| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 9, September 2024, pages 237-241
Respiratory Compromise Related to Primary Histoplasmosis Infection in Two Pediatric Patients
Collin Reevesa, Michael P. Tobiasb, Katherine Blinec, Joseph D. Tobiasb, d, e
aHeritage College of Osteopathic Medicine - Dublin Campus, Dublin, Ohio and Ohio University, Athens, OH, USA
bDepartment of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH, USA
cDivision of Pediatric Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital and The Ohio State University Wexner Medical Center, Columbus, OH, USA
dDepartment of Anesthesiology & Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
eCorresponding Author: Joseph D. Tobias, Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH 43205, USA
Manuscript submitted June 27, 2024, accepted August 15, 2024, published online August 22, 2024
Short title: Histoplasmosis and Respiratory Involvement
doi: https://doi.org/10.14740/jmc4279
| Abstract | ▴Top |
Primary infection related to the fungus, histoplasmosis, is generally asymptomatic in immunocompetent hosts. Calcified granulomas may be noted incidentally on radiologic imaging such as chest radiographs or computed tomography imaging. However, even in immunocompetent hosts, these primary infections occasionally result in end-organ involvement including respiratory compromise. Histoplasmosis should be included in the differential diagnosis of patients presenting with respiratory involvement and mediastinal adenopathy. We present two pediatric-aged patients who developed pulmonary involvement related to a primary histoplasmosis infection that resulted in mediastinal and tracheal lymphadenopathy. These led to respiratory compromise due to pleural effusion in the first patient and tracheal compression in the second. In this paper, the basic microbiology of Histoplasma capsulatum is presented, previous reports of primary respiratory involvement presented, and diagnostic and therapeutic options discussed.
Keywords: Histoplasma capsulatum; Histoplasmosis; Respiratory insufficiency; Pleural effusion; Lymphadenopathy
| Introduction | ▴Top |
Histoplasma capsulatum is a dimorphic fungus present worldwide, but most commonly found in endemic regions associated with river valleys. The endemic regions in the United States include the Ohio and Mississippi river valleys as well as the southeastern states [1]. H. capsulatum is a soil-based fungus commonly found in caves or areas with bat feces or bird feathers. When the fungus is disturbed, the conidia are aerosolized and can be inhaled, initiating a primary pulmonary infection [2]. H. capsulatum has a prevalence of 40 million cases in the United States with a yearly incidence of 500,000 new cases [2].
Primary infections are frequently asymptomatic or present with mild respiratory symptoms that resolve without treatment, especially in immunocompetent hosts [3, 4]. These initial infections may result in calcified granulomas which are seen incidentally on a chest radiograph or computed tomography (CT) imaging [3, 4]. In a minority of cases, a granulomatous inflammation results in pulmonary disease that may mimic pulmonary tuberculosis. In immunocompromised hosts, histoplasmosis can become disseminated, leading to morbidity, significant end-organ involvement, and mortality. Systemic involvement with H. capsulatum can present with pleural based disease or more widespread respiratory and mediastinal involvement and should be included in the differential diagnosis of patients presenting with mediastinal adenopathy [5, 6]. We present two immunocompetent pediatric patients who developed respiratory compromise related to pleural and mediastinal involvement during primary histoplasmosis infections. The basic microbiology of H. capsulatum is presented, previous reports of primary respiratory involvement reviewed, and diagnostic and therapeutic options discussed.
| Case Report | ▴Top |
Review of these cases and presentation in this format followed the guidelines of the Institutional Review Board of Nationwide Children’s Hospital.
Case 1
The patient was a 15-year-old male with no significant past medical history, who presented to their primary care provider with a 6-day history of progressively worsening cough and shortness of breath with pain on deep inspiration. A chest radiograph demonstrated left lower lobe pneumonia accompanied by a left-sided pleural effusion (Fig. 1). Despite a 3-day course of azithromycin, the patient developed worsening fever and pain on inspiration. He was admitted to the hospital for further evaluation and treatment. A repeat chest radiograph revealed worsening left lower lobe consolidation and increased pleural fluid. Routine laboratory parameters including a complete blood count and basic metabolic profile were within normal limits. The presence of pleural fluid was confirmed by ultrasound and antibiotic therapy was changed to oral amoxicillin/clavulanic acid. Other pertinent past history included no recent sick contacts and no prior history of respiratory illnesses. Physical examination revealed decreased and coarse breath sounds over the lower left lobes.
