| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 12, Number 4, April 2021, pages 164-171
Pulmonary Adenosquamous Cell Carcinoma With Systemic Lymphadenopathy due to Immunoglobulin G4-Related Disease: A Case Report
Saori Ikebea, Seigo Minamia, b, d, Shouichi Iharaa, b, Hironao Yasuokac, Kiyoshi Komutab
aDepartment of Respiratory Medicine, Osaka Police Hospital, 10-31 Kitayama-cho, Tennoji-ku, Osaka 543-0035, Japan
bDepartment of Respiratory Medicine, Daini Osaka Police Hospital, 2-6-40 Karasuga-tuji, Tennoji-ku, Osaka 543-0042, Japan
cDepartment of Pathology, Osaka Police Hospital, 10-31 Kitayama-cho, Tennoji-ku, Osaka 543-0035, Japan
dCorresponding Author: Seigo Minami, Department of Respiratory Medicine, Osaka Police Hospital, 10-31 Kitayama-cho, Tennoji-ku, Osaka-City, Osaka 543-0035, Japan
Manuscript submitted January 11, 2021, accepted January 27, 2021, published online February 8, 2021
Short title: Adenosquamous Carcinoma and IgG4-RD
doi: https://doi.org/10.14740/jmc3649
| Abstract | ▴Top |
A 75-year-old man with diabetes mellitus showed elevated C-reactive protein (CRP) level at his regular visit. Computed tomography scan showed a lung tumor in his left lower lobe and systemic lymphadenopathy including abdominal lymph nodes. The patient was diagnosed as primary pulmonary squamous cell carcinoma with systemic lymph node metastasis. Thereafter, unexpected steroid pulse therapy for accidental acute exacerbation of interstitial pneumonia rapidly shrank lymphadenopathy. At this time, we also found elevated serum immunoglobulin G4 (IgG4) level (385 mg/dL). Considering these findings, we doubted the lymph nodes metastases at the initial staging, and then corrected cancer-staging (C-staging) from inferior vena cava (IVC) to inferior abdomen (IA). In addition, during the steroid tapering, sudden onset and uncontrollable left pneumothorax required surgical approach. Curative-intent left lower lobectomy with lymphadenectomy was performed for the lung cancer. Pathological findings revealed coexistence of adenosquamous carcinoma and infiltration of IgG4-positive plasma cells in the resected mediastinal lymph node. We detected 384 IgG4-positive cells per high power field. IgG4/IgG-positive cell ratio was 54%. Based on these findings, the diagnosis of IgG4-related disease with primary adenosquamous carcinoma (p-stage IIIA) was confirmed. The patient died 24 days after surgery because of another acute exacerbation of interstitial pneumonia. Our case alerts oncologists to IgG4-related disease as a possible underlying comorbidity which may confuse pretreatment clinical stage.
Keywords: Adenosquamous cell carcinoma; IgG4-related disease; Interstitial pneumonia; Lymphadenopathy; Acute exacerbation; Fludeoxyglucose-positron emission tomography
| Introduction | ▴Top |
Immunoglobulin G4 (IgG4)-related disease is a systemic syndrome characterized by tissue infiltration of small lymphocytes and IgG4-positive plasma cells, and following fibrosis. This disease affects pancreas, salivary glands, artery, thyroid, lung, and many other organs. It remains controversial whether IgG4-related disease is associated with the risk of malignancy or not. In some studies, malignancy increased in IgG4-related disease [1, 2] or only in autoimmune pancreatitis [3, 4]. On the contrary, a Japanese study could not find any difference in the incidence of total malignancies between 113 patients with IgG4-related disease and a general population [5].
Serum level of IgG4 elevates in most cases. Although the diagnosis is based on biopsy, it is sometimes difficult to obtain pathological specimen. The diagnostic criteria of IgG4-related disease are defined by the following three findings: clinical manifestations, elevated serum IgG4 level, and pathological characteristics. Clinical manifestations of IgG4-related disease are similar to cancer-related manifestations in some cases. In a UK study of 53 patients with IgG4-related disease, 22 patients (41.5%) were categorized as definite, highly probable or probable thoracic involvement [6]. The following four patterns of radiological manifestation have been described for IgG4-related lung disease: solid nodular, bronchovascular, alveolar interstitial, and round shaped ground-glass opacity type [7]. These patterns mimic various lung diseases, including lung cancer, interstitial pneumonia and sarcoidosis.
