| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website http://www.journalmc.org |
Case Report
Volume 1, Number 1, August 2010, pages 23-26
Pure Primary Squamous Cell Carcinomas of the Breast: A Report of Eight Cases
Kaoutar Znatia, c, Sanaa Bennisa, Fouad Abbasa, Meriam Chraibia, Nawal Hammasa, Sanae Chahbounia, Taoufiq Harmoucha, Laila Chbania, Hinde Elfatemia, Yousra Aksbib, Samia Arifib, Omar Mesbahib, Afaf Amartia
aDepartment of Pathology, HASSAN II University Hospital, Fes, Morocco
bMedical Oncology Department, Hassan II University Hospital, Fez, Morocco
cCorresponding author: Residence Belair, Immeuble C Appartement 4, Route Immouzzer, Fes, Morocco
Manuscript accepted for publication May 20, 2010
Short title: Pure Primary Squamous Cell Carcinoma of Breast
doi: https://doi.org/10.4021/jmc2010.07.105e
| Abstract | ▴Top |
Pure primary squamous cell carcinoma (PPSCC) of the breast is a rare neoplasm included in World Health Organization Classification of tumors (2003) in metaplastic breast cancer. It accounts for less than 0.1% of all breast carcinomas. We performed a retrospective review of all women diagnosed or treated as PPSCC, from January 2004 to June 2009 to assess clinical presentation, surgical and pathological findings, treatment and outcome. Eight patients were identified. The median age was 48.2 (range: 40 - 57) years. The average tumor size was 7.3 cm, with a range of 3.5 to 18 cm. There were one well differentiated, three moderately differentiated and four poorly differentiated PPSCC. Two cases were stage IIA, two cases stage IIIA and three cases stage IIIB. Primary treatment was mastectomy in six patients and large local excision in one patient. There was lymph node (LN) involvement in one patient. Estrogen and progesterone receptors status and over expression of HER 2 were assessed and none tumors was positive. Cytokeratin 5/6 and/or Cytokeratin 14 were positive in all tumors. All patients were treated by radiotherapy and chemotherapy. Though PPSCC of the breast is a very rare and aggressive disease, in our experience it’s relatively frequent. Strict histologic criteria should be used to diagnose PPSCC, because they have significantly lower axillary lymph node metastasis, lower estrogen and PR positivity, and they are often treatment-refractory. The role of different new chemotherapy regimens needs to be explored.
Keywords: Pure squamous cell carcinoma; Metaplastic carcinoma; Breast; Morphology; Basal-like phenotype; Histogenesis; Prognosis; Treatment
| Introduction | ▴Top |
Pure primary squamous cell carcinoma (PPSCC) of the breast is a rare condition and is considered to arise through metaplastic change of ductal carcinoma cells [1, 2]. In World Health Organization Classification of tumors (2003), it’s included in metaplastic breast cancer [3]. Several pathological criteria are required to establish a firm diagnosis of PPSCC of the breast: (1) a tumor origin that is independent of the overlying skin and nipple or of adnexal elements; (2) more than 90% of the tumor must be squamous; (3) there can be no other invasive neoplastic elements, ductal or mesenchymal, on thorough sampling; and (4) other sites of primary squamous cell carcinoma must be excluded [1].
Clinical and radiological features are not specific and are generally characterized by the absence of expression of hormone receptor and human epidermal growth factor receptor 2 (HER2). Prognosis of this breast cancer is poor and the disease is associated with frequent locoregional and distant metastasis [1, 4]. There is no scientific evidence of the usefulness of adjuvant treatments and more studies are needed [5].
| Case Report | ▴Top |
Eight cases of PPSCC were diagnosed in the pathology department between January 2004 and June 2009 and were retrospectively reviewed.