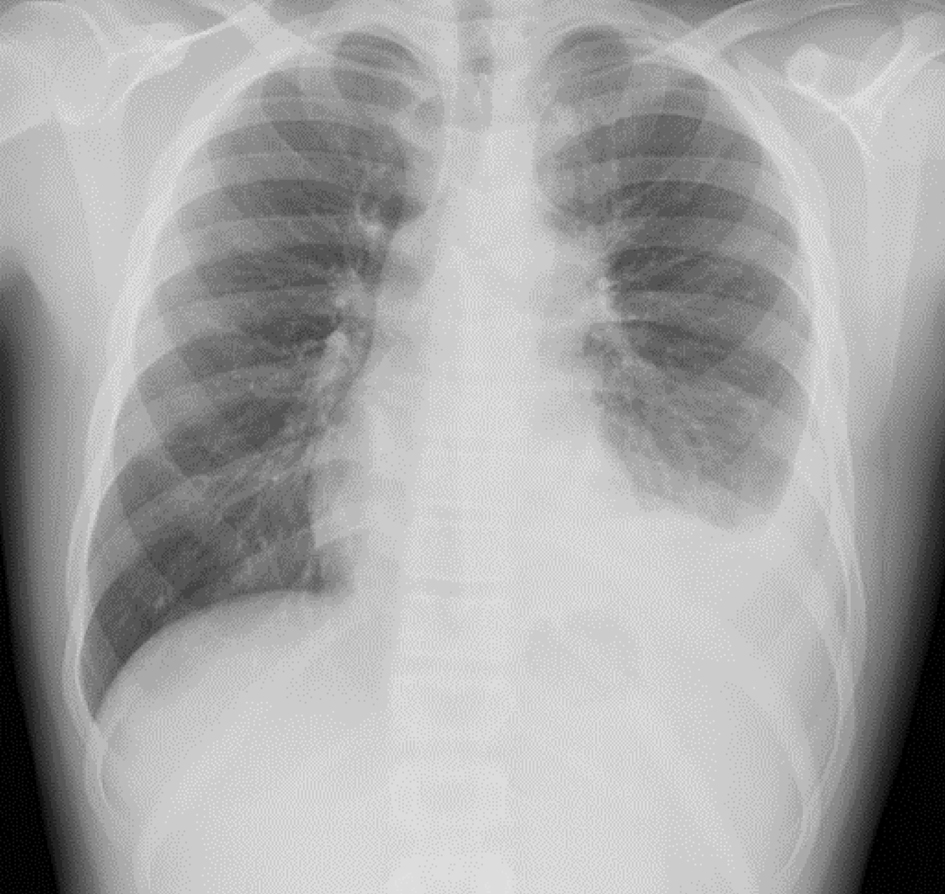 Click for large image | Figure 1. Initial chest radiograph showing left lower lobe pneumonia/infiltrate and left-sided pleural effusion. |
On hospital day 1, a left side pleural pigtail catheter was placed by interventional radiology and drained approximately 800 mL of pleural fluid. The pleural fluid had a total cell count of 9,668 cells/mm3 with a predominance of lymphocytes. Bacterial, fungal, and mycobacterium cultures were negative. The pleural fluid and urine were negative for histoplasmin antigen and a purified protein derivative (PPD) skin test was negative. Immunodiffusion fungal antibody tests were positive for histoplasmosis antibodies including the presence of an M band. Histoplasma yeast AB complement fixation titer was positive at a dilution of 1:256 (normal < 1:8). Chest CT demonstrated a moderate left-sided pleural effusion with suspected left lower lobe basilar atelectasis. The left hilar region displayed fullness suggestive of reactive adenopathy. There was fullness of the pulmonary parenchymal interlobular lymphatics suggestive of obstruction to lymphatic drainage. The pleural drain was left in place for 72 h and then removed without reaccumulation of the pleural fluid.
H. capsulatum infection was diagnosed by the pediatric infectious disease service based on the blood serology and the pre-vascular and supra-hilar lymphadenopathy present on CT imaging. Oral itraconazole was started and administered for 12 weeks. The patient was discharged home after 72 h. All symptoms resolved and the patient has done well on outpatient follow-up over the past 2 years.
