| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 10, October 2024, pages 304-309
Alpha-Fetoprotein-Producing Hepatoid Adenocarcinoma of the Stomach
Lefika Bathobakaea, e, Mohamed Elagamib, Anas Mahmouda, Jaydev Kesranic, Ruhin Yuridullahb, Gabriel Melkib, Amer Akmald, Yana Cavanaghb, Walid Baddourab
aInternal Medicine, St. Joseph’s University Medical Center, Paterson, NJ, USA
bGastroenterology and Hepatology, St. Joseph’s University Medical Center, Paterson, NJ, USA
cHematology and Oncology, St. Joseph’s University Medical Center, Paterson, NJ, USA
dPathology and Laboratory Medicine, St. Joseph’s University Medical Center, Paterson, NJ, USA
eCorresponding Author: Lefika Bathobakae, Internal Medicine, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
Manuscript submitted June 1, 2024, accepted August 27, 2024, published online September 20, 2024
Short title: AFP-Producing Hepatoid Adenocarcinoma of the Stomach
doi: https://doi.org/10.14740/jmc4263
| Abstract | ▴Top |
Hepatoid adenocarcinoma of the stomach (HAS) is a rare type of gastric cancer with unique clinicopathological features. HAS has a poor prognosis because of early liver, lung, and lymph node metastasis. Owing to its rarity and malignant potential, data on its pathophysiology and management are scarce. Herein, we describe a case of alpha-fetoprotein-producing HAS (AFP-HAS) with metastases to the liver, lungs, and spine. The patient presented with a 3-month history of epigastric pain and intractable emesis, initially thought to be gastroparesis given her uncontrolled diabetes mellitus. Contrast-enhanced computerized tomography (CECT) of the abdomen and pelvis revealed thickening of the gastric wall with hepatic metastases. Upper endoscopy revealed a fungating gastric mass, and the histopathology confirmed AFP-HAS. The patient did not tolerate palliative chemotherapy and died 6 months after her gastric cancer diagnosis.
Keywords: Alpha-fetoprotein-producing gastric cancer; Hepatoid adenocarcinoma of the stomach; Abdominal pain; Palliative chemotherapy
| Introduction | ▴Top |
Hepatoid adenocarcinoma of the stomach (HAS) is a rare and most malignant subtype of gastric cancer [1-6]. It accounts for 0.38-1.6% of all gastric cancers and has a very poor prognosis due to hepatic and early lymph node metastases [1, 2, 7, 8]. The term “hepatoid adenocarcinoma of the stomach” was coined in 1985 to describe a variant of gastric cancer with glandular and hepatoid differentiation [3, 9, 10]. Since then, more case reports have been published in the literature, most of which originated in East Asia [3]. Fewer cases have been reported in Europe and North America [3].
About 80% of patients with HAS have elevated serum alpha-fetoprotein (AFP) levels [9, 11]. AFP is an oncofetal glycoprotein secreted in the gastrointestinal tract, fetal liver, and yolk sac [8]. Elevated serum levels of AFP in adulthood are seen in hepatocellular carcinoma (HCC), yolk sac tumor (YST), and some gastric cancers such as HAS [8]. HAS is the most common AFP-producing gastric cancer, and serum AFP levels tend to correlate with the proportion of hepatoid-like cells in the tumor mass [1]. HAS cases with normal serum AFP levels have the same morphology, immunophenotype, and malignant potential as AFP-producing HAS (AFP-HAS) cases [11]. Herein, we present a case of AFP-HAS in an adult female.
| Case Report | ▴Top |
A 49-year-old female presented to the emergency room (ER) complaining of nausea, vomiting and abdominal pain for 3 months. Her medical history included gastroesophageal reflux disease (GERD) and diabetes mellitus with retinopathy. The pain was worse in the epigastric area, sharp in nature, constant, and rated 8/10 on a 0 - 10 pain scale. It was associated with early satiety and worsened with food intake. The patient endorsed a 15 pounds weight loss over 3 months and intermittent blood-stained stools. She denied having dysphagia, hematemesis, diarrhea, constipation, fever, dysuria, or gross hematuria. She also denied prior abdominal surgery, alcohol use, or use of non-steroidal anti-inflammatory drugs.
