| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 4, Number 10, October 2013, pages 654-659
Intraductal Papillary Neoplasm of the Bile Duct Arising From a Background of Autoimmune Hepatitis: A Case Report
Takafumi Saitoa, d, Tomohiro Katsumia, Kyoko Tomitaa, Chikako Satoa, Yuko Nishisea, Kazuo Okumotoa, Hisayoshi Watanabea, Naohiko Makinoa, Rintaro Oheb, Mitsunori Yamakawab, Wataru Kimurac, Yoshiyuki Uenoa
aDepartment of Gastroenterology, Yamagata University School of Medicine, Yamagata 990-9585, Japan
bDepartment of Pathology, Yamagata University School of Medicine, Yamagata 990-9585, Japan
cDepartment of Surgery, Yamagata University School of Medicine, Yamagata 990-9585, Japan
dCorresponding author: Takafumi Saito, Department of Gastroenterology, Yamagata University School of Medicine, Iida-nishi 2-2-2, Yamagata 990-9585, Japan
Manuscript accepted for publication August 5, 2013
Short title: IPNB With AIH
doi: https://doi.org/10.4021/jmc1439w
| Abstract | ▴Top |
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare cystic tumor of the liver defined separately from hepatic mucinous cystic neoplasm. Development of liver cancer in patients with autoimmune hepatitis (AIH) is also sporadic, and such cases reported previously have been hepatocellular carcinoma. Here we report a 50-year-old man with IPNB complicated by AIH who underwent a successful extended left hepatectomy. Histopathology and immunohistochemical staining of the resected tumor revealed that it was a pancreatobiliary-type IPNB expressing both MUC5AC and MUC6, accompanied by carcinoma in situ expressing p53, Ki-67 and CA19-9. The histopathology of the background liver was compatible with that of AIH showing interface hepatitis with accompanying plasma cell infiltration. The patient has remained free of cancer recurrence for 12 years following the hepatectomy, and liver dysfunction caused by AIH has been controllable by steroid administration. This is the first report of pancreatobiliary-type IPNB associated with AIH.
Keywords: Intraductal papillary neoplasm; Liver cancer; Mucinous cystic neoplasm; IPNB; Hepatitis; AIH; MUC
| Introduction | ▴Top |
Two types of cyst-forming epithelial neoplasms of the liver have been defined in the classification proposed by the World Health Organization (WHO) [1, 2]. Hepatic mucinous cystic neoplasm (MCN-H) is characterized by an ovarian-like stroma, and usually shows no communication to bile ducts. By contrast, intraductal papillary neoplasm of the bile duct (IPNB) shows predominant papillary intraductal growth with frequent communication to neighboring bile duct. Recent pathologic studies have suggested that IPNB and MCN-H are biliary counterparts of intraductal papillary mucinous neoplasm of the pancreas (IPMN) and MCN of the pancreas (MCN-P), respectively [3-6]. In some cases IPNB and MCN-H are known to be early neoplastic lesions that are followed by invasive cholangiocarcinoma, as is the case for IPMN and MCN-P, which progress to invasive carcinoma of the pancreas [4, 5].
Autoimmune hepatitis (AIH) rapidly leads to liver injury with a risk of progression to cirrhosis and liver failure, thus having a poor prognosis if left untreated. Hepatocellular carcinoma (HCC) has been reported to be the malignant tumor arising from the liver in patients with AIH [7-12]. HCC in patients with AIH occurs sporadically, and its incidence is low [7-12]. However, an increased incidence of HCC in AIH patients is expected because recent advances in treatment regimens based on steroid or azathioprine have achieved a high degree of efficacy, thus decreasing the mortality rates and achieving long-term survival rates of 80-90% or better [13, 14]. Therefore, the risk of malignant tumors other than HCC associated with AIH might increase in the future. Here, we report for the first time a case of IPNB in the liver of 50-year-old man with AIH, with special reference to the immunohistochemical characteristics of the resected tumor.
