| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 1, Number 3, December 2010, pages 103-107
Progressive Multifocal Leukoencephalopathy in an HIV Patient With High CD4 T Cell Count: A Case Report
Filipe Gaio Nerya, b, Margarida Francaa, Carlos Vasconcelosa
aClinical Immunology Unit, Centro Hospitalar do Porto – Hospital Sto Antonio, Porto, Portugal
bCorresponding author: Largo Prof Abel Salazar 4000 Porto, Portugal
Manuscript accepted for publication November 3, 2010
Short title: Progressive Multifocal Leukoencephalopathy
doi: https://doi.org/10.4021/jmc78w
| Abstract | ▴Top |
Progressive multifocal leukoencephalopathy is an AIDS defining disease often arising in HIV patients with low CD4 T cell count, and rarely among those with more than 500 CD4 T cell/mm3. Definite diagnosis requires JC Virus (JCV) isolation in cerebrospinal fluid (CSF) or in brain tissue. JCV PCR sensitivity in highly active antiretroviral therapy (HAART) era is lower, making progressive multifocal leukoencephalopathy (PML) definite diagnosis difficult. A 48-year-old woman was diagnosed with HIV1 in 1998, never having had any AIDS-defining illness. Combined antiretroviral therapy was started in 2004. In January 2009 she presented a tandem gait and gait ataxia. Her HIV viral load was undetectable and the CD4 T cell count was of 533/mm3. Brain CT scan was normal. In the following months a bilateral cerebellar syndrome installed and brain MRI was done showing asymmetrical demyelinating lesions. Normal cerebrospinal fluid (CSF) findings other than mononuclear pleocytosis and a negative JCV PCR were documented. PML was suspected, combined antiretroviral therapy (cARV) was altered, and cidofovir and mirtazapine were prescribed, associated with physiotherapy. She was clinically stable for some months. Almost one year later her neurological state got worse, CD4 T cell count was of 478/mm3, brain lesions progressed, and finally, JCV PCR became positive (5th determination). PML definite diagnosis was made. The patient died in June 2010 due to PML progression. Sensitivity of JCV PCR in CSF lowers under HAART with high CD4 T cell count, making definite PML diagnosis difficult even when a high grade of suspicion exists, based on clinical presentation and magnetic resonance imaging (MRI) findings. Differential diagnosis of demyelinating diseases should be considered, and HIV-leukoencephalitis should be taken in consideration when HIV replication exists in the brain. PML may arise in a patient under cARV and good immunological status. Treatment should not be delayed when a probable diagnosis exists even if CD4 T cell count is above 500/mm3. Repeated lumbar puncture with JCV determinations should be done in the advent of new/worsen neurological symptoms and evidence of demyelination showed in MRI. Sensitivity of JCV PCR increases with lower CD4 T cell count.
Keywords: Progressive multifocal leukoencephalopathy; JC Virus; AIDS; CD4 T cell count
| Introduction | ▴Top |
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system (CNS) first described in 1958. A polyomavirus was identified as the etiological agent in 1967, named in 1971 as JC Virus (JCV), after the initials of the patient where the virus was first isolated (John Cunningham) [1, 2]. It occurs in immunocompromised patients, nowadays mainly in HIV infected ones. HIV infection accounts for 85% of all cases of PML, with a prevalence estimated of 4-5% in this population [1, 3]. The probability of an HIV patient developing neurological symptoms and PML lesions is higher amongst the ones with a lower immunological status, expressed by a CD4 T cell count lower than 200/mm3 [4, 5]. There are very few reports of PML among HIV infected patients with a better immunological condition, with more than 500 CD4/mm3 [6].