Herein, we report a case of co-existence of pulmonary adenosquamous cell carcinoma and IgG4-related disease manifested by systemic lymphadenopathy, which confused our clinical staging and treatment strategy.
| Case Report | ▴Top |
A 75-year-old man with diabetes mellitus showed elevated C-reactive protein (CRP) level at his regular visit. Computed tomography (CT) scan found a tumor in the left lower lobe, interstitial pneumonia (Fig. 1a), and systemic lymphadenopathy including peri-gastric and peri-pancreatic lymph nodes. Bronchoscopic biopsy pathologically diagnosed the lung tumor as a squamous cell carcinoma in the middle of July 2016.
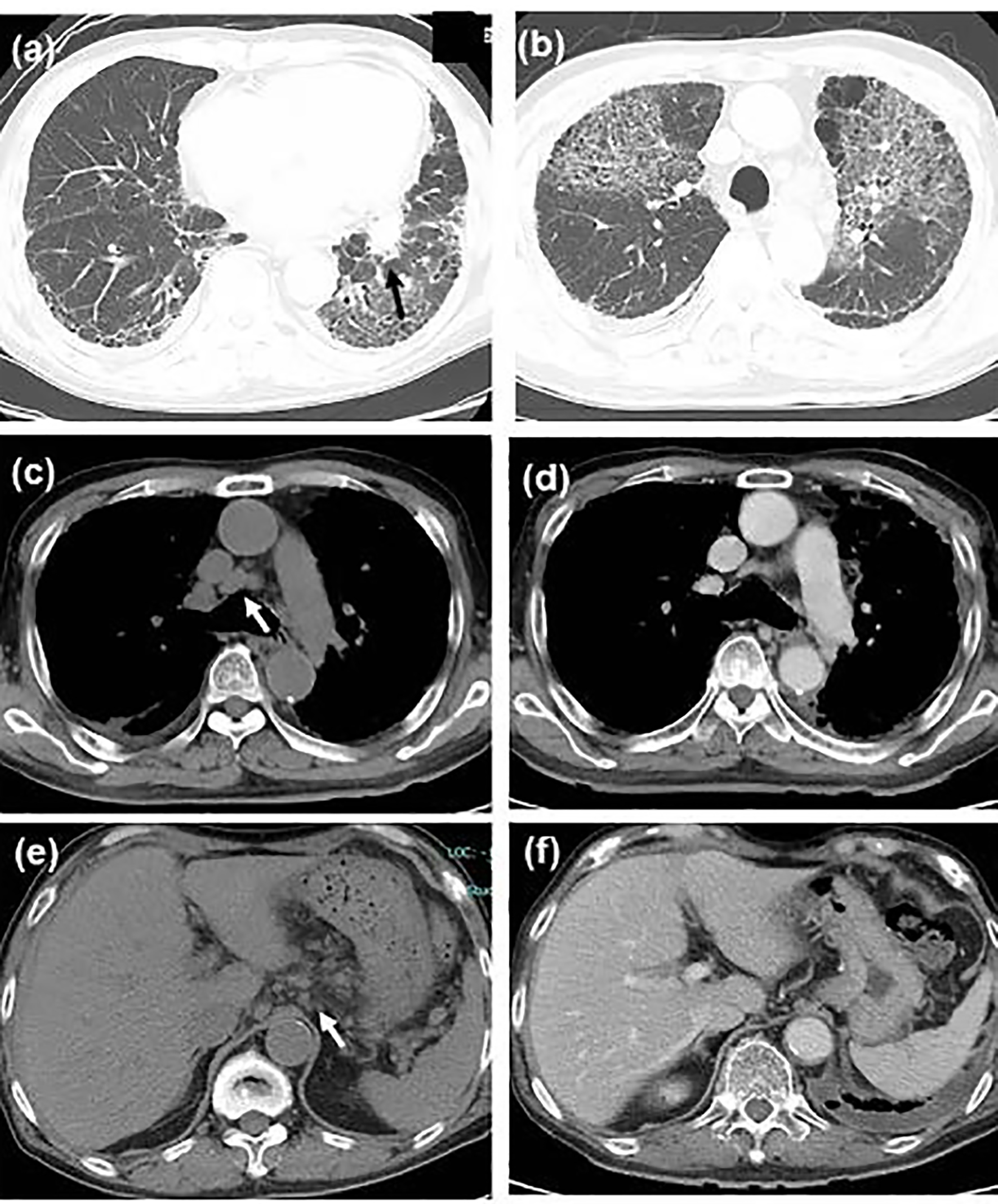 Click for large image | Figure 1. Chest computed tomography (CT) scan showing (a) the primary tumor in the left lower lobe (shown by black arrow) and bilateral sub-pleural reticular shadows in the bilateral lower lobes, (b) the first acute exacerbation of interstitial pneumonia, remarkable shrinkage of (c, d) mediastinal and (e, f) peri-gastric lymphadenopathy (c, e) before steroid therapy (shown by white arrows, in the middle of September) and (d, f) during steroid tapering (in the late October). |
Esophagogastroduodenoscopy, endoscopic sonography, and contrast-enhanced magnetic resonance imaging (MRI) could not detect abdominal malignancy. Enhanced brain CT did not find brain metastasis. Fludeoxyglucose-positron emission tomography (FDG-PET) showed abnormal uptake in the lung tumor in the left lower lobe (maximum standardized uptake value (SUVmax), 4.7), and systemic lymph nodes in the supraclavicular fossa (SUVmax, 6.8), subaortic arch (SUVmax, 4.0), gastric cardia (SUVmax, 3.5), peri-pancreas (SUVmax, 4.3), superior mesenteric arterial root (SUVmax, 5.1) (Fig. 2). Considering these findings, we diagnosed the lymphadenopathy as metastasis of lung cancer, and then planned chemotherapy for clinical stage IV cancer. However, acute exacerbation of interstitial pneumonia canceled the pre-planned chemotherapy in the middle of September (Fig. 1b). We immediately started intravenous pulse-steroid therapy at the dose of 1 g/day of methylprednisolone during the first consecutive 3 days, and thereafter gradually tapered steroid dose from 40 mg/day of prednisolone. During the steroid tapering, left pneumothorax suddenly occurred. Aspiration from two chest drainage tubes failed to control air leakage. Thus, we planned surgical approach for uncontrollable