| Case Report | ▴Top |
A 50-year-old man was admitted with a chief complaint of general fatigue and jaundice. He had no history of metabolic disorders, excess intake of alcohol or medication, as a potential cause of liver dysfunction. The laboratory data on admission are shown in Table 1. The levels of total bilirubin and liver enzymes including aspartate transferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (GGT) were high, and the liver functional reserve represented by serum albumin and the international normalized ratio (INR) of prothrombin time were impaired. Viral markers including hepatitis A, B and C viruses were all negative. Since anti-nuclear antibody was positive with a titer of 1:160 and the serum level of immunoglobulin G was high at 2,200 mg/dL, AIH was suspected as the cause of liver dysfunction. The levels of tumor markers including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA) and carbohydrate antigen (CA)19-9 were all within normal limits, but that of pancreatic cancer-associated protein-2 (DUPAN-2) was higher (470 U/mL) than the reference level (150 U/mL). Routine liver imaging including ultrasonography (US) and computed tomography (CT) showed a cyst-forming tumor with solid masses in its cavity in the medial segment of the liver. CT angiography demonstrated that the tumor masses had faint arterial staining in the early arterial phase (Fig. 1A), and intraductal ultrasonography (IDUS) of the choledochus clearly showed mass lesions that protruded into the lumen (Fig. 1B). Based on these results, the tumor was diagnosed as an intrahepatic cyst-forming neoplasm, possibly cystadenocarcinoma.
 Click to view | Table 1. Laboratory Data on Admission |
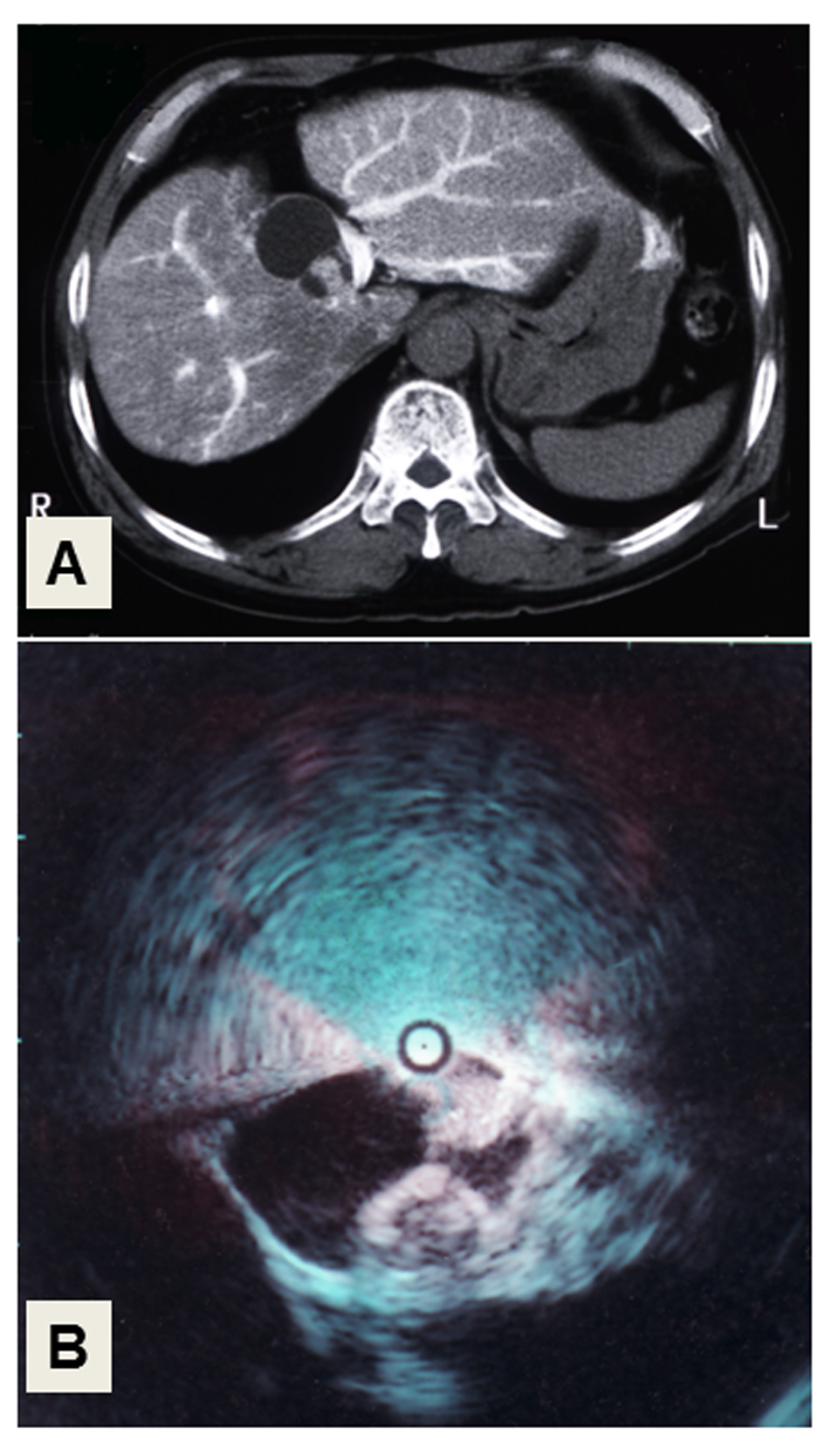 Click for large image | Figure 1. Liver imaging. (A) CT-angiography. The cystic tumor with solid masses in its cavity, showing faint arterial staining in the medial segment of the liver. (B) Intraductal ultrasonography (IDUS) of the choledochus. Two solid, irregular masses arise from the wall of the cystic tumor. |
