 38-40].
In this case, the proband’s clinic story includes a history of smoking abuse for 10 years (10 cigarettes per day), obesity, hypertension, and a family history of diabetes and hypertension. These risk factors, along with genetic variants, could trigger the early onset of AF. This emphasizes the importance of continuous medical campaigns that raise awareness about the risk factors associated with the onset of cardiac arrythmias and the beneficial role of exercise in prevention.
In terms of pharmacological intervention, the proband responded well to amiodarone (200 mg), which stabilized cardiac rhythm (heart rate: 60 bpm) and maintained blood pressure at 136/80 mm Hg. Additionally, Xarelto was prescribed to prevent embolic events; however, its use was discontinued due to the low risk of embolism. Nonetheless, regular monitoring of arrhythmias is advised for patients with AF to detect any changes that may require modifications in pharmacological management [41].
NGS allows for the identification of genetic variants that could contribute to CVDs. Furthermore, by identifying mutations associated with CVDs, NGS facilitates early diagnosis, personalized pharmacological approaches, and identification of novel biomarkers. Thus, NGS is a valuable tool in CVD management [7, 9].
Conversely, NGS also has limitations such as the need for bioinformatic tools, and higher costs that may be inaccessible for the majority of the population in developing countries, like Ecuador. Furthermore, another limitation is that due to budget constraints, we were unable to perform NGS for other family members [9].
Conclusion
CVDs are the product of a complex interplay between genetics and lifestyle factors. In this case report, the subject presents the signs and symptoms of AF; however, NGS yielded variants of uncertain significance not indicating a clear correlation between genetic factors and AF. It is important to highlight that several environmental factors associated with AF were present in the proband, which could suggest that the signs and symptoms have been primarily triggered by these factors. Additionally, further research is required in low- and middle-income countries, where resources are limited, which restricts an integral approach that includes the study of all disease-associated factors.
Learning points
The present case report highlights the implications and intricate interaction between lifestyle and genetic risk factors. The article presents a 56-year-old woman, who despite showing all the signs and symptoms of AF lacks any genetic variant that could be causing the disease. Although the genetic variants found in the proband have been mostly characterized as likely benign and benign, the SCN5A variant may be associated with a higher CVD risk in specific populations.
| Supplementary Material | ▴Top |
Suppl 1. Genes associated with AF, according to MedLine Plus, that are included in the TruSight Cardio Sequencing Panel by Illumina.
Acknowledgments
The authors are grateful to the Universidad UTE for supporting the researchers.
Financial Disclosure
The experimentation and publication fee of this article are funded by Universidad UTE.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
The participant provided their written informed consent to participate in this study. The study conducted with human participants followed the ethical standards of the 2013 Helsinki Declaration and was approved by the Committee on Ethics and Research in Human Subjects (CEISH)-UTE University (protocol code CEISH-2021-016, date of approval 18-05-2022).
Author Contributions
Conceptualization: RTT, EPC, SCU, and AKZ. Methodology: RTT, EPC, SCU, PGR, VARP, RIC, JLLB, and AKZ. Writing - original draft preparation: RTT, EPC, SCU, PGR, VARP, RIC, JLLB, and AKZ. Writing - review and editing: RTT, EPC, SCU, PGR, VARP, and AKZ. Supervision: AKZ. Project administration: AKZ. Funding acquisition: AKZ. All authors read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Zambrano AK, Cadena-Ullauri S, Guevara-Ramirez P, Paz-Cruz E, Tamayo-Trujillo R, Ruiz-Pozo VA, Domenech N, et al. The autosomal short tandem repeat polymorphisms are potentially associated with cardiovascular disease predisposition in the Latin American population: a mini review. Biomed Res Int. 2023;2023:6152905.
doi pubmed pmc - WHO. Ecuador: cardiovascular diseases profile demographic and socioeconomic profile all cause premature deaths (2011) [Internet]. 2014. Available from: http://bit.ly/1jMhEzO.
- World Health Organization. Noncommunicable Diseases (NCD) Ecuador Profile, 2018. 2018.