Case 2
The patient was a 12-year-old male who was transferred to the pediatric intensive care unit (ICU) from an outside emergency department with respiratory distress, orthopnea, and stridor with initial imaging concerning for a mediastinal mass. The patient developed a cough and fever 6 days prior to presentation. Initial chest radiograph displayed a prominent subcarinal density with severe narrowing of the air shadow of the left mainstem bronchus. Initial laboratory evaluation revealed a mild leukocytosis with a neutrophil predominance (89%) and anemia (hemoglobin 10 g/dL). COVID-19 polymerase chain reaction test from a nasal swab was negative. Past medical history revealed no sick contacts or recent travel. The patient had moved to Ohio at 5 years of age from Nepal with his three siblings.
The patient was admitted to the pediatric ICU and intravenous (IV) ampicillin/sulbactam was started to treat presumed community-acquired pneumonia. CT revealed complex mediastinal extensions of the subcarinal mass with extrinsic compression of the carina and left mainstem bronchus (Fig. 2). The subcarinal mass was comprised of a combination of a dominant septated cystic mass in the central subcarinal region with associated smaller microcystic components that extended bilaterally into the hilar/infra-hilar regions. The CT also displayed lymphadenopathy in the pre-tracheal and right paratracheal distributions. The complex mass resulted in tracheal compression and greater than 95% narrowing of the left bronchial lumen. The mass also resulted in extrinsic compression with diffuse mild narrowing of the right pulmonary artery. The pediatric oncology and infectious disease services were consulted. The oncology service offered a broad differential diagnosis including infectious, oncologic and rheumatoid etiologies with primary consideration for a lymphoid malignancy or a solid malignant tumor based on the CT imaging. The infectious disease service considered primary H. capsulatum infection to be likely based on the clinical presentation, diagnostic imaging, and living in an endemic area.
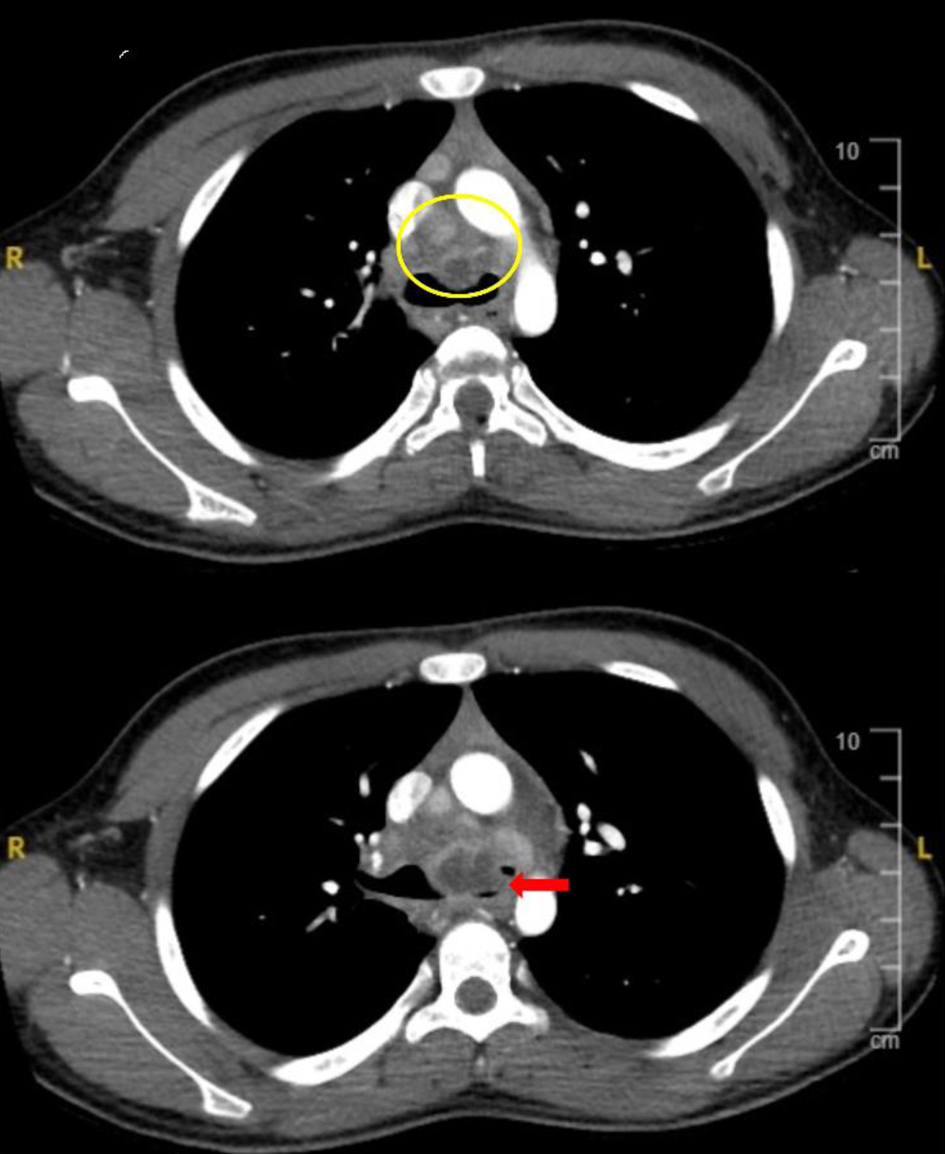 Click for large image | Figure 2. Computed tomography imaging demonstrating mediastinal extension of the subcarinal mass (yellow circle) with extrinsic compression of the carina and left mainstem bronchus (red arrow). |