Since no metastatic lesions were found and liver function parameters were all improved almost to within normal limits by glycyrrhizin administration after admission, the patient underwent an extended left hepatectomy. Neither lymph node metastasis nor invasion to the hepatic parenchyma or vessels adjacent to the tumor was detected. The resected specimen was a cyst-forming tumor measuring 54 × 42 × 32 mm. A cut section of the tumor demonstrated a large cyst with solid masses in the intraluminal space of the bile duct filled with 12 mL of fluid. There was a high level of CA19-9 (8,000 U/mL) in the fluid.
The histopathology of the resected tumor is shown in Figure 2. The epithelial tumor cells showed papillary growth accompanied by a fibrous stroma (Fig. 2A). No ovarian-like stroma was evident in the specimen. Adenocarcinoma components extended along the epithelium of the intrahepatic bile ducts, showing carcinoma in situ (Fig. 2B). Mucin production by the tumor cells was confirmed by both acian blue and periodic acid-Schiff staining. Immunohistochemical staining of the tumor cells demonstrated negativity for mucin core protein (MUC)1, MUC2, cytokeratin (CK)20, caudal-type homeobox (CDX)2, cluster of differentiation (CD)56, chromogranin A, synaptophysin, estrogen receptor (ER) and progesterone receptor, and positivity for CK7, CK19, MUC5AC, MUC6, p53, Ki-67, cyclin D1, CEA and CA19-9. The expression of MUC5A, MUC6, Ki-67 and CEA in the tumor cells is shown in Figure 3. The histology of the background liver tissue was compatible with that of AIH, showing interface hepatitis with accompanying plasma cell infiltration (Fig. 4A) and ductular proliferation of intrahepatic bile ducts (Fig. 4B). The overall features of this case were compatible with a diagnosis of pancreatobiliary-type IPNB, carcinoma in situ, associated with AIH.