Clinical information, including patient age, tumor size, tumor location, specimen type, estrogen receptor (ER), progesterone receptor (PR), and Her2/neu status was recorded from the pathology reports. The phenotype was designated as ‘‘triple negative’’ if ER and PR expression was recorded as 0% to 5%, Her2/neu expression was 0 or 1+. Expressions of Cytokeratins (Cks), including Ck5/6 and/or Ck14 were assessed to specify basal-like phenotype.
All patients were female, with a median age of 48.2 years (range: 40 - 57 years). The tumor was on the right in three cases, and on the left in five cases. Clinical presentation was non-specific in two patients and overlying skin was inflammatory in two cases with locally advanced tumors. One patient was initially diagnosed with a breast abscess. There was no evidence of lymphadenopathy on physical examination.
On mammography, all patients had well delineated mass densities with no specific data suggesting this specific diagnosis (Fig. 1).
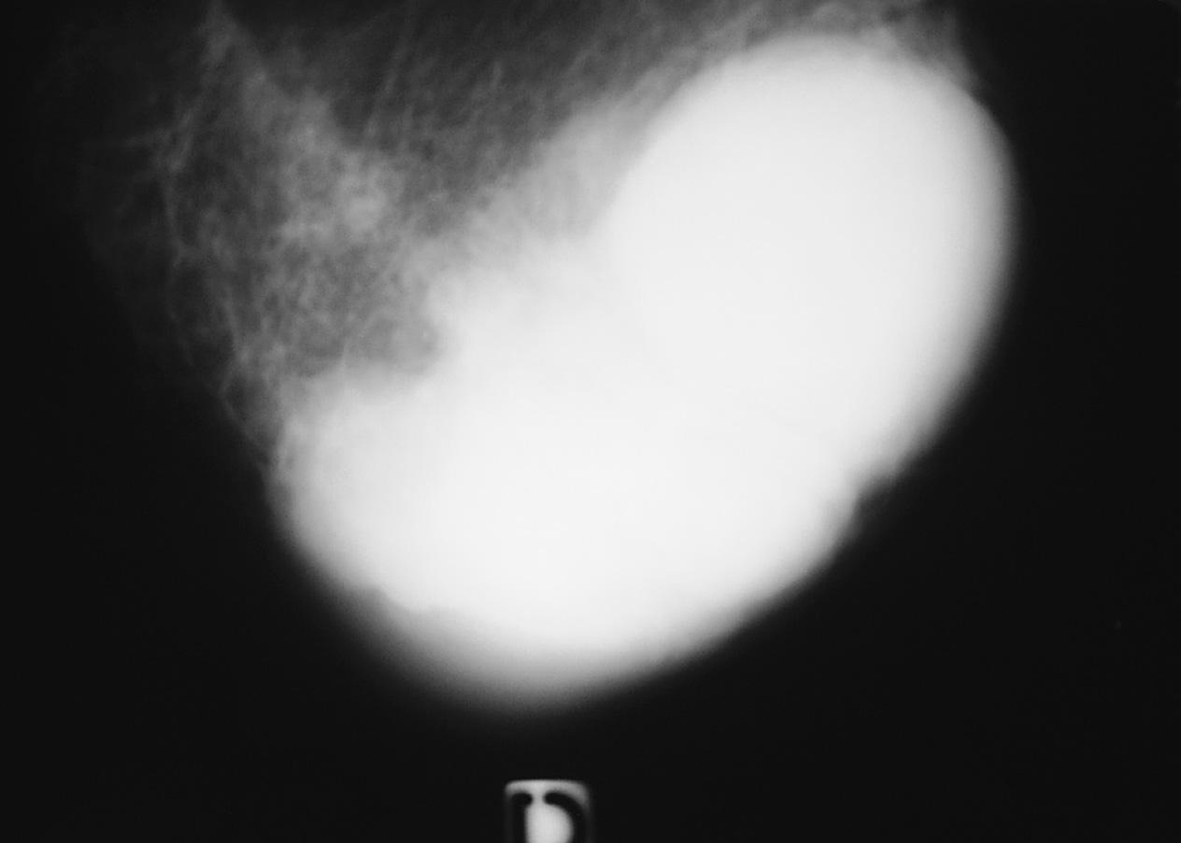 Click for large image | Figure 1. Mammography oblique view of the left breast shows a round mass highly dense with regular margins. |
Total body CT scan, bone scan, laboratory data (blood count, serum electrolytes, liver function tests, creatinine, prothrombin and partial thromboplastin time) and tumor markers were within normal range. No other tumor location was found.