- Hajar R. Genetics in cardiovascular disease. Heart Views. 2020;21(1):55-56.
doi pubmed pmc - Vrablik M, Dlouha D, Todorovova V, Stefler D, Hubacek JA. Genetics of cardiovascular disease: how far are we from personalized CVD risk prediction and management? Int J Mol Sci. 2021;22(8):4182.
doi pubmed pmc - Javed Z, Haisum Maqsood M, Yahya T, Amin Z, Acquah I, Valero-Elizondo J, Andrieni J, et al. Race, racism, and cardiovascular health: applying a social determinants of health framework to racial/ethnic disparities in cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2022;15(1):e007917.
doi pubmed - Guevara-Ramirez P, Cadena-Ullauri S, Ibarra-Castillo R, Laso-Bayas JL, Paz-Cruz E, Tamayo-Trujillo R, Ruiz-Pozo VA, et al. Genomic analysis of a novel pathogenic variant in the gene LMNA associated with cardiac laminopathies found in Ecuadorian siblings: A case report. Front Cardiovasc Med. 2023;10:1141083.
doi pubmed pmc - Paz-Cruz E, Ruiz-Pozo VA, Cadena-Ullauri S, Guevara-Ramirez P, Tamayo-Trujillo R, Ibarra-Castillo R, Laso-Bayas JL, et al. Associations of MYPN, TTN, SCN5A, MYO6 and ELN mutations with arrhythmias and subsequent sudden cardiac death: a case report of an Ecuadorian individual. Cardiol Res. 2023;14(5):409-415.
doi pubmed pmc - Cadena-Ullauri S, Guevara-Ramirez P, Ruiz-Pozo V, Tamayo-Trujillo R, Paz-Cruz E, Sanchez Insuasty T, Domenech N, et al. Case report: Genomic screening for inherited cardiac conditions in Ecuadorian mestizo relatives: Improving familial diagnose. Front Cardiovasc Med. 2022;9:1037370.
doi pubmed pmc - Zambrano-Mila MS, Agathos SN, Reichardt JKV. Human genetics and genomics research in Ecuador: historical survey, current state, and future directions. Hum Genomics. 2019;13(1):64.
doi pubmed pmc - Cleveland Clinic. Atrial Fibrillation (Afib). 2022.
- Atrial Fibrillation - StatPearls - NCBI Bookshelf [Internet]. [cited Jan 15, 2024]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526072/.
- MedlinePlus [Internet]. Familial atrial fibrillation [Internet]. Bethesda; 2017 Oct. Available from: https://medlineplus.gov/genetics/condition/familial-atrial-fibrillation/.
- Zambrano AK, Gaviria A, Cobos-Navarrete S, Gruezo C, Rodriguez-Pollit C, Armendariz-Castillo I, Garcia-Cardenas JM, et al. The three-hybrid genetic composition of an Ecuadorian population using AIMs-InDels compared with autosomes, mitochondrial DNA and Y chromosome data. Sci Rep. 2019;9(1):9247.
doi pubmed pmc - Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424.
doi pubmed pmc - Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, Gable AL, et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51(D1):D638-D646.
doi pubmed pmc - Elliott AD, Middeldorp ME, Van Gelder IC, Albert CM, Sanders P. Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol. 2023;20(6):404-417.
doi pubmed - Miyazawa K, Ito K, Ito M, Zou Z, Kubota M, Nomura S, Matsunaga H, et al. Cross-ancestry genome-wide analysis of atrial fibrillation unveils disease biology and enables cardioembolic risk prediction. Nat Genet. 2023;55(2):187-197.
doi pubmed pmc - Kim JA, Chelu MG, Li N. Genetics of atrial fibrillation. Curr Opin Cardiol. 2021;36(3):281-287.
doi pubmed pmc - Del Brutto OH, Costa AF, Cano JA, Penaherrera E, Plaza KJ, Ledesma EA, Tettamanti D, et al. Low prevalence of atrial fibrillation in Amerindians: a population-based study in frequent fish consumers living in rural coastal Ecuador (The Atahualpa Project). Aging Clin Exp Res. 2018;30(5):539-542.
doi pubmed - Li H, Song X, Liang Y, Bai X, Liu-Huo WS, Tang C, Chen W, et al. Global, regional, and national burden of disease study of atrial fibrillation/flutter, 1990-2019: results from a global burden of disease study, 2019. BMC Public Health. 2022;22(1):2015.
doi pubmed pmc - Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359(2):158-165.