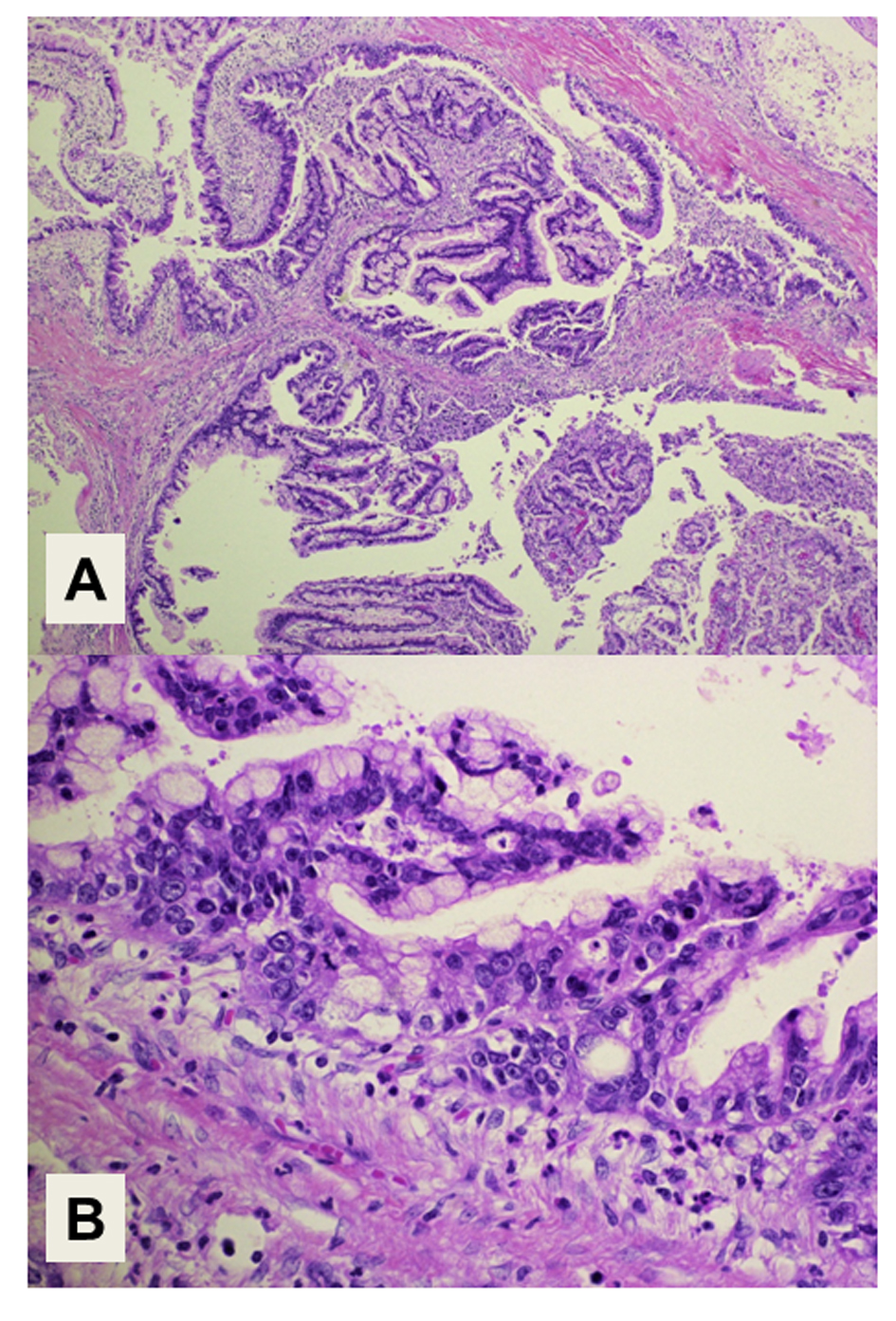 Click for large image | Figure 2. Histology of the tumor. (A) Intraductal papillary structures of the epithelial tumor cells with a fibrous stroma. Magnification: × 40. (B) Adenocarcinoma components extend along the epithelium of the intrahepatic bile ducts, showing a carcinoma in situ. Magnification: × 400. Hematoxylin and Eosin staining. |
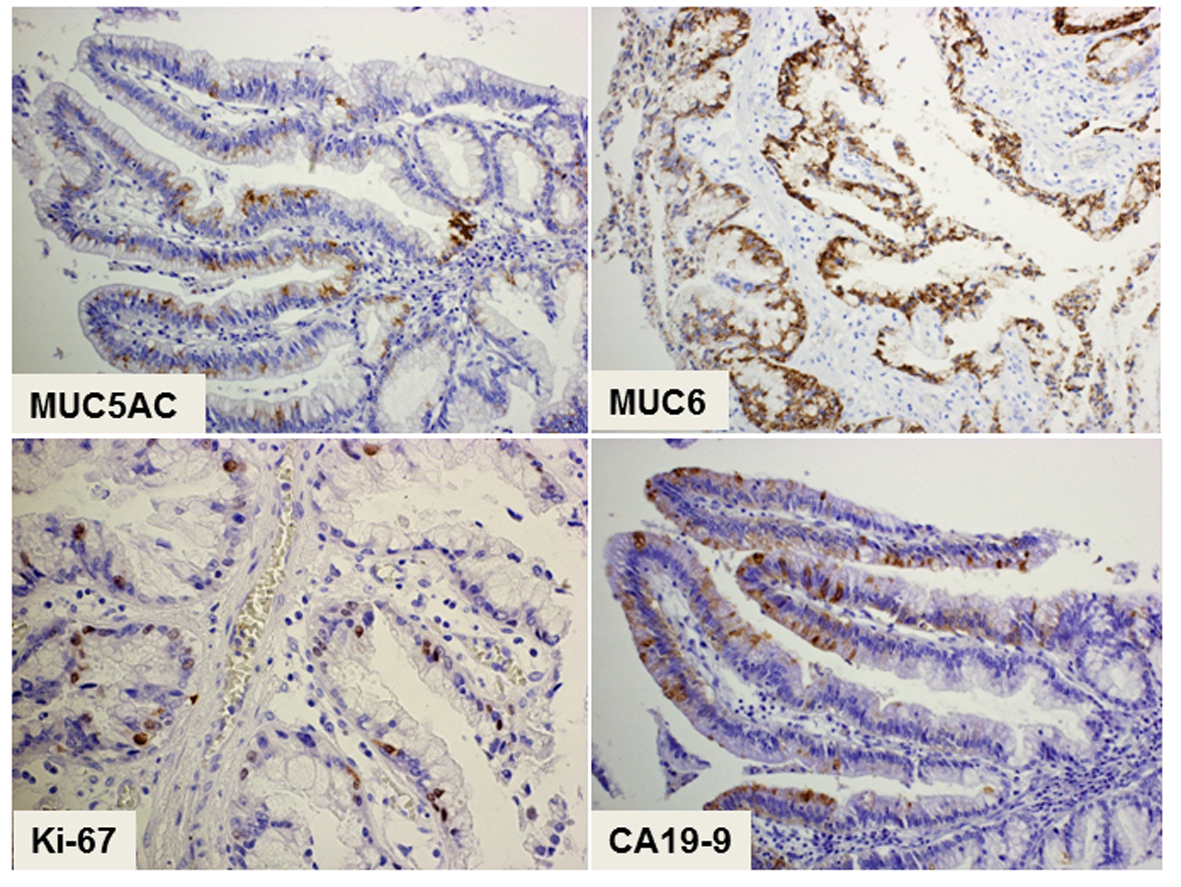 Click for large image | Figure 3. Immunohistochemical staining of the tumor showing a pancreatobiliary-type intraductal papillary neoplasm of the bile duct. The cytoplasmic expression of both MUC5A and MUC6 as mucin-related proteins is positive in the epithelial tumor cells. The cells show nuclear expression of Ki-67 and cytoplasmic expression of CA19-9, suggesting their malignant potential. Magnification: × 200. |