In the ER, the patient appeared in mild distress due to epigastric pain and nausea. She was afebrile and hemodynamically stable. She received symptomatic treatment for nausea, vomiting, and abdominal pain with improvement. On physical examination, her abdomen was soft, obese, and tender in the epigastrium. There was no guarding, rigidity or rebound tenderness. She had decreased sensation of touch in the lower extremities and lower thoracic spinal tenderness. The rest of the physical examination findings were unremarkable. Laboratory values were significant for anemia, thrombocytosis, elevated alkaline phosphatase, hypoalbuminemia, hyperglycemia, elevated AFP, elevated cancer antigen (CA) 19-9, elevated CA 125, and elevated carcinoembryonic antigen (CEA) levels (Table 1).
 Click to view | Table 1. Patient’s Admission Laboratory Values Compared to Normal Ranges |
Contrast-enhanced computed tomography (CECT) of the abdomen and pelvis showed gastric wall thickening, multiple necrotic lymph nodes in the upper abdomen, and hepatic lesions consistent with metastatic disease (Fig. 1). A bone nuclear scan revealed lesions at the T11 and L1 vertebrae, consistent with osseous metastasis. Upper endoscopy revealed a large, fungating, and ulcerated mass involving almost the entire lesser curvature of the stomach (Fig. 2). Biopsy results of the gastric mass revealed a moderately-to-poorly differentiated hepatoid gastric adenocarcinoma (Fig. 3). Histopathology of the liver lesions revealed tumor cells consistent with metastatic AFP-HAS (Fig. 4). The immunohistochemical staining (IHS) profiles of both the liver and gastric specimens were significant for AFP, caudal-type homeobox 2 (CDX2), and Sal-like protein 4 (SALL4) positivity (Figs. 3, 4).
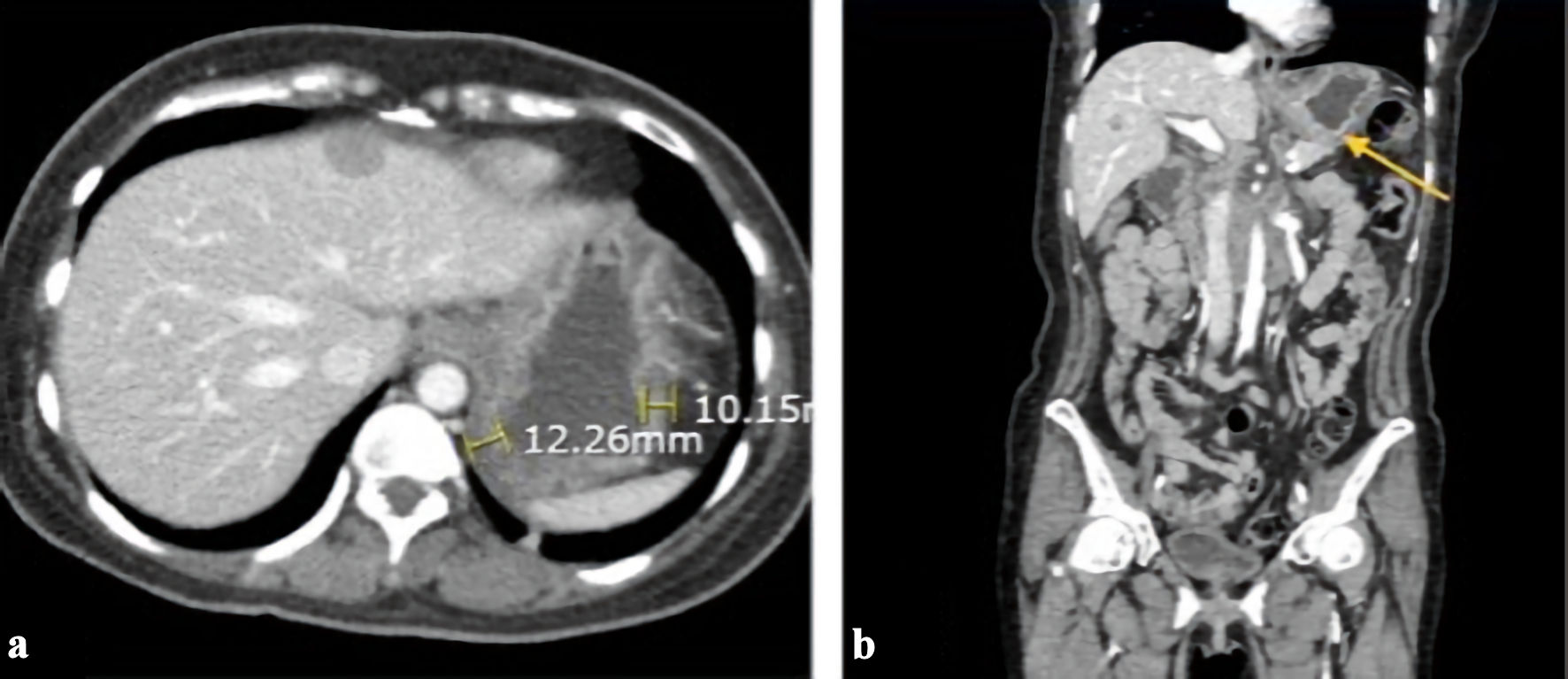 Click for large image | Figure 1. Contrast-enhanced CT scan of the abdomen/pelvis showing abnormal gastric wall thickening with extensive liver metastases. (a) An axial view of the CT scan with yellow lines estimating gastric wall thickness. (b) A coronal view with a yellow arrow showing stomach wall thickening consistent with gastric malignancy. CT: computed tomography. |
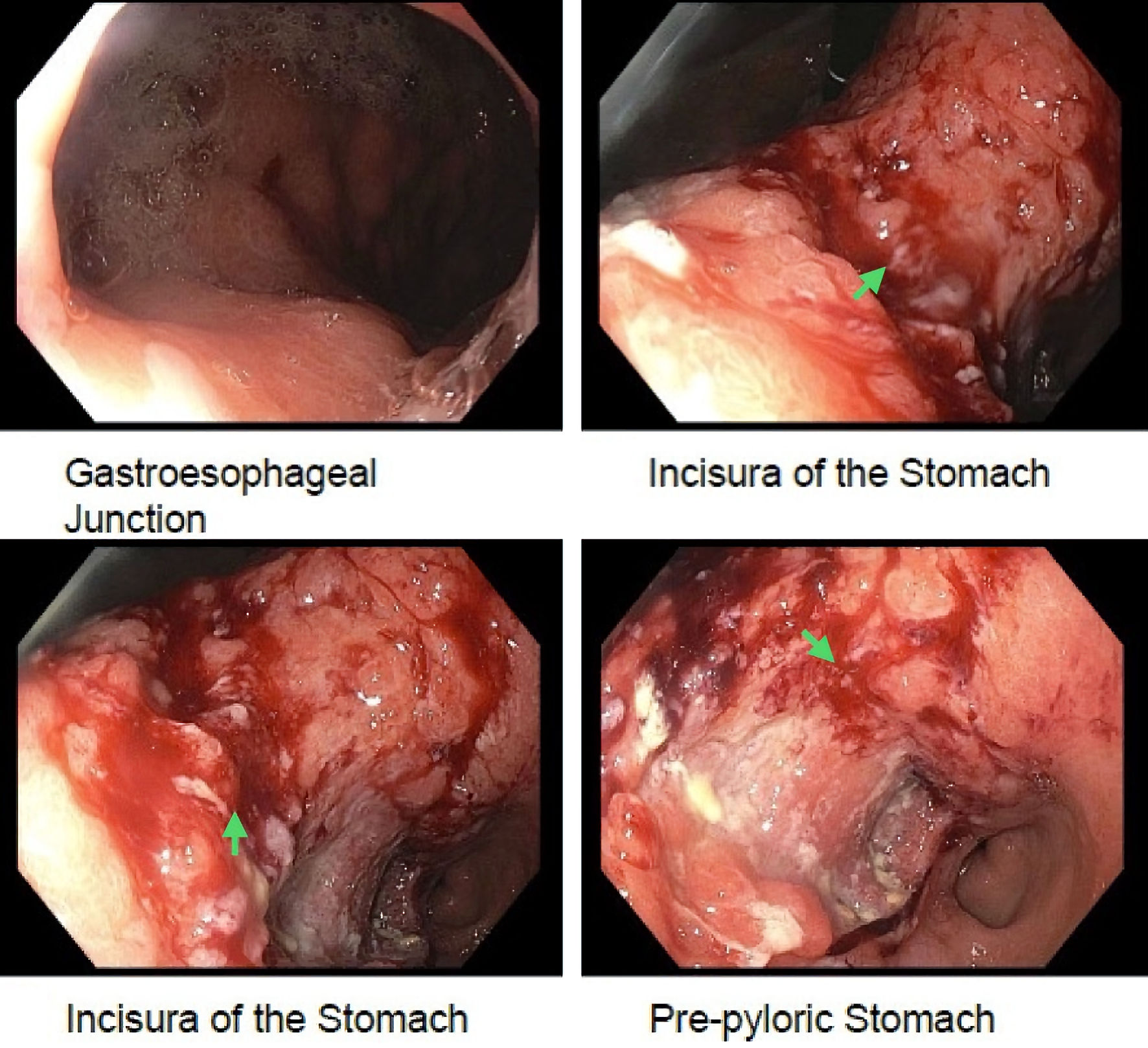 Click for large image | Figure 2. An endoscopy image showing a large, fungating, and ulcerated mass with friability involving almost the entire lesser curvature of the stomach (green arrows). |