One of the specimens was a large tumorectomy and six were mastectomy specimens. The average tumor size was 7.3 cm, with a range between 3.5 and 18 cm. There were one well differentiated (Fig. 2), three moderately differentiated and 4 poorly differentiated PPSCC (Fig. 3). Intermediate grade ductal carcinoma in situ was found in one case without squamous metaplasia. No other component has been observed. Three patients had neo adjuvant chemotherapy.
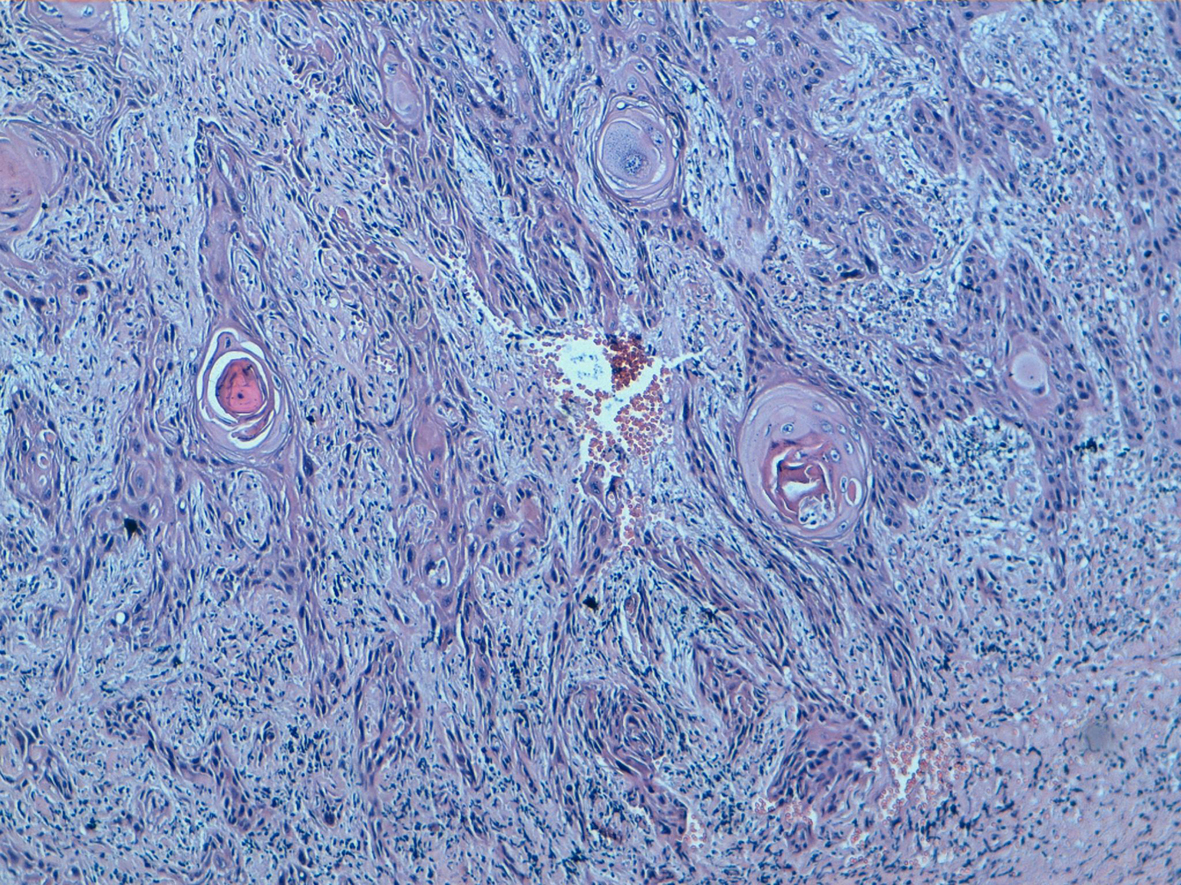 Click for large image | Figure 2. Well differentiated squamous cell carcinoma keratinising type with sheets of malignant squamous cells demonstrating keratin production (HE, 100). |
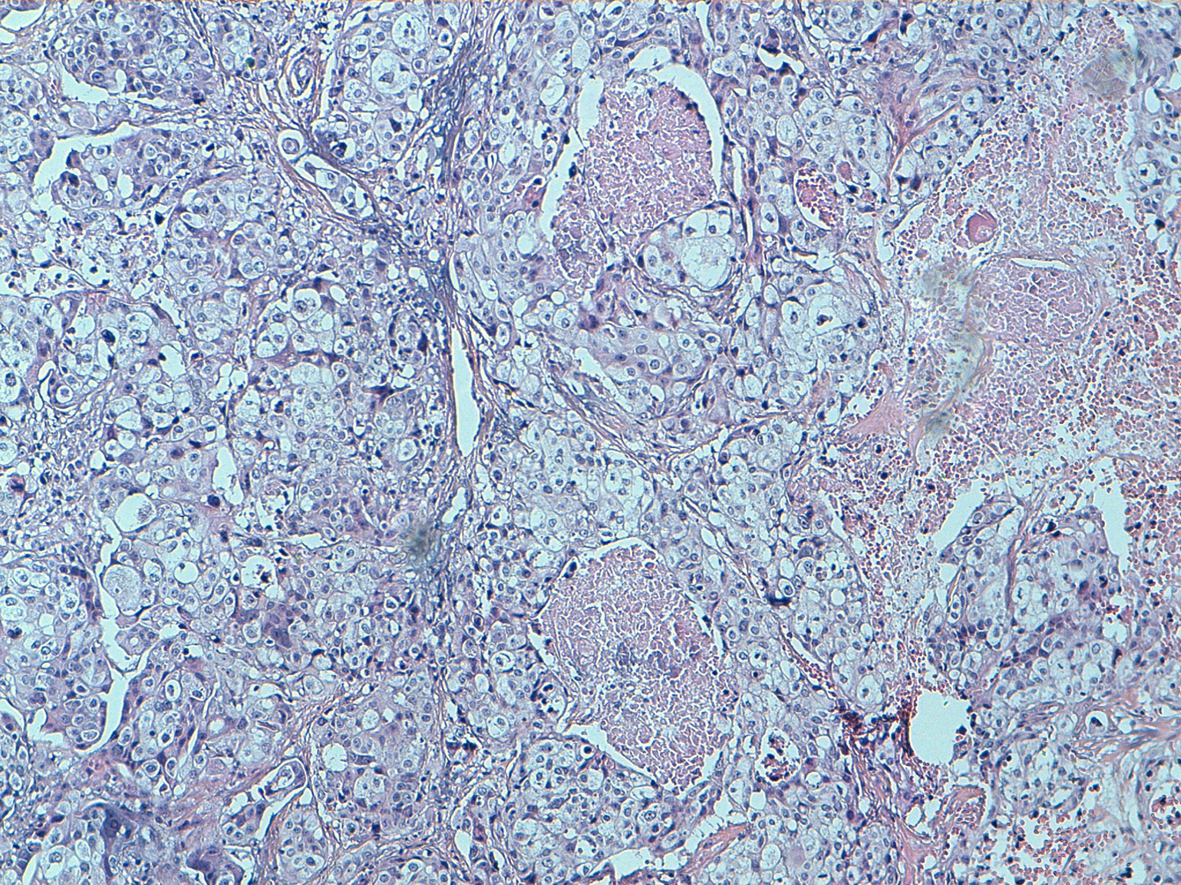 Click for large image | Figure 3. Poorly squamous cell carcinoma: no keratinising type showing variously shaped spaces lined by squamous cells (HE, 100). |
The evaluation of therapeutic response based on Sataloff classification was as following: two cases were T-C, N-B and one case was T-C, N-C.