Following admission, given the concern for impending respiratory failure, a combination of helium and oxygen was started which resulted in a decrease in the work of breathing. Methylprednisolone (20 mg IV every 6 h) was started due to the tracheal/bronchial compression and the concern for impending respiratory failure. Due to concerns of a primary fungal infection, IV amphotericin was administered. Over the ensuring 24 h, the patient’s respiratory status improved, and the helium-oxygen therapy was discontinued. On hospital day 2, bronchoscopy and bronchioalveolar lavage with biopsy was performed that demonstrated fistulation of the mediastinal mass into the bronchus. The patient tested positive for histoplasmosis antibodies with IgG enzyme immunoassay elevated at 46.5 µg/mL (normal < 8.0 µg/mL), IgM enzyme immunoassay (EIA) elevated at 10 µg/mL (normal < 8.0 µg/mL), Histoplasma mycelia antibody complement fixation (CF) elevated at 1:8 (normal < 1:8), Histoplasma yeast AB CF antibody elevated at 1:16 (normal < 1:8) and the presence of Histoplasma immunodiffusion M band. Treatment of H. capsulatum included an initial 8-day course of IV amphotericin B followed by a 12-weeek course of oral itraconazole. Methylprednisolone was continued for 5 days followed by a slow taper of the dose. Repeat CT imaging of the chest on hospital day 8 revealed an interval decrease in the size of the mediastinal mass and resolution of tracheal/bronchial compression.
The patient was discharged to the inpatient ward and then discharged home on hospital day 27. Follow-up bronchoscopy and CT imaging 29 days after admission and diagnosis showed resolution of the subcarinal mass and bronchial/tracheal compression with small residual nodules. The patient completed a 12-week course of anti-fungal therapy. The patient has done well and remains clinically asymptomatic on outpatient follow-up of more than 18 months.
| Discussion | ▴Top |
We present two immunocompetent hosts who developed significant end-organ involvement related to primary histoplasmosis infection. In our first patient, mediastinal adenopathy occluded lymphatic drainage resulting in a pleural effusion. In the second patient, peritracheal adenopathy resulted in tracheal compression and impending respiratory failure. The diagnosis of H. capsulatum infection was made by identification of serum antibody titers. Treatment included an extended oral course of itraconazole.
Identification and the original naming of the H. capsulatum organism are attributed to Dr. Samuel Darling, a pathologist in 1905 [7]. Its identification was somewhat serendipitous as Dr. Darling was studying malaria, which was prevalent at that time during the construction of the Panama Canal. He identified a protozoan-like microorganism in an autopsy specimen and coined the name, H. capsulatum, from what he observed microscopically. He reported that the organism invaded histiocyte-like cells (histo), involved primarily the cytoplasm (plasma), and had a halo around it that looked like a capsule (capsulatum) [6]. Although not commonly used in the literature, histoplasmosis is sometimes referred to as Darling’s disease.