pneumothorax. Another CT scan before surgery revealed remarkable shrinkage of systemic lymph nodes (Fig. 1 c-f). We doubted the decision of lymph nodes metastases at the initial staging, and then corrected C-staging from C-stage inferior vena cava (IVC) to C-stage inferior abdomen (IA) (cT1bN0M0). We also found elevated IgG4 level (385 mg/dL), KL-6 (661 U/mL) and SP-D (407 ng/mL) in the serum obtained before the steroid pulse therapy, and then decreased IgG4 level (193 mg/dL) and SP-D (67.6 ng/mL), but stable KL-6 (675 U/mL) in the serum obtained during the steroid tapering and just before the subsequent operation. There was no radiological indication of IgG4-related disease in other organs. Curative-intent left lower lobe lobectomy with lymphadenectomy was performed for the lung cancer at the end of October. The pneumothorax was successfully cured and did not recur thereafter. The resected left lower lobe contained mainly squamous cell carcinoma with keratinization (Fig. 3a), partial component of adenocarcinoma (Fig. 3b), and interstitial pneumonia (favor for non-specific interstitial pneumonia (NSIP)) with marked lymphoplasmacytic cell infiltration into interstitium of interlobular septal walls (Fig. 3c, d). Both resected lung and mediastinal lymph nodes contained epithelioid granulomas with necrosis, suggesting complication of pulmonary tuberculosis (Fig. 3e, f). Thereafter, mycobacterium tuberculosis was found to be negative in smear, but positive in culture of post-operative sputum specimen. Combination of four anti-tuberculosis drugs (isoniazid, rifampicin, ethambutol and pyrazinamide) started 18 days after the surgery. The resected mediastinal lymph nodes contained not only malignant cells (Fig. 3f) but also IgG4-positive plasma cells (Fig. 4). We could not detect typical pathological findings of IgG4-related disease, such as obliterative phlebitis or storiform-fibrosis. Pathological staging was determined as pT2aN2M0. We detected 384 IgG4-positive cells per high power field, and IgG4/IgG-positive cell ratio was 54% (Fig. 4). These findings confirmed the diagnosis of IgG4-related disease. Infiltration of IgG4-positive cells was also seen in lung tissue of diffuse ground-glass opacity, suggesting IgG4-related interstitial pneumonia. Another acute exacerbation of interstitial pneumonia occurred 8 days after surgery. Despite of steroid therapy, the patient died 24 days after surgery in late November.