The diagnosis may be presumptive with the combination of neurological symptoms (visual disturbance, cognitive and behavioural alterations, seizures, and motor disorders with paralysis or akinesia) and characteristic magnetic resonance imaging (MRI) findings. Positive JCV in cerebrospinal fluid (CSF) or brain tissue specific anomalies, in association with clinical features and MRI findings, confirm PML [7]. Patients with suspicion of having PML should undergo a lumbar puncture with JCV search by means of PCR. Some authors discourage the search for JCV in CSF of HIV patients with normal CD4 T cell counts, due to the low sensitivity of the exam in such patients [8]. Diagnostic sensitivity on detection of JCV DNA in CSF by PCR in patients with PML not treated with antiretroviral therapy is of 72-92% and specificity of 92-100%, but the likelihood of detecting JCV in CSF in patients undergoing combined antiretroviral therapy (cARV) is lower (58%) [7].
We describe a clinical case of an HIV infected woman under cARV with a high CD4 T cell count that developed neurological symptoms and characteristic PML brain lesions, with JCV becoming positive in CSF only at the 5th determination. Differential diagnosis and therapy is also discussed, as well as the difficulty of making a definite diagnosis of PML among patients under cARV and high CD4 T cell count.
| Case Report | ▴Top |
A 48-year-old heterosexual woman was diagnosed with HIV1 infection in October 1998, when she was found to have oral candidiasis. She started on zidovudine, lamivudine and efavirenz in November 2004. Since then, she had undetectable viraemia and progressive immunological recovery (stage B1-CDC).
In January 2009, she presented a two-month history of insidious progressive gait instability with tandem gait and gait ataxia with no motor, sensitivity or cognitive impairment. Her viral load was undetectable (< 40 copies/ml) and her CD4 T cell count was of 533/mm3. Her physical exam was almost normal, only showing a slightly wide base gait. A cerebral CT scan was done being described as normal. Neurological symptoms progressed and, two months later, she had slurred speech, bilateral limb (more evident on the left side) and gait