Axillary node dissection results were available for all patients. Eight lymph nodes metastasis were identified in one case, and no positive lymph nodes were identified among the remaining six cases. Two cases were stage IIA, three cases stage IIIA, and two cases stage IIIB.
The tumors had basal-like phenotype. Immunohistochemical staining for estrogen and progesterone receptors and HER-2/neu oncoprotein were negative whereas Ck5/6 and/or Ck14 were positive (Fig. 4). The patients’ Ki-67 proliferation index was high (in 50-75% of tumoral cells).
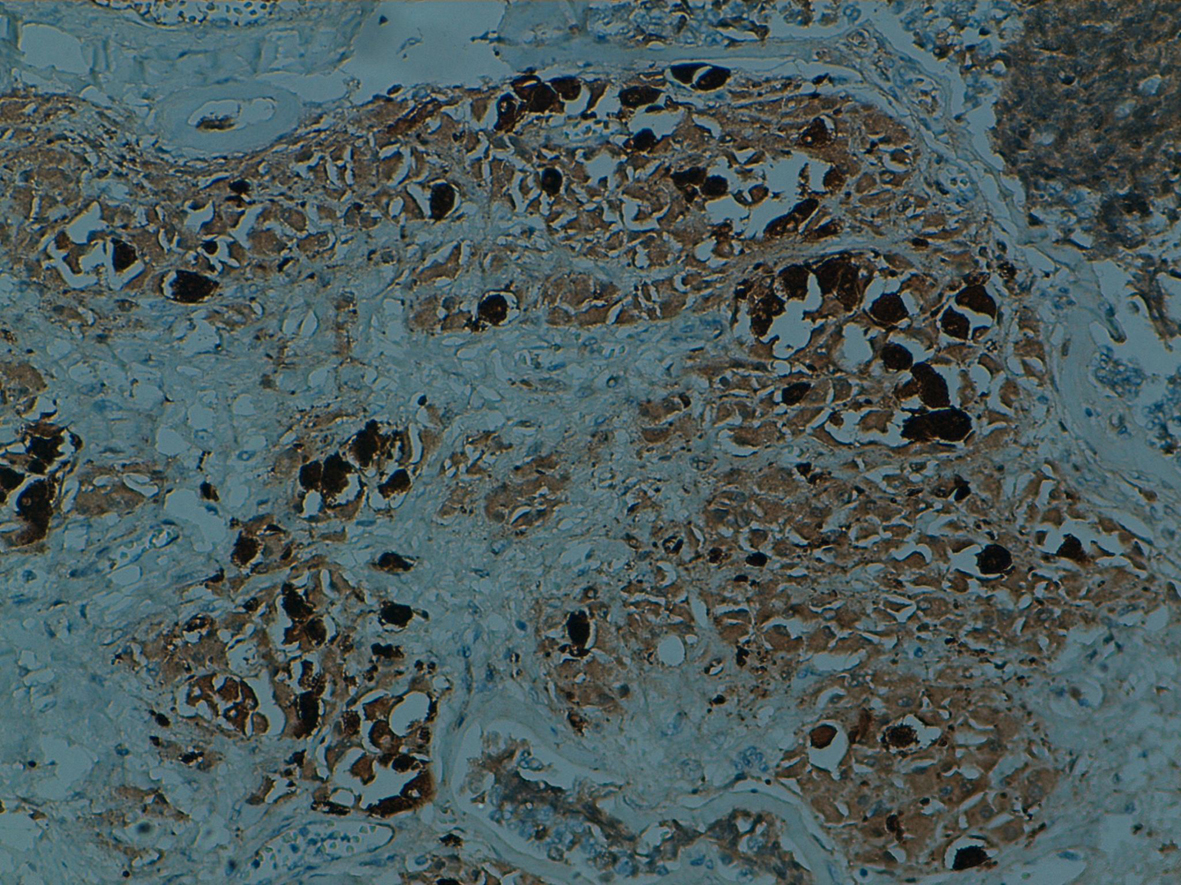 Click for large image | Figure 4. Immunostain for Ck5/6 is positive at squamous cells. |
The patients were treated with adjuvant radiotherapy, receiving a total dose of 50 Gy. Three patients, with locally advanced tumors, received neo-adjuvant chemotherapy with cisplatin/docetaxel, whereas four patients received only adjuvant chemotherapy. Three patients were followed from six months to three years with no evidence of recurrence and three patients died. The other two patients were lost to follow.