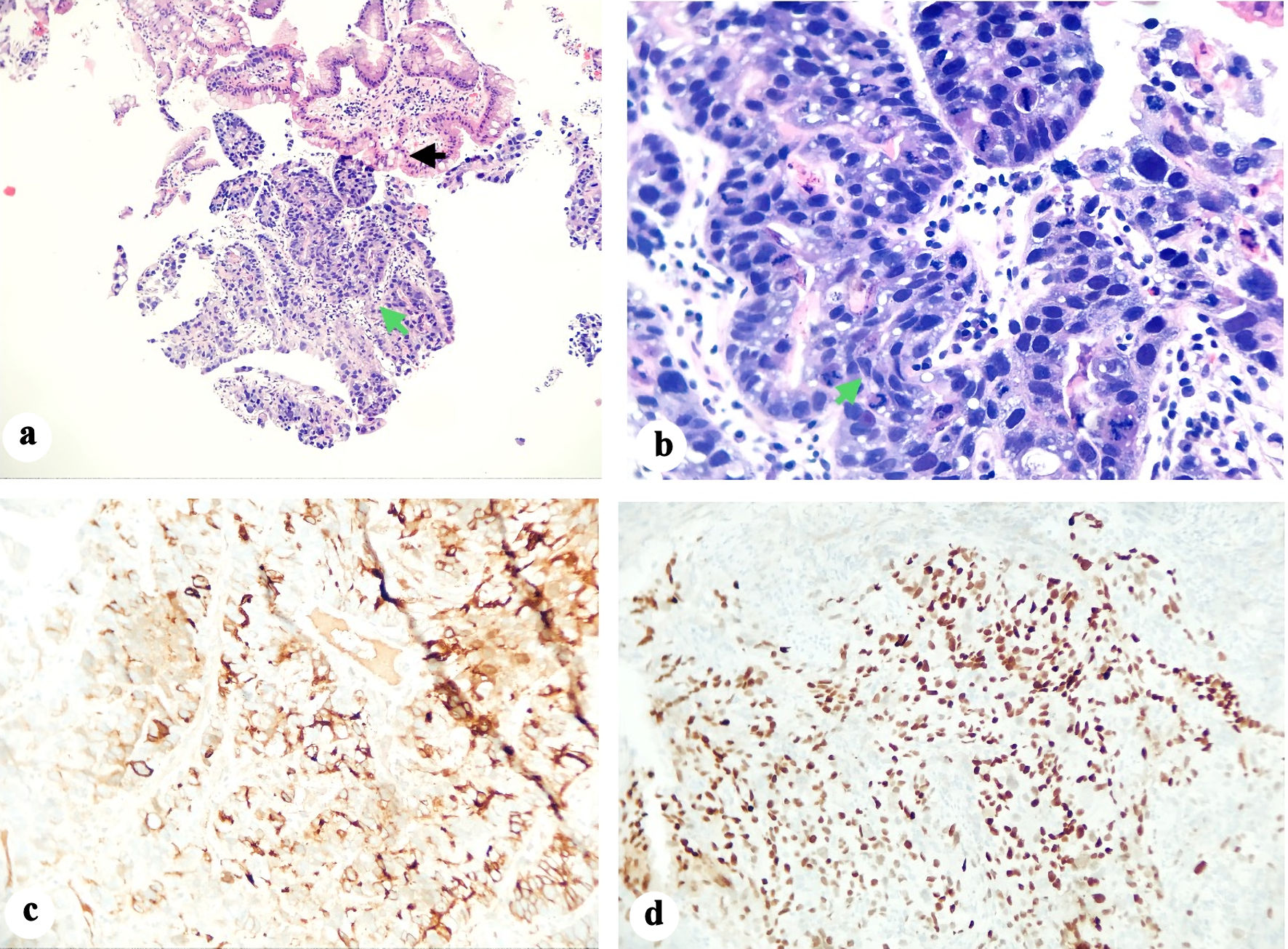 Click for large image | Figure 3. (a) Histological specimen showing mainly sheets of malignant cells (basophilic cells shown by green arrow) with focal glandular formation and prominent desmoplastic stromal response. The non-involved gastric glands (acidophilic staining and shown by black arrow) show focal intestinal metaplasia that is highlighted by PAS staining. (b) A closer look at the tumor showing sheets of malignant cells, pseudoglandular and hepatoid components (green arrow). Immunohistochemical staining was strongly positive for AFP (c) and positive for CDX2 (d). AFP: alpha-fetoprotein; CDX2: caudal-type homeobox 2. |
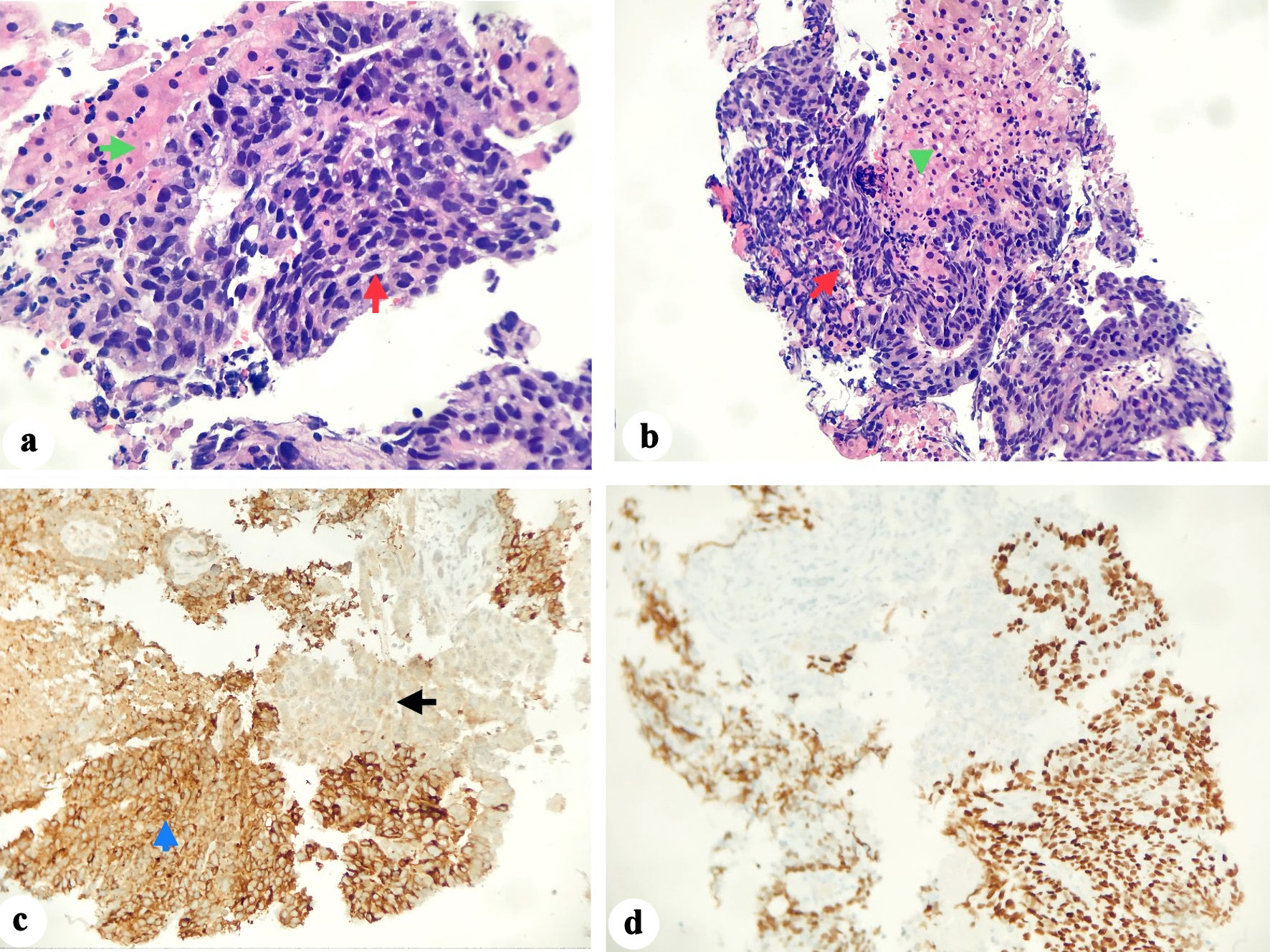 Click for large image | Figure 4. (a, b) The biopsy specimen shows liver parenchyma (green arrows) infiltrated by tumor cells (red arrows) forming nests and rare glands in the background of necrosis. (c) Tumor cells (blue arrow) in the background of normal liver tissue (black arrow). (d) Immunohistochemical staining showed positivity for SALL4 (d), CDX2, and AFP. AFP: alpha-fetoprotein; CDX2: caudal-type homeobox 2; SALL4: Sal-like protein 4. |