As a dimorphic fungus, the organism exists as a yeast at body temperature (36 °C) and as a mold at ambient temperatures (25 °C). H. capsulatum flourishes in moist soil and is transported in the gastrointestinal tract of bats and on the feathers of birds. Primary infections generally result in an asymptomatic response in immunocompetent individuals with the occasional development of asymptomatic nodules with calcifications that may persist within the lung parenchyma. Constitutional symptoms with the primary infection may include fever, cough, and malaise, which is generally self-limited even without therapy. These symptoms are frequently attributed to an upper respiratory viral infection. Erythematous and raised nodules on the scalp or shins are also common [8]. H. capsulatum can also mimic the signs and symptoms of Mycobacterium tuberculosis, making it challenging to identify histoplasmosis in endemic regions [9].
As noted in our patients, significant respiratory involvement from the primary disease process or its sequelae (lymphadenopathy) occasionally occurs even in immunocompetent patients. In our first patient, it was postulated that the hilar adenopathy resulted in obstruction to lymphatic drainage leading to a pleural effusion. This was supported by the characteristic findings on CT imaging with expansion of the interlobular lymphatic channels within the parenchyma of the lung as well, the predominance of lymphocytes in the fluid, negative pleural fluid cultures, and the absence of histoplasmin antigen suggestive of a passive effusion from lymphatic obstruction. As the effusion did not continue to drain or reaccumulate, additional therapy such as systemic corticosteroids to hasten resolution of the lymphoid hyperplasia and relieve obstruction to lymphatic drainage was not initiated. In our second patient, the hilar and peri-carinal adenopathy resulted in tracheal and bronchial compression with respiratory insufficiency and impending respiratory failure, which mandated the early administration of corticosteroids to prevent progressive respiratory decline. Traditionally, corticosteroid administration in primary fungal infections has been avoided due to concern for suppressing the immune response of the patient. However, corticosteroid administration in patients with H. capsulatum infection may be beneficial in patients with primary respiratory involvement [10].
The diagnosis of H. capsulatum requires a multifaceted approach that includes clinical, radiographic and laboratory evidence of the disease along with a high index of suspicion in endemic areas. Although primary involvement may be detected on a routine chest radiograph or CT imaging, the findings are non-specific as H. capsulatum can mimic many other conditions including pneumonia, malignancies, and tuberculosis.
Although the gold standard remains a positive culture for the organism or histologic identification of the yeast from lymph nodes, pleural fluid or lung nodules obtained by bronchoscopy or surgical procedures (thoracoscopy), more modern and less invasive techniques focus on antigen or antibody detection. These tests may provide a more rapid, noninvasive, and highly sensitive method of identification and marker of treatment response [10-13]. With acute infections, antigens can be detected in bodily fluids such as pleural fluid, serum or urine. New antigen detection techniques utilizing monoclonal antibodies as highly specific reagents have improved sensitivity and specificity [14]. When antigen testing is negative and biopsy specimens are not available or fail to reveal the organism, antibody titers can be used [14].
In both of our patients, in addition to a high clinical suspicion based on radiologic imaging and the clinical presentation, antibody titers to specific H. capsulatum antigens were used to confirm the diagnosis when cultures and antigen testing were negative. Titers are a measure of the antibody concentration to a specific antigen through a sequence of dilutions. Antibodies can be identified through various methods including EIAs, CF assays, and immunodiffusion (ID) assays. The latter two are used most commonly in clinical practice with CF titers identifying antibodies to the yeast or mycelial component and ID assays identifying antibodies in the H or M band. The presence of H and M bands is presumptive evidence of active infection. The serum of approximately 70% of patients with proven histoplasmosis contain the M band, whereas only 1% demonstrate both H and M bands. CF titers ≥ 1:8 are generally considered positive, indicating previous exposure to H. capsulatum while titers ≥ 1:32 or a fourfold rise in antibody titers from acute to convalescent phase serum is strongly suggestive of a recent active infection.
Although rare, mediastinal and pleural involvement during H. capsulatum infections can present with detrimental consequences in both immunocompetent and immunocompromised patients [5, 6, 15-18]. Previous reports have included both primary pleural involvement with empyema or mediastinal spread with a mediastinal mass or fibrosing mediastinitis.