ataxia. No vertigo, diplopia, dysphagia, motor or sensitive symptoms were reported, besides a two weight loss and some nausea with occasional vomit. The clinical picture was compatible with a bilateral cerebellar syndrome. A cerebral MRI was done revealing a hyperintense sign in T2w and hypointensity in T1w, with discrete mass effect involving the bilateral precentral gyrus, right medium cerebellar pedunculus, white matter, right protuberance with extension to homolateral mesencephalic and periaqueductal regions, and left medium cerebellar pedunculus, found to be characteristic of PML (Fig. 1). Lumbar puncture was done and CSF revealed normal glucose (0.62 g/L) and protein (0.22 g/L) levels, with 13 leucocytes (9 mononuclear). CSF was negative for JCV. The immunoglobulin G index was more than 1 (1.3) and one oligoclonal band was present.
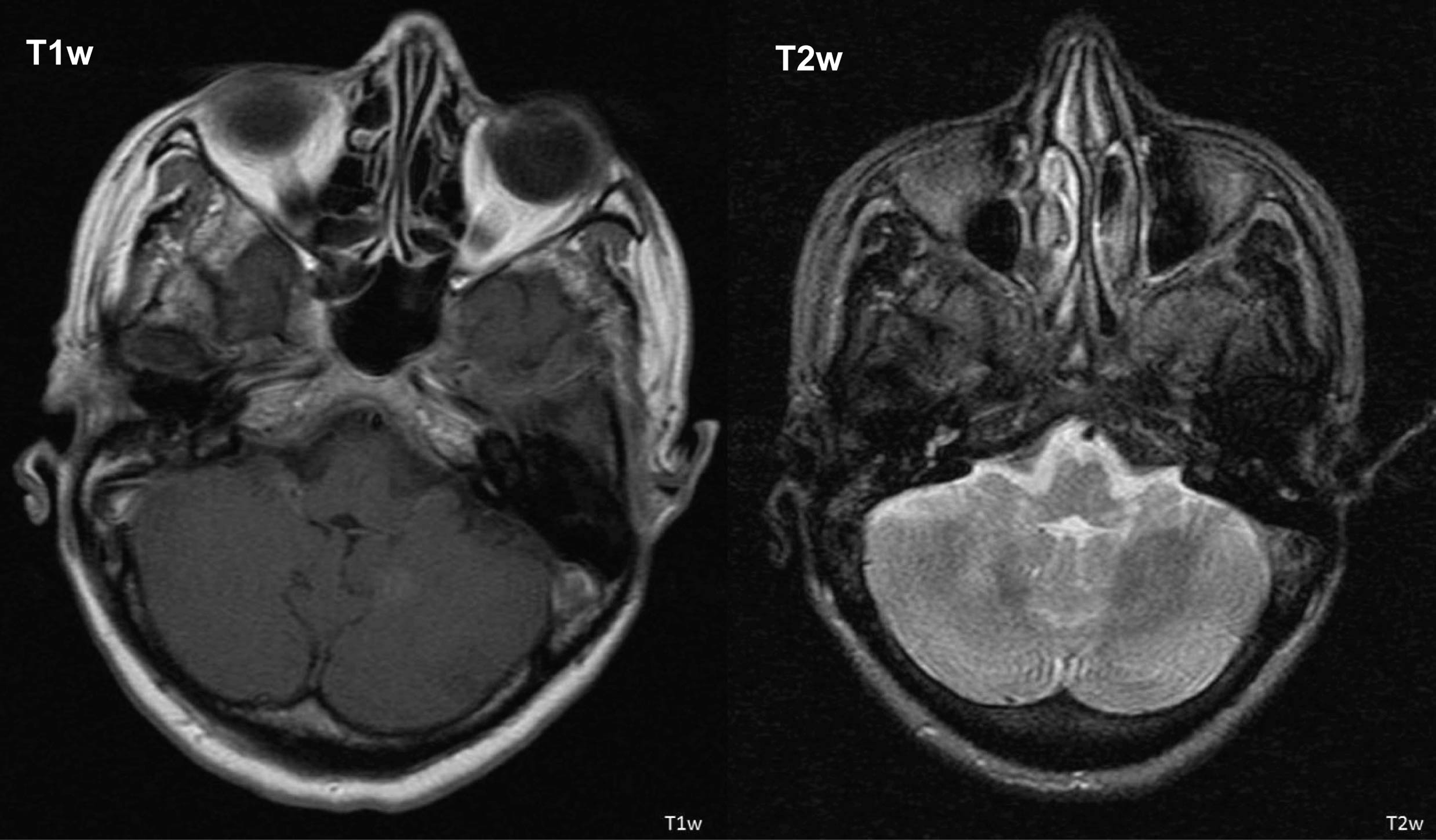 Click for large image | Figure 1. Cerebral MRI showing T2w and T1w sequences (description within the text). |
Her neurological state got worse. A severe gait ataxia with vomit, dizziness and frequent falls, and a speech disorder with dysarthria were documented. She had no visual complains. Due to her state, at the beginning of July 2009, she was admitted in hospital for further investigation. By that time she had 717 CD4/mm3, undetectable serum HIV viral load and a CSF HIV RNA of 7119 copies/mL. The neurological exam revealed a left hemiparesis with face (grade 4-/4+ to superior/inferior limb respectively), with normal muscular strength on the right side. Left Babinski, exacerbated reflexes on the left side, and a diminished sensitivity on the left side (including face) were recorded. She was not able to walk without help. She had no fever and her vital signs were normal. Concerning the number of pills per day and due to nausea and vomit, AZT was suspended giving place to abacavir, maintaining lamivudine and efavirenz.
She had no evidence of infectious disease elsewhere and no signs of systemic infection were found. Thyroid, renal and hepatic profile was normal and there were no vitamin deficiencies. A lympho- or myeloproliferative disorder and an inflammatory or granulomatosis disease were also ruled out.