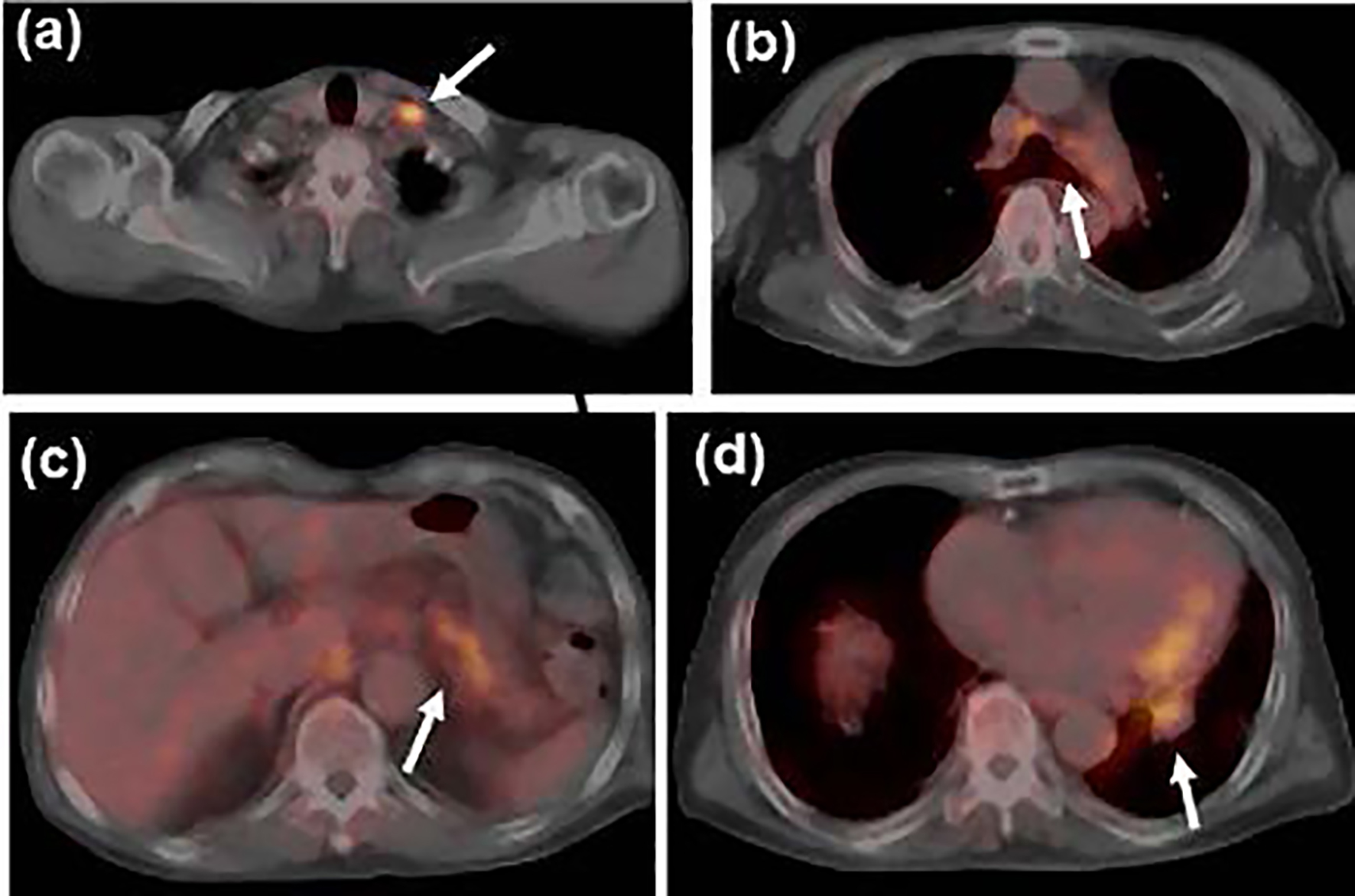 Click for large image | Figure 2. Fludeoxyglucose-positron emission tomography (FDG-PET) scan at the initial staging before steroid therapy showed uptake in (a) the left supraclavicular, (b) mediastinal and (c) peri-gastric lymph nodes, and (d) the primary tumor in the left lower lobe (shown by white arrows). |
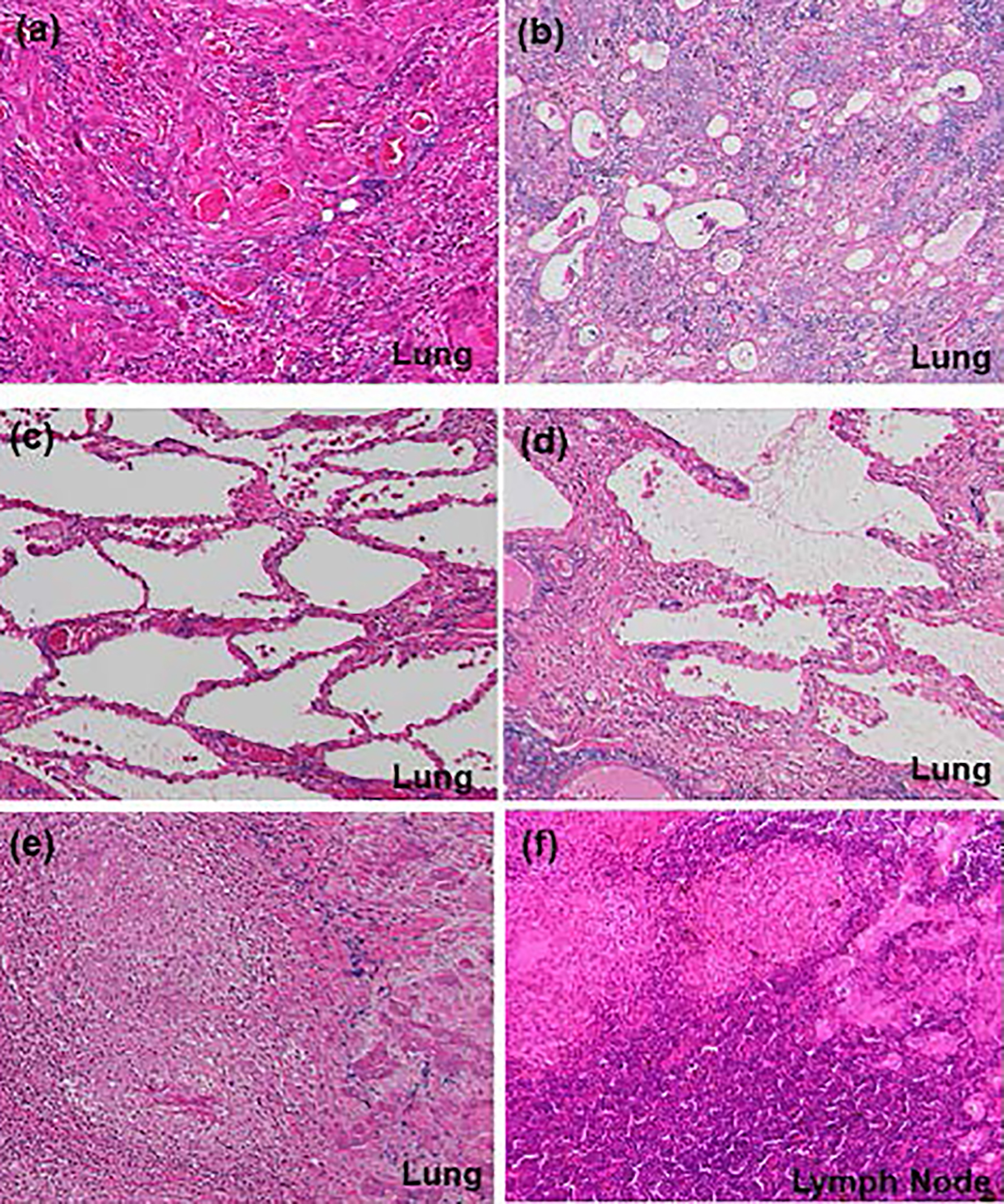 Click for large image | Figure 3. Histological findings (hematoxylin and eosin (H&E) stain, × 10) of (a) main component of squamous cell carcinoma