| Discussion | ▴Top |
PPSCC is a very rare tumor, with a reported incidence of approximately 0.1% of all ductal carcinomas [6]. In our experience, it accounts for more than 0.5% of all invasive breast cancer and 68% of all metaplastic carcinoma. In PPSCC, all or the majority of the cells, are squamous type with keratinization, and the presence of some glandular features should make us disregard this diagnosis [1-9]. These criteria are critical to distinguish true squamous breast cancers from the common ductal carcinoma not otherwise specified with squamous metaplasia.
The tumors are most often seen in postmenopausal women. Our patients’ median age is 48.2 years. Clinical presentation is not different from that of infiltrating duct carcinoma. Most patients present with a well circumscribed palpable mass, with a median size of 3 - 4 cm. In some reports more than half of these tumors measure over 5cm, with some massive lesions (> 20cm) which may displace the nipple and ulcerate through the skin [1-10]. Like our patients, the tumor occurs very rarely as breast abscess. To the best of our knowledge, only six patients with these tumors, which presented clinically as breast abscess, have been reported previously [11]. There are no findings on mammography specific to this diagnosis, which may explain the more-advanced disease stage at diagnosis [12, 13]. Breast ultrasound has been reported to be more helpful with these tumors appearing as solid hypoechogenic masses with complex cystic components [14].
Histogenesis remains controversial. This breast cancer occurs in two clinical situations: (1) complicating benign squamous metaplasia in benign breast conditions without evidence of intraductal carcinoma; and (2) extensive and prominent squamous metaplasia in infiltrating duct carcinoma. The concept of a disease continuum with varying degrees of squamous metaplasia and glandular features was well supported by Stevenson et al [2]. In our report, one tumor included ductal carcinoma in situ without squamous metaplasia which is discordant with that hypothesis.
Macroscopically, the PPSCC present often as large tumors (> 4 cm) at diagnosis. Cystic changes are observed in 50% of the cases [2, 11, 15]. In our experience, the average tumor size was 7.3 cm, with a range of 3.5 - 18 cm.
Microscopically, PPSCC is entirely composed of metaplastic squamous cells that may be keratinizing, non-keratinizing, and less frequently spindle cell and acantholytic types; some show a combination of these patterns. The most bland appearing and well differentiated cells often line cystic spaces. As the tumor cells emanate out to infiltrate the surrounding stroma, they become spindle shaped and loose their squamous features. PPSCC can be graded based mainly on nuclear features and, to a lesser degree, cytoplasmic differentiation. Grading the squamous carcinoma is of uncertain utility [1]. The grading system of usual ductal carcinoma (Nottingham modification of the Bloom-Richardson system) is not applicable to these tumors. Pathologic staging is similar to that used for any mammary carcinoma.
Most of PPSCC are ER, PR, and HER2 (“triple”) negative. The expression of Cks of high molecular weight, including Ck5/6, Ck14 and Ck17, can be used to identify these lesions reliably on tissue sections fixed in formalin and embedded in paraffin [16-18]. Our results support this observation. Indeed all tumors were negative of ER, PR, Her 2 neu or expressed Ck5/6 and/ or Ck14.