doi pubmed pmc - Orr N, Arnaout R, Gula LJ, Spears DA, Leong-Sit P, Li Q, Tarhuni W, et al. A mutation in the atrial-specific myosin light chain gene (MYL4) causes familial atrial fibrillation. Nat Commun. 2016;7:11303.
doi pubmed pmc - Goodyer WR, Dunn K, Caleshu C, Jackson M, Wylie J, Moscarello T, Platt J, et al. Broad genetic testing in a clinical setting uncovers a high prevalence of titin loss-of-function variants in very early onset atrial fibrillation. Circ Genom Precis Med. 2019;12(11):e002713.
doi pubmed pmc - Wilde AAM, Amin AS. Clinical Spectrum of SCN5A Mutations: Long QT Syndrome, Brugada Syndrome, and Cardiomyopathy. JACC Clin Electrophysiol. 2018;4(5):569-579.
doi pubmed - Li W, Yin L, Shen C, Hu K, Ge J, Sun A. SCN5A Variants: Association With Cardiac Disorders. Front Physiol. 2018;9:1372.
doi pubmed pmc - ClinVar. National Institutes of Health. 2024. Genomic variation as it relates to human health. Available from: https://www.ncbi.nlm.nih.gov/clinvar/variation/9393/.
- Omasits U, Ahrens CH, Muller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30(6):884-886.
doi pubmed - Cheng J, Tester DJ, Tan BH, Valdivia CR, Kroboth S, Ye B, January CT, et al. The common African American polymorphism SCN5A-S1103Y interacts with mutation SCN5A-R680H to increase late Na current. Physiol Genomics. 2011;43(9):461-466.
doi pubmed pmc - Wada Y, Yang T, Shaffer CM, Daniel LL, Glazer AM, Davogustto GE, Lowery BD, et al. Common ancestry-specific ion channel variants predispose to drug-induced arrhythmias. Circulation. 2022;145(4):299-308.
doi pubmed pmc - Sun AY, Koontz JI, Shah SH, Piccini JP, Nilsson KR, Jr., Craig D, Haynes C, et al. The S1103Y cardiac sodium channel variant is associated with implantable cardioverter-defibrillator events in blacks with heart failure and reduced ejection fraction. Circ Cardiovasc Genet. 2011;4(2):163-168.
doi pubmed pmc - ClinVar. National Institutes of Health. 2023. Genomic variation as it relates to human health. Available from: https://www.ncbi.nlm.nih.gov/clinvar/variation/535247/.
- Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605-D612.
doi pubmed pmc - Gomez S, Blumer V, Rodriguez F. Unique cardiovascular disease risk factors in Hispanic individuals. Curr Cardiovasc Risk Rep. 2022;16(7):53-61.
doi pubmed pmc - Guevara-Ramirez P, Cadena-Ullauri S, Ruiz-Pozo VA, Tamayo-Trujillo R, Paz-Cruz E, Simancas-Racines D, Zambrano AK. Genetics, genomics, and diet interactions in obesity in the Latin American environment. Front Nutr. 2022;9:1063286.
doi pubmed pmc - Brundel B, Ai X, Hills MT, Kuipers MF, Lip GYH, de Groot NMS. Atrial fibrillation. Nat Rev Dis Primers. 2022;8(1):21.
doi pubmed - Xiang J, Yang J, Chen L, Chen Q, Yang H, Sun C, Zhou Q, et al. Reinterpretation of common pathogenic variants in ClinVar revealed a high proportion of downgrades. Sci Rep. 2020;10(1):331.
doi pubmed pmc - Andersen JH, Andreasen L, Olesen MS. Atrial fibrillation-a complex polygenetic disease. Eur J Hum Genet. 2021;29(7):1051-1060.
doi pubmed pmc - Dong XJ, Wang BB, Hou FF, Jiao Y, Li HW, Lv SP, Li FH. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2019. Europace. 2023;25(3):793-803.
doi pubmed pmc - Kany S, Reissmann B, Metzner A, Kirchhof P, Darbar D, Schnabel RB. Genetics of atrial fibrillation-practical applications for clinical management: if not now, when and how? Cardiovasc Res. 2021;117(7):1718-1731.
doi pubmed pmc - Okutucu S, Gorenek B. Current recommendations on atrial fibrillation: a comparison of the recent European and Canadian guidelines. Cardiology. 2022;147(1):81-89.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


