| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 3, Number 2, April 2012, pages 140-143
Long-Term Remission After Gefitinib Therapy in an Elderly Patient With Advanced Non-Small-Cell Lung Carcinoma
Yao Wei Zhanga, Jian Guana, Yi Dinga, Long Hua Chena, b
aDepartment of Radiation Oncology, Nanfang Hospital, Southern Medical University, China
bCorresponding author: Long Hua Chen, Department of Radiation Oncology, Nanfang Hospital, Southern Medical University, China
Manuscript accepted for publication January 13, 2012
Short title: Therapy for NSCLC
doi: https://doi.org/10.4021/jmc513w
| Abstract | ▴Top |
The prognosis for non-small-cell lung carcinoma (NSCLC) patients in advanced stages is poor. Gefitinib inhibits the tyrosine kinase activity of epidermal growth factor receptor (EGFR) and have been studied extensively. Oral epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) have been as the 2nd-line treatment for NSCLC. It is widely accepted that some clinicopathologic characteristics (female, nonsmoking status, Asian, and EGFR mutations) are the main clinical positive predictive factors when using EGFR-TKIs. The elder patients often suffer from deterioration of performance status (PS). The side reaction caused by chemotherapy is serious and unavoidable. For the elder patients with positive predictive factors and poor PS, there is no report about anti-NSCLC using gefitinib as the 1st-line treatment. We report the case of an 84-year-old woman with diffuse bone metastases from lung cancer. She received oral gefitinib 150 mg/day, combined with three dimensional conformal radiation therapies (3DCRT). A total tumor dose of 36Gy/12fractions was delivered to the tumor bed and localized metastatic bone pain areas, respectively. After concurrent gefitinib-3DCRT, gefitinib was continued as maintenance therapy. She experienced total regression of the metastases under gefitinib treatment for 30 months. Gefitinib therapy provided effective anti-tumor results. Therefore, for NSCLC patients of multiple metastases with favorable predictive factors such as EGFR mutations, adenocarcinoma, Asian, female gender and nonsmoking status, we suggest that gefitinib may become the 1st-line treatment even with poor PS.
Keywords: Gefitinib; Lung cancer; Bone metastasis
| Introduction | ▴Top |
Human epidermal growth factor receptor (EGFR) is a target for anticancer therapy. EGFR is expressed on the cell surface of cancer cells [1]. Gefitinib, an orally active, is a selective EGFR tyrosine kinase inhibitor (EGFR-TKI) that inhibits EGFR signaling transduction pathways, thus decreasing tumor growth [2]. Gefitinib is currently approved as monotherapy for patients against chemotherapy-refractory non-small-cell lung carcinoma (NSCLC) as the 2nd-line treatment.
| Case Report | ▴Top |