and (b) partial component of adenocarcinoma in the primary tumor, (c) interstitial pneumonia (favor for non-specific interstitial pneumonia), (d) lymphoplasmacytic cell infiltration into interstitium of interlobular septal walls, (e) coexistence of epithelioid granulomas and primary squamous cell carcinoma in the left lower lobe, and (f) coexistence of epithelioid granulomas and metastatic lesions in the mediastinal lymph nodes. (e, f) These findings suggest complication of pulmonary tuberculosis and lung cancer. |
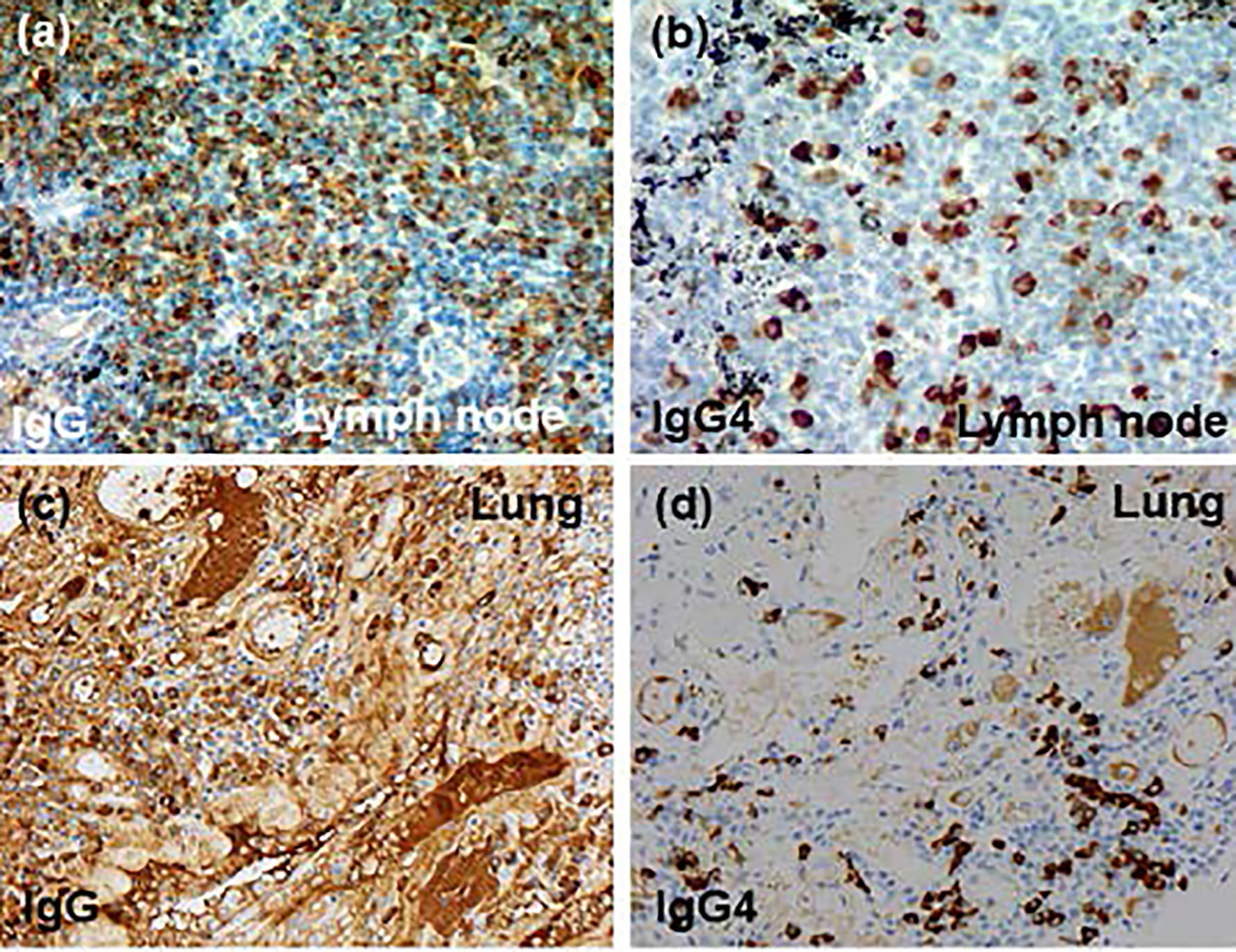 Click for large image | Figure 4. Immunohistochemical findings (× 20): (a, b) mediastinal lymph node, (c, d) lung tissue, (a, c) anti-IgG antibody and (b, d) anti-IgG4 antibody. IgG4: immunoglobulin G4. |
| Discussion | ▴Top |
This was a rare case of co-existence of locally advanced pulmonary adenosquamous cell carcinoma and IgG4-related disease manifested by systemic lymphadenopathy. To our knowledge, there were only nine case reports of lung cancer concomitant with IgG4-related disease [8-16] (Table 1).