A cerebral MRI was repeated, revealing the same lesions, with the same characteristics, at the same locations. Lumbar puncture was repeated and CSF findings were similar: JCV was negative, as was the bacterial and virological study (PCR for Mycobacterium tuberculosis, CMV, enterovirus, herpes 6 virus, VZV and herpes simplex 1 and 2). As in the previous lumbar puncture, EBV was positive in the CSF.
In spite of a negative JCV determination and a high CD4 T cell count, PML was considered as the most probable diagnosis. Considering the ongoing cARV and the neurological worsening manifestations, cidofovir treatment was started in a bimonthly regular basis together with mirtazapine in a daily dose of 30 mg and physiotherapy. Considering lesion locations, brain biopsy was not an option.
Three months later the patient’s clinical state was better, walking with orthopaedic aid, feeling less dizzy, with a perfectly comprehensible speech, and a better humor. All JCV CSF determinations (4 in the total) continued negative.
Other demyelinating diseases were considered, namely acute disseminated encephalomyelitis (ADEM) (but CSF immunoglobulin studies were not diagnostic) and HIV-leukoencephalitis (but signs of dementia or behavioural disturbances were not noticed). Cidofovir treatment was sustained and a surveillance program with physiotherapy was proposed together with cARV.
In May 2010 she presented herself at the outpatient clinic on a wheelchair, not being able to walk, and dysarthric. Lesion progression was suspected. At this time she had 478 CD4 T cell/mm3 and a serum HIV load of 186 copies/mL. Antiretroviral therapy was changed once again to zidovudine, lamivudine, lopinavir and ritonavir, based on better lopinavir penetration in the blood-brain barrier. A cerebral MRI was ordered. While waiting, she was admitted in the hospital due to prostration and emetic activity. She was aphasic, not following orders. She had discrete facial left central paresis, left hemiparesis with crural involvement, and plegic at the level of the superior left limb, with generalized hyperreflexia. As suspected, cerebral MRI revealed lesions progression, with hyperintense sign in T2W and hypointense in T1w, with involvement of the white matter of the frontal, subcortical and profound lobes, without mass effect.
CSF analysis was similar with normal glucose (0.61 g/L) and protein (0.37 g/L) levels, with 11 leucocytes/uL and 8 mononuclear/uL. JCV PCR analysis of the CSF was finally positive and PML definitive diagnosis was done. The patient died in June 2010 due to neurological disorder attributed to PML progression.
| Discussion | ▴Top |
This case report shows how a definite PML diagnosis is rare and difficult to achieve in a patient with a high CD4 T cell count and under cARV. It is known that in HIV patients, PML often arises in severe immunocompromised states, when CD4 T cell count is lower than 200/mm3. Nevertheless, cases have been described with CD4 T cell count higher than that value [4, 5, 7, 9]. Yet, case reports of definite PML with more than 500/mm3 CD4 T cell count are quite rare. It is well known that JCV cannot infect T lymphocytes or bind to T cell membranes. It seems that JCV uses B lymphocytes as a ‘Trojan horse’ to reach the brain exceeding the blood-brain barrier, and it happens when the reactivation process starts, when an immunosuppression state is achieved, because it can induce the loss of specific immune cells that may allow the beginning of active replication and infection, as well as changes in JCV regulatory region [10].
Nowadays, it is not known which ‘level’ of immunodepression is needed in an HIV patient for JCV to turn from a latent to a replicative state, or if there are some other means for JCV to reach the brain and infect oligodendrocytes rather than the ‘B lymphocyte way’.
The cARV was probably one of the causes for the consecutive negative results of JCV determination, as it is well known that highly active antiretroviral therapy (HAART) lowers the sensitivity of JCV PCR in CSF [7].
JCV isolation was possible only at the fifth determination, when CD4 T cell count was slightly lower than500/mm3 and neurological deficits were severe. It is known that the profitability of JCV determination in CSF is higher with lower serum CD4 T cell count [7] and that sample collected in the early course of the disease with a negative result does not exclude the possibility of the presence of JCV in the brain [11].