Learning points
Primary infections related to H. capsulatum are frequently asymptomatic or present with mild respiratory symptoms, mimicking a self-limited viral disease process with mild upper respiratory tract involvement. These primary infections may result in calcified granulomas which are seen incidentally on radiologic imaging that may mimic pulmonary tuberculosis. Rarely in immunocompetent hosts, systemic involvement with H. capsulatum can present with pleural based disease or more widespread respiratory and mediastinal involvement. In our two patients, we would postulate that the end-organ involvement was related to lymphoid hyperplasia more than active infection. The lymphoid hyperplasia and resultant lymphadenopathy resulted in obstruction to pleural lymphatic drainage in our first patient and tracheal/bronchial compression in our second patient. After initiation of anti-fungal therapy, corticosteroids were administered to our second patient given the risk of impending respiratory failure. The diagnosis of H. capsulatum includes clinical, radiographic and laboratory evidence of the disease along with high index of suspicion in endemic areas. The gold standard remains culture of the organism or its histologic identification from tissue or bodily fluids. Other techniques focus on antigen or antibody detection for a more rapid, noninvasive and highly sensitive method of diagnosis. Treatment includes long courses (12 weeks or more) of anti-fungal agents such as itraconazole.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained for hospital/anesthetic care and the use of de-identified information for publication.
Author Contributions
Preparation of initial, subsequent, and final drafts: CR and MPT; concept, writing, and review of all drafts: JDT; review of final draft: KB.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Hage CA, Wheat LJ, Loyd J, Allen SD, Blue D, Knox KS. Pulmonary histoplasmosis. Semin Respir Crit Care Med. 2008;29(2):151-165.
doi pubmed - Kurowski R, Ostapchuk M. Overview of histoplasmosis. Am Fam Physician. 2002;66(12):2247-2252.
pubmed - Di Mango AL, Zanetti G, Penha D, Menna Barreto M, Marchiori E. Endemic pulmonary fungal diseases in immunocompetent patients: an emphasis on thoracic imaging. Expert Rev Respir Med. 2019;13(3):263-277.
doi pubmed - Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am. 2016;30(1):207-227.
doi pubmed - Kilburn CD, McKinsey DS. Recurrent massive pleural effusion due to pleural, pericardial, and epicardial fibrosis in histoplasmosis. Chest. 1991;100(6):1715-1717.
doi pubmed - Cui N, Wang L, Zhao J. A case report of histoplasma-associated empyema treated with intravenous injection and local thoracic irrigation of amphotericin B plus medical thoracoscopy. Front Public Health. 2022;10:914529.
doi pubmed pmc - Darling ST. A protozoan general infection producing pseudotubercules in the lungs and focal necrosis in the liver, spleen, and lymph nodes. JAMA. 1906;46:1283-1285.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20(1):115-132.
doi pubmed pmc - Adenis A, Nacher M, Hanf M, Basurko C, Dufour J, Huber F, Aznar C, et al. Tuberculosis and histoplasmosis among human immunodeficiency virus-infected patients: a comparative study. Am J Trop Med Hyg. 2014;90(2):216-223.
doi pubmed pmc - Wheat J, Sarosi G, McKinsey D, Hamill R, Bradsher R, Johnson P, Loyd J, et al. Practice guidelines for the management of patients with histoplasmosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30(4):688-695.
doi pubmed - Wheat J. Histoplasmosis: recognition and treatment. Clin Infect Dis. 1994;19(Suppl 1):S19-27.
doi pubmed - Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol. 2017;55(6):1612-1620.
doi pubmed pmc - Guimaraes AJ, Nosanchuk JD, Zancope-Oliveira RM. Diagnosis of histoplasmosis. Braz J Microbiol. 2006;37(1):1-13.
doi pubmed pmc - Fida M, Misra A, Harring JA, Kubbara A, Theel ES. Histoplasma capsulatum complement fixation and immunodiffusion assay sensitivity in culture-confirmed cases of histoplasmosis: a 10-year retrospective review (2011 to 2020). J Clin Microbiol. 2022;60(10):e0105722.
doi pubmed pmc - Ghaye B, Szapiro D, Fanchamps JM, Dondelinger RF. Mediastinal involvement in histoplasmosis: Report on two cases and literature review. Ann Thor Surg. 2012;93(2):e27-e29.
- Khalid M, Khan I, Rahman Z, Alazzeh A, Youssef D. Fibrosing mediastinitis: uncommon life-threatening complication of histoplasmosis. Cureus. 2018;10(4):e2532.
doi pubmed pmc - Kortsik C, Elmer A, Tamm I. Pleural effusion due to Histoplasma capsulatum and idiopathic CD4 lymphocytopenia. Respiration. 2003;70(1):118-122.
doi pubmed - Brewer PL, Himmelwright JP. Pleural effusion due to infection with histoplasma capsulatum. Chest. 1970;58(1):76-79.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