These tumors are also associated with a significantly lower rate of axillary lymph node metastasis compared to usual ductal adenocarcinoma of the breast [1, 5]. Metastases to axillary nodes in all patients with PPSCC have been reported to range from 10% to 30% [1, 4, 5]. This is lower than the reported rates of 40-60% in usual ductal adenocarcinomas. This is unlike cutaneous squamous cell carcinomas that tend to metastasize to regional lymph nodes. In fact, many authors report that PPSCC is likely to skip regional nodes and present with distant metastasis, with rates reported in the range of 30-33% [1, 4, 5].
The prognosis is still a subject of controversy; some reports suggest that it is aggressive, with an outcome comparable to poorly differentiated ductal carcinoma of the breast [1, 5, 7, 9]. Large tumor size and positive lymph node status were found to be the principle features of poor prognosis [1].
Rapidly progressive PPSCC is reported to be associated with a prominent spindle cell component or the presence of necrosis and acantholytic features. Yamagachi et al indicate that the presence of high-grade spindle cells in PPSCC was at least one important prognostic factor [19]. The 5-year disease-free survival for this tumor has been reported to be 63% [20] and 52% [21].
Because of its rarity the most appropriate therapeutic regimen for PPSCC of the breast is still unclear. PPSCC is usually a hormone receptor-negative tumor [3]. This means that hormonebased therapy may not be effective in these tumors. HER2/neu is also usually not over-expressed or amplified in this disease [12]. A little review suggests that PPSCC is not sensitive to chemotherapeutic agents commonly used for ductal carcinoma [6, 22]. In our experience, three patients received neo adjuvant chemotherapy and had a low therapeutic response on histologic examination. The high frequency of EGFR positivity is interesting. The use of anti-EGFR, combined with synergic cytotoxic agents such as Platin or Taxanes, should be investigated in clinical trials which should be ideally multicentric, given the low frequency of this tumor [23]. The role of radiation has been reported as unclear in many studies. Indeed locoregional relapse occurred frequently also in irradiated field.
In conclusion, PPSCC of the breast is a very rare and aggressive disease. In our experience it’s relatively frequent. Strict histologic criteria should be used to diagnose PPSCC, because they have significantly lower axillary lymph node metastasis, lower estrogen and PR positivity, and are often treatment-refractory. The role of different new chemotherapy regimens needs to be explored and biologic studies are needed to determine the tumor chemoresistance mechanisms and the potential use of other treatment targets that improve patients’ survival.
Conflict of Interest
There is no conflict of interest and no study sponsors. The authors have obtained the patients’ written informed consent for print and electronic publication of the case report.
| References | ▴Top |
- Behranwala KA, Nasiri N, Abdullah N, Trott PA, Gui GP. Squamous cell carcinoma of the breast: clinico-pathologic implications and outcome. Eur J Surg Oncol 2003;29(4):386-389.
pubmed doi - Stevenson JT, Graham DJ, Khiyami A, Mansour EG. Squamous cell carcinoma of the breast: a clinical approach. Ann Surg Oncol 1996;3(4):367-374.
pubmed doi - World Health Organization classification of Tumours. In: Tavassoli FA, Devilee P, editors. Tumours of. Pathology and genetics of tumors of the breast and female genital organs. Lyon, France: IARC Press; 2003. p. 37-41.