The patient was an 84-year-old Chinese woman with no smoking history and performance status (PS) 3. She suffered from prolonged occiput, left neck and chest pain on our 1st visit in January 2009. With a personal history of resecting colon adenocarcinoma in February 2007, we strongly demanded to do 18F-FDG-PET/CT examination and rule out metastasis. Evaluation revealed hypermetabolism in the left lung, suprarenal gland, multiple bone (occipita, right scapula, side of right atlas, C3, left side of C5, spinous process of C7, T6, T10 and T11, L3 spinous process, side of the left 2nd rib, side of the right 5th rib, right iliac body and the upper of right femur). Chest computed tomography (CT) showed a soft tissue mass measuring 2.6 × 2.7 cm in the left below lung (Fig. 1a). To define a clear diagnosis and choose a therapeutic strategy, we suggested pathologic examination by transbronchial biopsy from the left lung mass and radiotherapy as a treatment to relieve bone pain caused by cancer that has spread into the bone. She confused for transbronchial biopsy, surgery and chemotherapy due to serious side effects. The therapy with gefitinib was chosen because of favorable predictive factors such as Asian, female gender and nonsmoking status. At this point, written informed consent was obtained from the patient, and treatment with gefitinib was initiated as the 1st-line treatment. The patient was treated with oral gefitinib at 150 mg/day. At the same time, complementary radiotherapy, delivered to the left lung soft tissue mass region, was initiated to a dose of 36Gy in 12 fractions over 2.5 weeks. Complementary radiotherapy, delivered to occipita, side of right atlas, C3, left side of C5, T10 and the upper of right femur, was initiated to a dose of 36Gy in 12 fractions over 2.5 weeks. Radiotherapy relieved bone pain completely. After 3 months of treatment, the patient showed a good clinical response. Hypermetabolism shadowing in the all last bone metastatic regions (including hypermetabolism regions received no radiotherapy) were not detected on PET/CT. But new hypermetabolism regions (sternum, T10, T11, L3 and the upper of both of femur) were detected (Fig. 1b). We thought gefitinib was effective. Treatment with gefitinib was continuing in the outpatient clinic. After 6 months of treatment with gefitinib, she had been without evidence of disease progression (Fig. 1c). After 16 months of treatment with gefitinib, C7, T12 and right side of the 7th rib hypermetabolism regions were detected. IMRT, delivered to C7, T12 and right side of the 7th rib metastatic regions, was initiated to a dose of 30Gy in 5 fractions over 1 week. After 30 months of treatment with gefitinib, the left chest wall pain. Occipita, C7, T10, T11, right side of the 4th, 8th rib and left side of 2nd rib and left ilium hypermetabolism regions were detected on PET/CT (Fig. 1d). There were evidences of disease progression. Then, 3DCRT delivered to the left chest wall region, was initiated to a dose of 15Gy/5F +15Gy/3F over 1.5 weeks. We suggested treatment with erlotinib, 150 mg/day administered orally, was then initiated starting in July 2011. The latest whole PET/CT showed no evidence of disease progression (Fig. 1e). The patient was alive now.
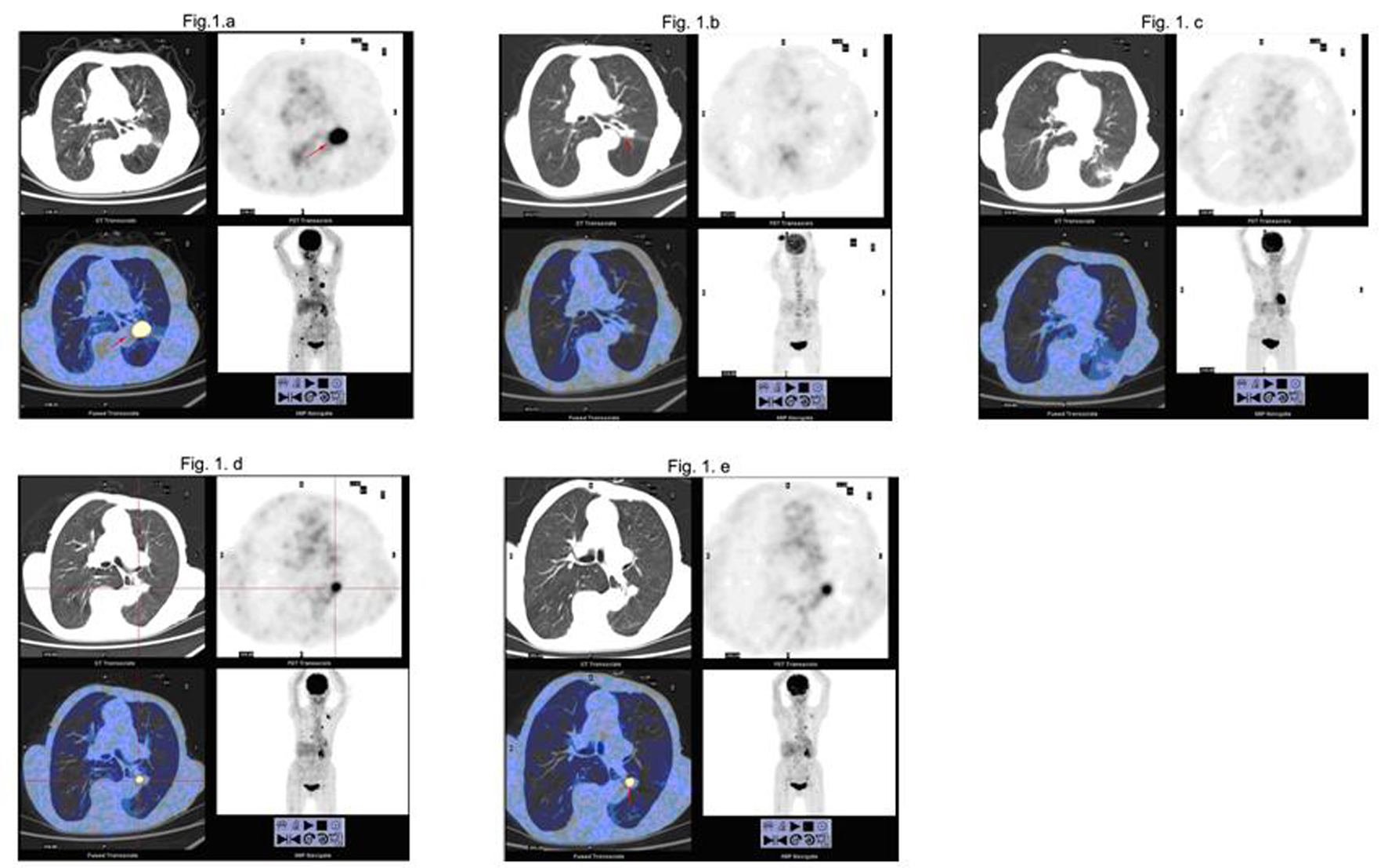 Click for large image | Figure 1. PET/CT before and after treatment with gefitinib and radiotherapy. |
| Discussion | ▴Top |