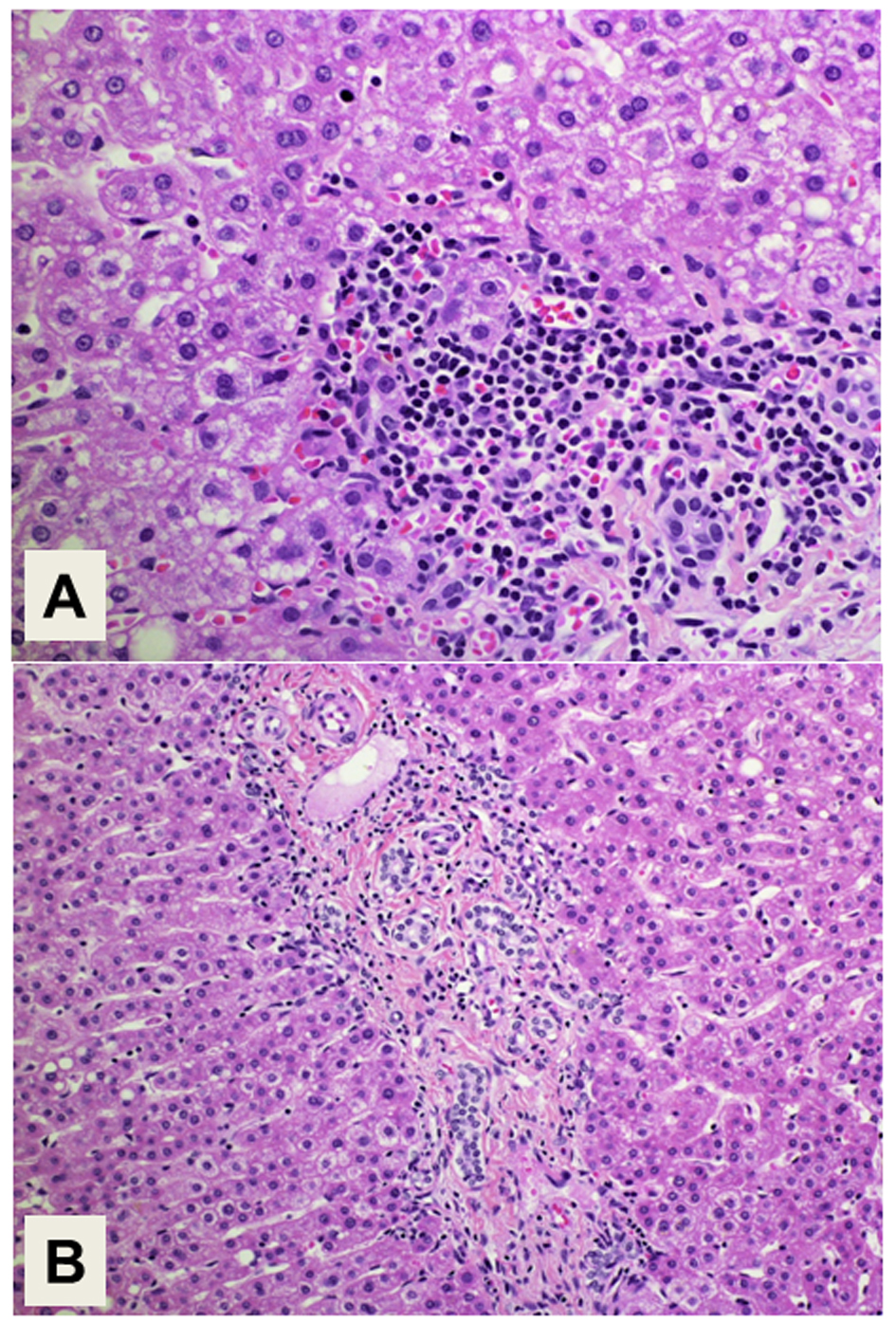 Click for large image | Figure 4. Histology of the background liver. (A) Interface hepatitis with plasma cell infiltration is compatible with the histology of autoimmune hepatitis. Magnification: × 400. (B) Proliferation of intrahepatic bile ducts is also demonstrated. Magnification: × 200. Hematoxylin and Eosin staining. |
The postoperative course was good, and the patient was discharged 35 days after surgery. The patient has shown no sign of cancer recurrence for 12 years after hepatectomy. The liver dysfunction associated with AIH has been controllable with steroid maintenance therapy on an outpatient basis.
| Discussion | ▴Top |
IPNB is a rare neoplasm of the liver, which was recently defined in the 2010 WHO classification [2]. The present case was diagnosed as an intrahepatic cystadenoma/cystadenocarcinoma at the time of surgery, before this classification had been published. The rarity of IPNB and paucity of clinical information may cause it to be confused with other cyst-forming tumors of the liver. IPNB has malignant potential [2], and cystadenoma and cystadenocarcinoma, which are both histological phenotypes of IPNB in the liver, share similar radiological characteristics and clinicopathological features, making it difficult to distinguish a malignant tumor from a benign adenoma. It is important for clinicians to diagnose this tumor correctly in order to develop an appropriate treatment plan.