The patient started palliative chemotherapy consisting of cisplatin 75 mg/m2 on day 1 and etoposide 100 mg/m2 on days 1 - 3 and completed the first cycle while in the hospital. The patient was not a candidate for adjunctive trastuzumab therapy due to her negative human epidermal growth factor receptor 2 (HER-2) status. The patient experienced severe adverse reactions during consecutive chemotherapy sessions that required multiple hospitalizations. She had febrile neutropenia, intractable nausea and vomiting, acute kidney injury, decreased appetite, poor nutrition, and rapid weight loss, leading to a decline in performance status from Eastern Cooperative Oncology Group (ECOG) 1 to ECOG 3. Ultimately, a decision was made to transition the patient to comfort care with at-home hospice services, and she died 6 months after her diagnosis.
| Discussion | ▴Top |
Herein, we describe a novel case of AFP-HAS in a female patient with a 3-month history of constant epigastric pain, weight loss, and occasional hematochezia. Our patient is an outlier owing to her age, gender, and ethnicity. HAS is a rare type of gastric cancer that morphologically mimics HCC [3, 5, 9] and is often associated with elevated AFP levels. It has enteroblastic and hepatocellular differentiation and a poor prognosis due to liver and early lymph node metastasis [9]. HAS cases typically involve older males in Asian countries such as Japan and China [9, 12]. This rarity highlights the need for clinicians to consider HAS in differential diagnoses when encountering gastric tumors with unusual features, even in populations where this condition is exceedingly infrequent.
HAS typically presents with vague symptoms, but epigastric pain and fatigue are the most common [3, 9, 11]. Due to its rarity and ambiguous symptoms, HAS can easily be missed, leading to a delayed diagnosis [9]. Persistent abdominal pain is a common initial symptom in patients with HAS, which often warrants a computed tomography (CT) scan of the abdomen and pelvis. Gastric wall thickening is noted in most cases [5]. The tumor size usually ranges from 1.6 to 14.0 cm, and a heterogeneous texture of the mass correlates with tumor necrosis and hemorrhage [3, 5]. On histology, HAS consists of glandular and hepatocellular carcinoma-like foci [3], which can be confirmed by immunostaining of SALL4 and AFP [1]. Our patient had gastric wall thickening on imaging, fungating mass on esophagogastroduodenoscopy (EGD), and the IHS profile was positive for both SALL4 and AFP.