The prognosis for NSCLC patients in advanced stages is poor [3]. EGFR is highly expressed on NSCLC cell surfaces. Therefore, it is considered a valuable therapeutic target. The pathways including ras-raf-1-MAPK and PI3K-Akt are involved in downstream EGFR signaling. Activation of these pathways results in a mitogenic signaling cascade responsible for a number of processes crucial to tumor progression, including proliferation, decreased apoptosis, angiogenesis, and invasion [4]. EGFR-TKIs are new anticancer agents that act by inhibiting EGFR signaling transduction pathways, thus decreasing tumor growth [5]. Two classes of EGFR mutations, in-frame deletion in exon 19 and missense mutation L858R in exon 21, are the most frequent mutations, representing > 85% of EGFR mutations reported [1, 6]. These EGFR mutations have been associated with response to EGFR-TKI in some studies [7, 8]. The rate of exon-19 mutations, female gender, and nonsmoking status were identified as additional predictors of outcome at meta-regression analysis [9]. Anti-EGFR therapy is largely ineffective in NSCLC with activating KRAS mutations [10]. The EGFR mutation is rare in small cell lung cancer (SCLC) patients. Gefitinib may not be effective in treating SCLC patients [1].
Radiotherapy is often used to control pain. Radiotherapy for cancer that has spread to bones can also help to prevent new painful areas developing [11]. Having this treatment may slow down the cancer and give patients a better quality of life for a longer time.
Here, we report the case of a woman with diffuse bone metastases from lung cancer. Although there was no pathologic examination, the efficacy NSCLC therapy confirmed pathologic lung cancer. She experienced total regression of the metastases under gefitinib treatment for 30 months. The rate of exon-19 or exon-21 mutations, Asian female gender, adenocarcinoma, and nonsmoking status were identified as predictors of outcome after the treatment of EGFR-TKI at meta-regression analysis [9, 12]. The patient showed a good clinical response with EGFR-TKI because of favorable predictive factors such as Asian female gender and nonsmoking status. There should be other favorable factors such as adenocarcinoma and EGFR mutations. Treatment of EGFR-TKI should be the first choice in the previously untreated NSCLC patients with activated EGFR mutation. Gefitinib could be used as a single agent in select subsets of patients with advanced NSCLC [13]. The patient's condition was exacerbated during gefitinib treatment but treatment with another EGFR-TKI, erlotinib, may be effective [5, 14, 15]. Moreover, addition of standard-dose gefitinib to radiotherapy was feasible and without evidence of increased toxicities.
Targeted molecules are valuable weapons in the management of certain cancers. Gefitinib provided clinically significant antitumor activity, clinically significant improvements in disease-related symptoms, and improvements in quality of life. To our knowledge, this is the longest-term survive case report of NSCLC associated with the EGFR-TKI gefitinib. No evidence severe adverse events have been observed. Before patients can be offered first-line treatment with gefitinib, deletion of exon 19 or exon 21 mutation of EGFR gene should be detected in carcinoma cells. These results suggest that there is the superiority of first-line gefitinib therapy in a clinically selected population consisting of Asian female patients with adenocarcinoma, EGFR mutation, and no smoking history.