 Click to view | Table 1. A Review of English or Japanese Case Reports of Coexistence of Lung Cancer and IgG4-Related Disease |
A unifying diagnostic criterion has not been established, because various organs are involved in this disease. The current diagnosis of IgG4-related disease is based either on comprehensive or organ-specific diagnostic criteria. According to the comprehensive diagnostic criteria for IgG4-related disease published in 2011 by the Japanese IgG4 team, organized by the Ministry of Health, Labor and Welfare (MHLW) of Japan [17], our case met the three diagnostic factors: chest imaging, serology and histology. Based on the eight weighted inclusion criteria domains, the third step of classification process, of the 2019 American College of Rheumatology/European League against Rheumatism Classification Criteria for IgG4-related Disease [18], our case presented dense lymphocytic infiltrate (+4 points), the IgG4/IgG ratio 41-70% and the number of IgG4-positive cells/high power field > 10 cells (+14 points), and 2 - 5 × upper limit of normal serum IgG4 concentration (+6 points). Thus, our case exceeded the threshold of 20 points. In light of the diagnostic criteria for IgG4-related respiratory disease proposed by Matsui et al in 2016 [19], our case met the chest imaging of hilar and mediastinal lymphadenopathy, the elevated serum IgG4 concentration, marked lymphoplasmacytic cell infiltration into interstitium of interlobular septal wall, and the IgG4/IgG-positive cell ratio > 40% and > 10 IgG4-positive cells/high power field. Thus, our case was a possible diagnosis of IgG4-related respiratory disease. The latter two diagnostic criteria were published after our patient was already dead or at the same time of our surgery, respectively. Despite of satisfaction of these various inclusion criteria, our case did not meet the exclusion criteria due to pulmonary comorbidities of lung cancer and tuberculosis. A significant correlation between malignancy and IgG4-related disease has indicated IgG4-related disease as a paraneoplastic syndrome, rather than an independent disease [20]. Furthermore, we could not completely exclude other similar diseases, because we did not examine various serological markers suggestive of another systemic autoimmune disease, vasculitis or sarcoidosis such as antinuclear antibody, rheumatoid factor, and anti-neutrophil cytoplasmic antibody (ANCA). Thus, in the current and latest diagnostic criteria, our diagnosis was not enough for the strict definition of IgG4-related disease.
We thought it impossible to distinguish IgG4-related lymphadenopathy from lymph node metastases only by routine images of clinical staging. Either endobronchial ultrasound-guided transbronchial needle biopsy (EBUS-TBNB) or mediastinoscopical biopsy should be considered aggressively for confusing lymphadenopathy. In our case, neither pre-staging enhanced CT scan nor FDG-PET could detect the underlying IgG4-related disease. As a result, the remarkable shrinkage of lymph nodes after unexpected steroid therapy reminded us of IgG4-related disease. Surgical resection finally determined the IgG4-related systemic lymphadenopathy, and then down-staged pre-surgical clinical staging from IV to IIIA. In fact, most previous cases required surgical biopsy for the diagnosis of IgG4-related disease (Table 1). We oncologists have to keep in mind a malignancy mimicker, IgG4-related disease [21].
The second important finding was post-surgical acute exacerbation of IgG4-related lung disease, NSIP pattern. In a UK study, interstitial lung disease of NSIP pattern was observed in 22.7% of IgG4-related thoracic disease. In this study, among four dead patients with thoracic involvement, NSIP was considered to have contributed to the patient’s death in two cases [6]. On the other hand, according to two Japanese nationwide surveys of 744 and 1,763 lung surgeries for lung cancer with idiopathic interstitial pneumonia, the prevalence of acute exacerbation following surgery was 8.3% (in 62 cases) and 9.3% (in 164 patients ), and the mortality rate was 3.5% (in 26 cases) and 2.6% (in 46 cases), respectively [22, 23]. Among seven independent risk factors of acute exacerbation identified in the previous study [23], our case met at least four risk factors: surgical procedure of lobectomy, male gender, history of exacerbation and preoperative steroid use. We could not access percent vital capacity preoperatively. Thus, in terms of interstitial lung diseases, post-surgical acute exacerbation might be very likely in our case. There was no report of post-operative acute exacerbation and mortality in IgG4-related lung disease. Our case, in which acute exacerbation occurred soon after lung surgery, calls physicians’ attention to a risk of post-operative acute exacerbation.
Conclusion
In conclusion, our case alerts oncologists to IgG4-related disease as a possible underlying comorbidity which confuses pretreatment clinical staging and treatment strategy.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable because the manuscript has been sufficiently de-identified to protect the patient.
Author Contributions
S. Ikebe, S. Minami, S. Ihara and K. Komuta were involved in treatment of this patients. H. Yasuoka performed the pathological diagnosis. S. Ikebe drafted the report. All authors read and critically reviewed the report. All authors approved the final submitted version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Ahn SS, Song JJ, Park YB, Lee SW. Malignancies in Korean patients with immunoglobulin G4-related disease. Int J Rheum Dis. 2017;20(8):1028-1035.
doi pubmed - Yamamoto M, Takahashi H, Tabeya T, Suzuki C, Naishiro Y, Ishigami K, Yajima H, et al. Risk of malignancies in IgG4-related disease. Mod Rheumatol. 2012;22(3):414-418.
doi pubmed - Gupta R, Khosroshahi A, Shinagare S, Fernandez C, Ferrone C, Lauwers GY, Stone JH, et al. Does autoimmune pancreatitis increase the risk of pancreatic carcinoma?: a retrospective analysis of pancreatic resections. Pancreas. 2013;42(3):506-510.