Sometimes in the course of the disease, PML diagnosis was questioned, mainly due to the relatively high CD4 T cell count and consecutive negative results for JCV, in spite of the initial findings in MRI. MRI is the best imaging tool to diagnose PML, with typical appearance in 90% of the patients, usually with patchy and commonly non-enhancing white matter lesions, usually bilateral and asymmetric, and with no mass effect. Yet, some demyelinating diseases may present almost with the same characteristics, but some of them with mass effect[12]. HIV-leukoencephalitis is the main differential diagnosis, but it seems that lesions are less diffuse, more symmetric, not visible on T1w and less intense on T2w sequence [13, 14]. There is also evidence of HIV active replication in brain in this last situation [15]. Our patient had, in fact, a higher HIV count in CSF than in serum, indicating an active replication in that reservoir, pending the balance to this last diagnosis. Yet, there were no signs of dementia or cognitive disturbance. It was the neurological evolution and the final isolation of JCV in CSF that changed to the definite diagnosis more than six months later.
One year and a half was the time that elapsed between the initial presentation of the symptoms and death. Neurological symptoms progressed in the beginning, then stabilized (and even got a little bit better in the summer 2009) and got worse once again. The patient was under antiretroviral therapy since the beginning, the only well demonstrated therapy to PML in HIV patients, in order to rise CD4 T cell count, to ameliorate the immunological status of the patient and diminish JCV replication and subsequently neurological manifestations [9, 16]. Cidofovir is a deoxycytidine monophosphate analogue that inhibits viral DNA synthesis by interfering with viral DNA polymerase. There have been some positive reports concerning the use of cidofovir in HIV patients with PML in the past [1]. However, a recent paper concludes that cidofovir does not influence mortality or morbidity related to PML in HIV infected patients [17]. Mirtazapine has been described in some reports as of value in the treatment of PML in HIV patients, inducing the down-regulation of 5HT2a receptors, which are used by JCV to enter glial cells [18, 19].
It is not possible to say if the transitory recovery was due to cidofovir, mirtazapine or even physiotherapy, started at the same time. The only therapy that was maintained was HAART. AZT and abacavir have similar penetration on the CNS. Cidofovir was not repeated when definite PML diagnosis was done, due to the extension of the lesions and the presumed irreversibility of the neurological symptoms.
There is also some evidence that vital prognosis (lesion progression and death) and the speed of the onset correlate directly with JCV CSF count [3, 20]. Our patient had no JCV detected in the first determinations. It was when neurological symptoms aggravated that it turned positive. She died one month after evidence of JCV replication in CSF. Could the absence of JCV detection be a factor of ‘good prognosis’ related to a prolonged survival?
In conclusion, clinical symptoms and signs together with typical MRI findings among patients with high CD4 T cell count and under cARV should be considered and treated as PML. The progression of the disease may correlate with a higher replication of the JCV. Several lumbar punctures with JCV PCR in CSF should be done, but a negative result should not delay the start of antiretroviral treatment in those patients with CD4 T cell count above 500/mm3.
Conflict of Interest
There is no conflict of interest to report.
| References | ▴Top |
- Focosi D, Marco T, Kast RE, Maggi F, Ceccherini-Nelli L, Petrini M. Progressive multifocal leukoencephalopathy: what's new? Neuroscientist 2010;16(3):308-323.
pubmed doi - Zheng HC, Yan L, Cui L, Guan YF, Takano Y. Mapping the history and current situation of research on John Cunningham virus - a bibliometric analysis. BMC Infect Dis 2009;9:28.
pubmed - Taoufik Y, Gasnault J, Karaterki A, Pierre Ferey M, Marchadier E, Goujard C, Lannuzel A, et al. Prognostic value of JC virus load in cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. J Infect Dis 1998;178(6):1816-1820.