Imaging modalities for diagnosis of INPB in relation to pathological findings have made some degree of progress. IDUS is able to clearly demonstrate a malignant biliary tumor [15]. In addition, arterial blood flow or enhancement of the tumor wall/nodule tends to be demonstrated more commonly in biliary cystadenocarcinoma by CT or CT angiography than in cystadenoma [16]. No tumor markers with reliable sensitivity and specificity have yet been assigned to INPB. In the present case, solid masses in the cystic cavity showing an irregular surface were clearly demonstrated by IUDS, and faint arterial staining of the intraluminal masses evident by CT angiography suggested malignancy. Although the serum level of CA19-9 was within the normal range in this case, but it was high (8,000 U/mL) in the tumor fluid, and the serum DUPAN-2 level was high. Thus, markers of pancreatobiliary tumors are worth measuring as an adjunct for diagnosis of IPNB with malignancy.
Recently, IPNB has been considered a biliary counterpart of IPMN [3-6], and the immunohistochemical similarities between them have been noted [17]. IPNB and IPMN differ from MCN-H and MCN-P in lacking an ovarian-like stroma expressing ER [5]. Histopathologically, it has been proposed that IPNB should be classified into four subgroups along the lines of those for IPMN, i.e. the gastric type, intestinal type, oncocyte type and pancreatobiliary type [18]. Among them, both the frequency of invasive carcinoma and survival are reportedly worse for pancreatobiliary-type IPNB, as is the case for IPMN [19, 20]. It has been suggested that the nuclear expression of p53 or cyclin D1 is involved in the carcinogenesis of IPNB [21], and that Ki-67 is associated with malignancy in cases of IPMN [22]. In accordance with these tumor characteristics, the present case was diagnosed as a pancreatobiliary-type IPNB, carcinoma in situ, with accompanying malignant potential.
In addition to the results of serologic tests, the histopathology of the background liver showed interface hepatitis with plasma cell infiltration, compatible with that of AIH. The incidence of complications ascribable to malignant liver tumors with AIH remains unclear. So far, liver cancer in patients with AIH reported previously has been HCC [7-12]. One of the proposed mechanisms of hepatocarcinogenesis in AIH is long-standing liver inflammation, similar to that resulting from virus-related hepatitis. However, the low incidence of hepatocarcinogenesis in AIH [7-12] in comparison with virus-related hepatitis suggests that this theory may not be wholly valid. One of the major histopathological features of AIH is interface hepatitis, involving severe inflammation of the portal and periportal areas. Proliferation of intrahepatic bile ducts is also often observed, as in the present case. The occurrence of biliary carcinoma in patients with AIH might be associated with both abnormality of the immune system and persistent inflammation of the bile duct. Although IPNB itself is a rare tumor, further investigation is needed to clarify whether it is a malignancy associated with AIH. It has been proposed that curative resection of IPNB with malignant potential may offer a favorable prognosis with a chance of long-term survival [23-25], and in fact the present patient has remained free of cancer recurrence for 12 years after hepatectomy. To our knowledge, this is the first report of pancreatobiliary-type IPNB, carcinoma in situ, arising from the liver in a patient with AIH.
Conflict of Interest
None reported.
| References | ▴Top |
- Tsui WMS, Adsay NV, Crawford JM, Hruban R, Klöppel G, Wee A. Mucinous cystic neoplasm of the liver. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. World Health Organization of Tumours, 4th ed. Lyon: IARC Press, 2010: 236-238.
- Nakanuma Y, Curabo MP, Franceschi S, Gores G, Paradis V, Sripa B, Tsui WMS, et al. Intrahepatic cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. World Health Organization of Tumours, 4th ed. Lyon: IARC Press, 2010: 217-224.
- Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, Chiu CT, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34(4 Pt 1):651-658.
doi pubmed - Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44(5):1333-1343.
doi pubmed - Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, Quaglia A, et al. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011;24(8):1079-1089.
doi pubmed - Lim JH, Zen Y, Jang KT, Kim YK, Nakanuma Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects. AJR Am J Roentgenol. 2011;197(5):1111-1120.
doi pubmed - Hino-Arinaga T, Ide T, Kuromatsu R, Miyajima I, Ogata K, Kuwahara R, Hisamochi A, et al. Risk factors for hepatocellular carcinoma in Japanese patients with autoimmune hepatitis type 1. J Gastroenterol. 2012;47(5):569-576.
doi pubmed - Park SZ, Nagorney DM, Czaja AJ. Hepatocellular carcinoma in autoimmune hepatitis. Dig Dis Sci. 2000;45(10):1944-1948.