HAS is a rare type of gastric cancer that morphologically mimics HCC [1, 5]. HAS is genetically unique and does not fit any of the Cancer Genome Atlas Program (TCGA) primary gastric cancer profiles [1]. As a result, there are no set treatment protocols for this cancer [1]. Since TP53 is frequently mutated in both HAS and conventional gastric cancer cases, it can help differentiate AFP-HAS from HCC [1, 3]. Both AFP-HAS and YST-like carcinoma of the stomach can present with elevated AFP level; it is therefore important to distinguish between the two clinical entities through histopathological analysis. YST-like carcinoma of the stomach is a rare type of gastric carcinoma that histologically resembles YSTs, featuring patterns like microcystic, reticular, and solid structures, and often showing aggressive behavior with a poor prognosis [13-15]. While both conditions involve elevated AFP levels, they differ in their histology, with the YST-like carcinoma showing distinct YST-like patterns, which are not present in most AFP-producing gastric cancers.
Patients with HAS have a poor prognosis, with a median survival of 6 months [16] and a 5-year survival rate of 9% [5]. In early-stage cancers, surgery is the primary treatment with curative intent, but the role of endoscopic resection and neoadjuvant and adjuvant chemotherapy is unclear [3, 11]. Given the rarity of this variant of gastric cancer, almost all evidence is retrospective in the form of case reports [3, 16]. Although infrequent, there are isolated cases of successful and curative endoscopic resection of AFP-producing gastric cancers. Ishikawa et al described a unique case of AFP-producing gastric cancer which was successfully resected via endoscopic submucosal dissection [17]. At the 1-year follow-up, the patient remained disease free. Similarly, Hirasaki et al shared a unique case of AFP-producing early gastric cancer that was managed through a hybrid approach [18]. The patient underwent endoscopic mucosal resection followed by distal gastrectomy with lymph node dissection. Interval tumor marker levels show no evidence of disease recurrence 3 years after intervention.
Due to the paucity of data on this HAS [19], there are no clear National Comprehensive Cancer Network (NCCN) guidelines for the treatment of advanced stages of the disease [11, 19]. Patients with advanced HAS have limited therapeutic options, and palliative chemotherapy is the mainstay of treatment [3, 19]. Cisplatin-based chemotherapy regimens have been used in some cases, resulting in mixed results [11]. Palliative gastrectomy and resection of hepatic metastases have also been reported in some studies, but data are limited [11]. Our patient did not tolerate cisplatin-based palliative chemotherapy and was transitioned to comfort care after a total of four cycles.
Learning points
AFP-HAS is a unique and malignant type of stomach cancer. With a propensity to metastasize to the liver and lymph nodes, HAS has a very poor prognosis and limited therapeutic interventions. Presently, there are no specific chemotherapy treatment protocols for the advanced stages of this disease. Gastrectomy with adjuvant chemotherapy has been used in most cases with limited efficacy. Since current evidence is only in the form of case reports and case series, more studies are needed to understand this malignancy’s clinicopathologic features and identify effective treatments.
Acknowledgments
We are grateful to the patient for allowing us to share this interesting case report with the rest of the medical community.
Financial Disclosure
No funding was obtained for the writing or submission of this case report.
Conflict of Interest
A portion of this case report was presented as a poster at the American College of Gastroenterology Annual Conference, in October 2023, and published as an abstract in the American Journal of Gastroenterology.
Informed Consent
The patient consented to the publication of this case report.
Author Contributions
LB and ME conceptualized the idea for this case report and crafted an outline. AM, JK, RY, and GM wrote some sections of the case report. AA prepared the pathology slides and helped with the interpretations. YC and WB edited and proofread the final version of this case report.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
HAS: hepatoid adenocarcinoma of the stomach; AFP: alpha-fetoprotein; AFP-HAS: alpha-fetoprotein-producing hepatoid adenocarcinoma of the stomach; ER: emergency room; CA 19-9: cancer antigen 19-9; CEA: carcinoembryonic antigen; CECT: contrast-enhanced computed tomography; CDX2: caudal-type homeobox 2; SALL4: Sal-like protein 4; ECOG: Eastern Cooperative Oncology Group; HCC: hepatocellular carcinoma; TCGA: Cancer Genome Atlas Program; NCCN: National Comprehensive Cancer Network
| References | ▴Top |
- Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, Yuan J, et al. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer. 2019;22(6):1183-1192.
doi pubmed pmc - Zhou RU, Cai Y, Yang YI, Xiang J, Chen Z. Hepatoid adenocarcinoma of the stomach: A case report and review of the literature. Oncol Lett. 2015;9(5):2126-2128.