pubmed doi - Khanna N, Elzi L, Mueller NJ, Garzoni C, Cavassini M, Fux CA, Vernazza P, et al. Incidence and outcome of progressive multifocal leukoencephalopathy over 20 years of the Swiss HIV Cohort Study. Clin Infect Dis 2009;48(10):1459-1466.
pubmed doi - Engsig FN, Hansen AB, Omland LH, Kronborg G, Gerstoft J, Laursen AL, Pedersen C, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis 2009;199(1):77-83.
pubmed doi - Delobel P, Brassat D, Delisle MB, Scaravilli F, Clanet M. Progressive multifocal leukoencephalopathy in an HIV patient with normal CD4 T-cell count and magnetic resonance imaging. AIDS 2004;18(4):702-704.
pubmed - Cinque P, Koralnik IJ, Gerevini S, Miro JM, Price RW. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis 2009;9(10):625-636.
pubmed doi - Wang Y, Kirby JE, Qian Q. Effective use of JC virus PCR for diagnosis of progressive multifocal leukoencephalopathy. J Med Microbiol 2009;58(Pt 2):253-255.
pubmed doi - Falco V, Olmo M, del Saz SV, Guelar A, Santos JR, Gutierrez M, Colomer D, et al. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J Acquir Immune Defic Syndr 2008;49(1):26-31.
pubmed doi - Sabath BF, Major EO. Traffic of JC virus from sites of initial infection to the brain: the path to progressive multifocal leukoencephalopathy. J Infect Dis 2002;186 Suppl 2:S180-186.
pubmed - Hammarin AL, Bogdanovic G, Svedhem V, Pirskanen R, Morfeldt L, Grandien M. Analysis of PCR as a tool for detection of JC virus DNA in cerebrospinal fluid for diagnosis of progressive multifocal leukoencephalopathy.J Clin Microbiol 1996;34(12):2929-2932.
pubmed - Kingsley PB, Shah TC, Woldenberg R. Identification of diffuse and focal brain lesions by clinical magnetic resonance spectroscopy. NMR Biomed 2006;19(4):435-462.
pubmed doi - Sarrazin JL, Soulie D, Derosier C, Lescop J, Schill H, Cordoliani YS. [MRI aspects of progressive multifocal leukoencephalopathy]. J Neuroradiol 1995;22(3):172-179.
pubmed - Shah R, Bag AK, Chapman PR, Cure JK. Imaging manifestations of progressive multifocal leukoencephalopathy. Clin Radiol 2010;65(6):431-439.
pubmed doi - Luer W, Gerhards J, Poser S, Weber T, Felgenhauer K. Acute diffuse leukoencephalitis in HIV-1 infection. J Neurol Neurosurg Psychiatry 1994;57(1):105-107.
pubmed doi - Clifford DB, Yiannoutsos C, Glicksman M, Simpson DM, Singer EJ, Piliero PJ, Marra CM, et al. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology 1999;52(3):623-625.
pubmed - De Luca A, Ammassari A, Pezzotti P, Cinque P, Gasnault J, Berenguer J, Di Giambenedetto S, et al. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: a multicohort analysis. AIDS 2008;22(14):1759-1767.
pubmed - Verma S, Cikurel K, Koralnik IJ, Morgello S, Cunningham-Rundles C, Weinstein ZR, Bergmann C, et al. Mirtazapine in progressive multifocal leukoencephalopathy associated with polycythemia vera. J Infect Dis 2007;196(5):709-711.
pubmed doi - Cettomai D, McArthur JC. Mirtazapine use in human immunodeficiency virus-infected patients with progressive multifocal leukoencephalopathy. Arch Neurol 2009;66(2):255-258.
pubmed doi - Eggers C, Stellbrink HJ, Buhk T, Dorries K. Quantification of JC virus DNA in the cerebrospinal fluid of patients with human immunodeficiency virus-associated progressive multifocal leukoencephalopathy—a longitudinal study. J Infect Dis 1999;180(5):1690-1694.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