Grant Support
This study was supported by grants from Science and Technology Commission projects of Guangdong Province (2010B031600245) and Natural Science Foundation of Guangdong Province (8451051501001344).
Conflict of Interest
The authors report no conflicts of interest.
| References | ▴Top |
- Shiao TH, Chang YL, Yu CJ, Chang YC, Hsu YC, Chang SH, Shih JY, et al. Epidermal growth factor receptor mutations in small cell lung cancer: a brief report. J Thorac Oncol. 2011;6(1):195-198.
pubmed doi - Ko HW, Tsai YH, Yu CT, Huang CY, Chen CH. Good response to gefitinib for lung adenocarcinoma with hyperamylasemia: a case report. Chang Gung Med J. 2008;31(6):606-611.
pubmed - Watanabe S, Tanaka J, Ota T, Kondo R, Tanaka H, Kagamu H, Ichikawa K, et al. Clinical responses to EGFR-tyrosine kinase inhibitor retreatment in non-small cell lung cancer patients who benefited from prior effective gefitinib therapy: a retrospective analysis. BMC Cancer. 2011;11:1.
pubmed - Watanabe S, Tanaka J, Ota T, Kondo R, Tanaka H, Kagamu H, Ichikawa K, et al. Clinical responses to EGFR-tyrosine kinase inhibitor retreatment in non-small cell lung cancer patients who benefited from prior effective gefitinib therapy: a retrospective analysis. BMC Cancer. 2011;11:1.
pubmed - Cataldo VD, Gibbons DL, Perez-Soler R, Quintas-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364(10):947-955.
pubmed doi - Zhou Q, Zhang XC, Chen ZH, Yin XL, Yang JJ, Xu CR, Yan HH, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(24):3316-3321.
pubmed doi - Tang WH, Chen JH, Ye RH, Ho CL. Near Total Regression of Diffuse Brain Metastases in Adenocarcinoma of the Lung with an EGFR Exon 19 Mutation: A Case Report and Review of the Literature. Case Rep Oncol. 2011;4(3):445-451.
pubmed - Asahina H, Yamazaki K, Kinoshita I, Yokouchi H, Dosaka-Akita H, Nishimura M. Non-responsiveness to gefitinib in a patient with lung adenocarcinoma having rare EGFR mutations S768I and V769L. Lung Cancer. 2006;54(3):419-422.
pubmed doi - Bria E, Milella M, Cuppone F, Novello S, Ceribelli A, Vaccaro V, Sperduti I, et al. Outcome of advanced NSCLC patients harboring sensitizing EGFR mutations randomized to EGFR tyrosine kinase inhibitors or chemotherapy as first-line treatment: a meta-analysis. Ann Oncol. 2011;22(10):2277-2285.
pubmed doi - Sun L, Zhang Q, Luan H, Zhan Z, Wang C, Sun B. Comparison of KRAS and EGFR gene status between primary non-small cell lung cancer and local lymph node metastases: implications for clinical practice. J Exp Clin Cancer Res. 2011;30:30.
pubmed - Pennell NA. Integration of EGFR inhibitors and conventional chemotherapy in the treatment of non-small-cell lung cancer. Clin Lung Cancer. 2011;12(6):350-359.
pubmed doi - Soo RA, Loh M, Mok TS, Ou SH, Cho BC, Yeo WL, Tenen DG, et al. Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J Thorac Oncol. 2011;6(6):1030-1038.
pubmed doi - Heigener DF, Reck M. Mutations in the epidermal growth factor receptor gene in non-small cell lung cancer: Impact on treatment beyond gefitinib and erlotinib. Adv Ther. 2011;28(2):126-133.
pubmed doi - Chang JW, Chou CL, Huang SF, Wang HM, Hsieh JJ, Hsu T, Cheung YC. Erlotinib response of EGFR-mutant gefitinib-resistant non-small-cell lung cancer. Lung Cancer. 2007;58(3):414-417.
pubmed doi - Song ZB, Yu YF, Chen ZW, Lu S. Erlotinib as a salvage treatment for patients with advanced non-small cell lung cancer after failure of gefitinib treatment. Chin Med J (Engl). 2011;124(15):2279-2283.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