doi pubmed pmc - Vlachostergios PJ, Voutsadakis IA, Barbanis S, Karasavvidou F, Papandreou CN. AFP-producing hepatoid adenocarcinoma of the stomach: a case report. Cases J. 2009;2:9296.
doi pubmed pmc - Liu XM, Chen GQ, Li SL, Zai TS. Hepatoid adenocarcinoma of the stomach: A case report and literature review. Exp Ther Med. 2015;9(6):2133-2136.
doi pubmed pmc - Fakhruddin N, Bahmad HF, Aridi T, Yammine Y, Mahfouz R, Boulos F, Awada A, et al. Hepatoid adenocarcinoma of the stomach: a challenging diagnostic and therapeutic disease through a case report and review of the literature. Front Med (Lausanne). 2017;4:164.
doi pubmed pmc - Zhou K, Wang A, Ao S, Chen J, Ji K, He Q, Ji X, et al. The prognosis of hepatoid adenocarcinoma of the stomach: a propensity score-based analysis. BMC Cancer. 2020;20(1):671.
doi pubmed pmc - Liu D, Li B, Yan B, Liu L, Jia Y, Wang Y, Ma X, et al. The clinicopathological features and prognosis of serum AFP positive gastric cancer: a report of 16 cases. Int J Clin Exp Pathol. 2020;13(9):2439-2446.
pubmed pmc - Takada J, Araki H, Ozawa N, Sugiyama T, Kubota M, Ibuka T, Shimizu M. Effective treatment of cytotoxic agent refractory alpha-fetoprotein-producing gastric cancer with ramucirumab: a case report and review of the literature. J Gastrointest Cancer. 2019;50(3):556-559.
doi pubmed - Zhou K, Wang A, Wei J, Ji K, Li Z, Ji X, Fu T, et al. The value of perioperative chemotherapy for patients with hepatoid adenocarcinoma of the stomach undergoing radical gastrectomy. Front Oncol. 2021;11:789104.
doi pubmed pmc - Liu XM, Di LJ, Zhu JX, Wu XL, Li HP, Wu HC, Tuo BG. Localized primary gastric amyloidosis: Three case reports. World J Clin Cases. 2020;8(19):4667-4675.
doi pubmed pmc - Xia R, Zhou Y, Wang Y, Yuan J, Ma X. Hepatoid adenocarcinoma of the stomach: current perspectives and new developments. Front Oncol. 2021;11:633916.
doi pubmed pmc - Wang CJ, Sparano J, Palefsky JM. Human immunodeficiency virus/AIDS, human papillomavirus, and anal cancer. Surg Oncol Clin N Am. 2017;26(1):17-31.
doi pubmed pmc - Bihari C, Rastogi A, Chandan KN, Yadav V, Panda D. Gastric adenocarcinoma with yolk sac tumor differentiation and liver metastasis of yolk sac tumor component. Case Rep Oncol Med. 2013;2013:923596.
doi pubmed pmc - Magni E, Sonzogni A, Zampino MG. Primary pure gastric yolk sac tumor. Rare Tumors. 2010;2(1):e10.
doi pubmed pmc - Qureshi A, Al-Moundhri M, Al-Shaibi M, Al-Haddabi I, Mittal A. Primary gastric yolk sac tumour. Sultan Qaboos Univ Med J. 2018;18(3):e383-e385.
doi pubmed pmc - Yang J, Wang R, Zhang W, Zhuang W, Wang M, Tang C. Clinicopathological and prognostic characteristics of hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract. 2014;2014:140587.
doi pubmed pmc - Ishikawa A, Nakamura K. Gastric adenocarcinoma with enteroblastic differentiation resected through endoscopic submucosal dissection: a case report. Case Rep Gastroenterol. 2024;18(1):68-73.
doi pubmed pmc - Hirasaki S, Tanimizu M, Tsuzuki T, Tsubouchi E, Hidaka S, Hyodo I, Tajiri H. Seronegative alpha-fetoprotein-producing early gastric cancer treated with endoscopic mucosal resection and additional surgery. Intern Med. 2004;43(10):926-930.
doi pubmed - Ye MF, Tao F, Liu F, Sun AJ. Hepatoid adenocarcinoma of the stomach: a report of three cases. World J Gastroenterol. 2013;19(27):4437-4442.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