doi pubmed - Yeoman AD, Al-Chalabi T, Karani JB, Quaglia A, Devlin J, Mieli-Vergani G, Bomford A, et al. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: Implications for follow-up and screening. Hepatology. 2008;48(3):863-870.
doi pubmed - Montano-Loza AJ, Carpenter HA, Czaja AJ. Predictive factors for hepatocellular carcinoma in type 1 autoimmune hepatitis. Am J Gastroenterol. 2008;103(8):1944-1951.
doi pubmed - Werner M, Almer S, Prytz H, Lindgren S, Wallerstedt S, Bjornsson E, Bergquist A, et al. Hepatic and extrahepatic malignancies in autoimmune hepatitis. A long-term follow-up in 473 Swedish patients.J Hepatol. 2009;50(2):388-393.
doi pubmed - Teufel A, Weinmann A, Centner C, Piendl A, Lohse AW, Galle PR, Kanzler S. Hepatocellular carcinoma in patients with autoimmune hepatitis. World J Gastroenterol. 2009;15(5):578-582.
doi pubmed - Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996;110(3):848-857.
doi pubmed - Czaja AJ. Treatment of autoimmune hepatitis. Semin Liver Dis. 2002;22(4):365-378.
doi pubmed - Takanami K, Hiraide T, Kaneta T, Hayashi H, Unno M, Fujishima F, Fukuda H, et al. FDG PET/CT findings in malignant intraductal papillary mucinous neoplasm of the bile ducts. Clin Nucl Med. 2010;35(2):83-85.
doi pubmed - Wang C, Miao R, Liu H, Du X, Liu L, Lu X, Zhao H. Intrahepatic biliary cystadenoma and cystadenocarcinoma: an experience of 30 cases. Dig Liver Dis. 2012;44(5):426-431.
doi pubmed - Matsubara T, Sato Y, Sasaki M, Harada K, Nomoto K, Tsuneyama K, Nakamura K, et al. Immunohistochemical characteristics and malignant progression of hepatic cystic neoplasms in comparison with pancreatic counterparts. Hum Pathol. 2012;43(12):2177-2186.
doi pubmed - Zen Y, Fujii T, Itatsu K, Nakamura K, Konishi F, Masuda S, Mitsui T, et al. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19(9):1243-1254.
doi pubmed - Kim KM, Lee JK, Shin JU, Lee KH, Lee KT, Sung JY, Jang KT, et al. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol. 2012;107(1):118-125.
doi pubmed - Takasu N, Kimura W, Moriya T, Hirai I, Takeshita A, Kamio Y, Nomura T. Intraductal papillary-mucinous neoplasms of the gastric and intestinal types may have less malignant potential than the pancreatobiliary type. Pancreas. 2010;39(5):604-610.
doi pubmed - Nakanishi Y, Zen Y, Kondo S, Itoh T, Itatsu K, Nakanuma Y. Expression of cell cycle-related molecules in biliary premalignant lesions: biliary intraepithelial neoplasia and biliary intraductal papillary neoplasm. Hum Pathol. 2008;39(8):1153-1161.
doi pubmed - Takeshita A, Kimura W, Hirai I, Takasu N, Moriya T, Tezuka K, Watanabe T. Clinicopathologic study of the MIB-1 labeling index (Ki67) and postoperative prognosis for intraductal papillary mucinous neoplasms and ordinary ductal adenocarcinoma. Pancreas. 2012;41(1):114-120.
doi pubmed - Nanashima A, Sumida Y, Tamaru N, Nakanuma Y, Abo T, Tanaka K, Sawai T, et al. Intraductal papillary neoplasm of the bile duct extending superficially from the intrahepatic to extrahepatic bile duct. J Gastroenterol. 2006;41(5):495-499.
doi pubmed - Paik KY, Heo JS, Choi SH, Choi DW. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol. 2008;97(6):508-512.
doi pubmed - Li T, Ji Y, Zhi XT, Wang L, Yang XR, Shi GM, Zhang W, et al. A comparison of hepatic mucinous cystic neoplasms with biliary intraductal papillary neoplasms. ClinGastroenterolHepatol. 2009;7(5):586-593.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