- Zanconati F, Zanella M, Falconieri G, Di Bonito L. Gestational squamous cell carcinoma of the breast: an unusual mammary tumor associated with aggressive clinical course. Pathol Res Pract 1997;193(11-12):783-787; discussion 789-790.
pubmed - Menes T, Schachter J, Morgenstern S, Fenig E, Lurie H, Gutman H. Primary squamous cell carcinoma (SqCC) of the breast. Am J Clin Oncol 2003;26(6):571-573.
pubmed doi - Aparicio I, Martinez A, Hernandez G, Hardisson D, De Santiago J. Squamous cell carcinoma of the breast. Eur J Obstet Gynecol Reprod Biol 2008;137(2):222-226.
pubmed doi - Moisidis E, Ahmed S, Carmalt H, Gillett D. Primary squamous cell carcinoma of the breast. ANZ J Surg 2002;72(1):65-67.
pubmed doi - Macia M, Ces JA, Becerra E, Novo A. Pure squamous carcinoma of the breast. Report of a case diagnosed by aspiration cytology. Acta Cytol 1989;33(2):201-204.
pubmed - Tayeb K, Saadi I, Kharmash M, Hadadi K, El Omari-Alaoui H, El Ghazi E, Mansouri A, et al. [Primary squamous cell carcinoma of the breast. Report of three cases]. Cancer Radiother 2002;6(6):366-368.
pubmed doi - Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K. Characteristics and treatment of metaplastic breast cancer: analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol 2007;14(1):166-173.
pubmed doi - Gupta C, Malani AK, Weigand RT, Rangineni G. Pure primary squamous cell carcinoma of the breast: a rare presentation and clinicopathologic comparison with usual ductal carcinoma of the breast. Pathol Res Pract 2006;202(6):465-469.
pubmed doi - Siegelmann-Danieli N, Murphy TJ, Meschter SC, Stein ME, Prichard J. Primary pure squamous cell carcinoma of the breast. Clin Breast Cancer 2005;6(3):270-272.
pubmed doi - Comellas N, Marin Gutzke M. Primary pure squamous cell carcinoma of the breast presenting as a breast abscess. J Plast Reconstr Aesthet Surg 2009;62(6):e178-179.
pubmed doi - Shigekawa T, Tsuda H, Sato K, Ueda S, Asakawa H, Shigenaga R, Hiraide H, et al. Squamous cell carcinoma of the breast in the form of an intracystic tumor. Breast Cancer 2007;14(1):109-112.
pubmed doi - Nakayama K, Abe R, Tsuchiya A, Watanabe T, Furukawa Y, Nihei M, Kimijima I. Squamous cell carcinoma of the breast. Report of a case diagnosed by fine needle aspiration cytology. Acta Cytol 1993;37(6):961-965.
pubmed - Reis-Filho JS, Milanezi F, Steele D, Savage K, Simpson PT, Nesland JM, Pereira EM, et al. Metaplastic breast carcinomas are basal-like tumours. Histopathology 2006;49(1):10-21.
pubmed doi - Banerjee S, Reis-Filho JS, Ashley S, Steele D, Ashworth A, Lakhani SR, Smith IE. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol 2006;59(7):729-735.
pubmed doi - Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol 2006;37(9):1217-1226.
pubmed doi - Yamaguchi R, Horii R, Maeda I, Suga S, Makita M, Iwase T, Oguchi M, et al. Clinicopathologic study of 53 metaplastic breast carcinomas: their elements and prognostic implications. Hum Pathol 2010;41(5):679-685.
pubmed doi - Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast. IV. Squamous cell carcinoma of ductal origin. Cancer 1990;65(2):272-276.
pubmed doi - Grenier J, Soria JC, Mathieu MC, Andre F, Abdelmoula S, Velasco V, Morat L, et al. Differential immunohistochemical and biological profile of squamous cell carcinoma of the breast. Anticancer Res 2007;27(1B):547-555.
pubmed - Cardoso F, Leal C, Meira A, Azevedo R, Mauricio MJ, Leal da Silva JM, Lopes C, et al. Squamous cell carcinoma of the breast. Breast 2000;9(6):315-319.
pubmed doi - Geyer FC, Weigelt B, Natrajan R, Lambros MB, de Biase D, Vatcheva R, Savage K, et al. Molecular analysis reveals a genetic basis for the phenotypic diversity of metaplastic breast carcinomas. J Pathol 2010;220(5):562-573.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