doi pubmed - Shiokawa M, Kodama Y, Yoshimura K, Kawanami C, Mimura J, Yamashita Y, Asada M, et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol. 2013;108(4):610-617.
doi pubmed - Hirano K, Tada M, Sasahira N, Isayama H, Mizuno S, Takagi K, Watanabe T, et al. Incidence of malignancies in patients with IgG4-related disease. Intern Med. 2014;53(3):171-176.
doi pubmed - Corcoran JP, Culver EL, Anstey RM, Talwar A, Manganis CD, Cargill TN, Hallifax RJ, et al. Thoracic involvement in IgG4-related disease in a UK-based patient cohort. Respir Med. 2017;132:117-121.
doi pubmed - Inoue D, Zen Y, Abo H, Gabata T, Demachi H, Kobayashi T, Yoshikawa J, et al. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology. 2009;251(1):260-270.
doi pubmed - Abbass K, Krug H. Granulomatosis with polyangiitis in a patient with biopsy-proven IgG4-related pulmonary disease and coincident small cell lung cancer. BMJ Case Rep. 2019;12(3):e226280.
doi pubmed - Choi S, Park S, Chung MP, Kim TS, Cho JH, Han J. A rare case of adenosquamous carcinoma arising in the background of IgG4-related lung disease. J Pathol Transl Med. 2019;53(3):188-191.
doi pubmed - Gomez Hernandez MT, Rodriguez Alvarado I, Novoa N, Jimenez Lopez MF. Immunoglobulin G4-Related Lung Disease as an Incidental Finding After Surgical Resection of Lung Cancer. Arch Bronconeumol. 2019;55(5):276-278.
doi pubmed - Ikari J, Kojima M, Tomita K, Nakamura T, Toyoda F, Otsuki Y, Shimizu S, et al. A case of IgG4-related lung disease associated with multicentric Castleman's disease and lung cancer. Intern Med. 2010;49(13):1287-1291.
doi pubmed - Inoue T, Hayama M, Kobayashi S, Oyaizu T, Nakazato Y, Honma K, Chida M. Lung cancer complicated with IgG4-related disease of the lung. Ann Thorac Cardiovasc Surg. 2014;20(Suppl):474-477.
doi pubmed - Ito Y, Harada M, Kagoo N, Kubota T, Ichijyo K, Mochizuki E, Uehara M, et al. Axillary lymphadenopathy with IgG4 positive plasma cell infiltration as differential diagnosis of metastatic lung adenocarcinoma. Respir Med Case Rep. 2020;31:101196.
doi pubmed - Tashiro H, Takahashi K, Nakamura T, Komiya K, Kimura S, Sueoka-Aragane N. Coexistence of lung cancer and immunoglobulin G4-related lung disease in a nodule: a case report. J Med Case Rep. 2016;10(1):113.
doi pubmed - Terashima T, Iwami E, Shimada T, Kuroda A, Matsuzaki T, Nakajima T, Sasaki A, et al. IgG4-related pleural disease in a patient with pulmonary adenocarcinoma under durvalumab treatment: a case report. BMC Pulm Med. 2020;20(1):104.
doi pubmed - Tokuda K, Nakaya T, Nakayama M, Bando M, Sugiyama Y, Hagiwara H. Primary lung adenocarcinoma associated with IgG4-related lung disease. Annals of the Japanese Respiratory Society. 2018;7(1):20-24.
- Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22(1):1-14.
doi pubmed - Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire JF, Hart PA, et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. 2020;79(1):77-87.
doi pubmed - Matsui S, Yamamoto H, Minamoto S, Waseda Y, Mishima M, Kubo K. Proposed diagnostic criteria for IgG4-related respiratory disease. Respir Investig. 2016;54(2):130-132.
doi pubmed - Wang Y, Zhu J. Need for caution in diagnosing IgG4-Related disease: a possible paraneoplastic syndrome? Comment on the Article by Wallace et al. Arthritis Rheumatol. 2017;69(3):681-682.
doi pubmed - Haider M, Haji F, Alalwan O, Aljufairi E, Shah TS. IgG4-Related Disease, the Malignancy Mimicker: Case Series from Bahrain. Case Rep Rheumatol. 2018;2018:4057024.
doi pubmed - Miyamoto A, Kishi K, Yoshimura K. [A nationwide survey concerning lung surgery for lung cancer associated with idiopathic interstitial pneumonia]. Nihon Kokyuki Gakkai Zasshi. 2011;49(2):148-150.
- Sato T, Teramukai S, Kondo H, Watanabe A, Ebina M, Kishi K, Fujii Y, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2014;147(5):1604-1611 e11603.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


