| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 1, January 2022, pages 5-10
Difficulties in Differentiating Hypertensive Disorders of Pregnancy With Polyarteritis Nodosa
Tamaki Wadaa, Emi Kondoa, b, Eiji Shibataa, Toshihide Sakuragia, Kazuaki Iioa, Takayuki Uchimuraa, Yasuyuki Kinjoa, Midori Murakamia, Kiyoshi Yoshinoa
aDepartment of Obstetrics and Gynecology, Institution of University of Occupational and Environmental Health, Kitakyushu, Fukuoka, Japan
bCorresponding Author: Emi Kondo, Department of Obstetrics and Gynecology, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu, Fukuoka 857-8556, Japan
Manuscript submitted October 18, 2021, accepted November 27, 2021, published online January 17, 2022
Short title: Polyarteritis Nodosa in Pregnancy
doi: https://doi.org/10.14740/jmc3813
| Abstract | ▴Top |
Polyarteritis nodosa (PAN) is characterized by medium- or small-sized artery vasculitis with vessel wall inflammation and necrosis of muscular arteries, commonly presenting with fatigue, fever, weight loss, and joint pain. PAN in pregnancy is rare and is associated with worsening of vasculitis after delivery, resulting in myocardial infarction and heart failure which frequently lead to maternal death. We report a case of hypertensive disorders of pregnancy (HDP), which is difficult to differentiate from PAN. A 27-year-old multigravida was diagnosed with PAN 4 years prior after experiencing fever and lower extremity skin rash. During her PAN remission, she conceived her second pregnancy and opted to discontinue PAN medication and declined antihypertensive medications. At 22 weeks of gestation, her blood pressure was elevated to 200/100 mm Hg without proteinuria, for which she was admitted to our hospital. She was diagnosed with HDP-chronic hypertension without PAN recurrence due to the absence of PAN-specific skin or joint symptoms according to the PAN diagnostic criteria. Antihypertensive medication was administered. At 30 weeks of gestation, her blood pressure was poorly controlled and she developed proteinuria, which led to a diagnosis of superimposed preeclampsia that necessitated emergency cesarean section delivery. After delivery, her blood pressure was immediately controlled using antihypertensive medication. Our case report highlights the importance of carefully managing HPD as a serious complication of PAN.
Keywords: Polyarteritis nodosa; Fibrinoid necrosis; Hypertensive disorders of pregnancy; Preeclampsia; Vasculitis
| Introduction | ▴Top |
Polyarteritis nodosa (PAN), first described in 1986, is necrotizing arteritis of medium or small arteries without glomerulonephritis, or arteriolar, venular, or capillary vasculitis [1]. It is not associated with antineutrophil cytoplasmic antibodies (ANCA) [2]. PAN can affect the whole body, manifesting as inflammation-induced constitutional symptoms, such as fever, fatigue, weight, loss, and hypertension. Additionally, it can cause ischemia or infarction-induced organ damage causing cardiac, respiratory, digestive, peripheral nervous, dermatologic, and musculoskeletal symptoms [3].
PAN is diagnosed based on the above clinical symptoms, according to the 1990 classification criteria of the American College of Rheumatology [4], the exclusion of ANCA-related vasculitis, and the histological confirmation of small- and medium-sized artery vasculitis. The most important component of the diagnostic process is to provide pathologic evidence regarding blood vessel inflammation of the affected organ, which necessitates biopsy of the affected area.
The difficulty in diagnosing and classifying vasculitis syndromes has led to uncertainties regarding the incidence of PAN. A previous study found that PAN affects approximately 31 individuals per 1 million [5], and has a predilection toward men aged 40 - 60 years [3]. Without accounting for pregnant women, the 5-year survival rate for PAN is 75-93% [6]. On the contrary, the 5-year survival rate is 88% when the following five prognostic factors are not considered: proteinuria > 1g/day, renal failure, cardiomyopathy, gastrointestinal tract involvement, and central nervous system involvement [6]. If more than two factors are present, the 5-year survival rate decreases to 54% [6, 7].
PAN in pregnancy is rare, with only three reported cases in the past decade [8-10]. Pregnancy outcomes are dependent on PAN severity during pregnancy [11]. It is rare for pregnancy to occur after PAN; most cases of PAN occur during pregnancy [12]. Pregnant women during the remission period have relatively good pregnancy outcomes; however, the prognosis is poor if PAN is first diagnosed during pregnancy, which may necessitate therapeutic abortion in 5-10% of patients [11, 12]. Regardless of the time of onset, approximately 40% of PAN in pregnancy is associated with hypertensive disorders of pregnancy (HDP), which can cause vasculitis in the maternal blood vessels of the placental bed [8]. Herein, we report a case of HDP, which was difficult to differentiate from PAN, and present a review of related literature.
| Case Report | ▴Top |
Investigations
A 27-year-old, woman, gravida 2 para 1, visited the hospital for prenatal check-up. At 18 years of age, she was diagnosed with hypertension; at 19 years, she conceived and underwent normal spontaneous delivery of her first child. At 23 years of age, she experienced spontaneous high-grade fever (40 °C), joint pain, and leg rash, in addition to hypertension. She sought medical advice and was diagnosed with PAN because histological skin biopsy showed medium-sized artery vasculitis with fibrinoid necrosis. She was treated with oral corticosteroids (60 mg/day) and methotrexate (8 mg/day) as remission induction therapy; subsequently, at 25 - 27 years of age, remission maintenance therapy was initiated with oral corticosteroids (2.5 - 5.0 mg/day) and azathioprine (100 mg/day).
At 27 years of age, her second pregnancy was conceived during PAN remission. During the first trimester, she was diagnosed with chronic hypertension (CH) (blood pressure (BP), 160/90 mm Hg) by her previous doctor. She declined antihypertensive medication or to continue PAN medication.
At 22 weeks of gestation, her BP was greatly elevated (200/100 mm Hg), which necessitated hospital admission. On admission, vital sign measurements revealed BP of 166/98 mm Hg and pulse rate of 82 beats per minute; however, physical examination revealed no leg edema. Her laboratory examinations are shown in Table 1. Liver and kidney functions were unremarkable with no proteinuria. Additionally, soluble fms-like tyrosine kinase-1 (sFLt-1) and placental growth factor (PlGF) were 2,620 pg/mL and 45.8 pg/mL, respectively. Fetal ultrasound showed that the estimated fetal body weight was within the normal range (560 g ± 0.5 standard deviation (SD)) with normal amniotic fluid index (21.7 cm). Fetal monitoring with non-stress tests showed reactive tracings with no uterine contraction.
 Click to view | Table 1. Clinical Findings at Admission and at Delivery |
Diagnosis
The patient was diagnosed with HDP (CH); PAN recurrence was excluded due to the absence of PAN-specific skin or joint symptoms indicated in the diagnostic criteria. To control BP, nifedipine (80 mg/day) and labetalol (150 mg/day) were initiated. Although we diagnosed HDP rather than recurrent PAN, we monitored her carefully, considering the possibility of recurrent PAN.
At 30 weeks of gestation, she was diagnosed with superimposed preeclampsia (SPE) because of severe hypertension (230/125 mm Hg), proteinuria of 4+ on dipstick testing (protein/creatinine 1.98 mg/dL ≥ 0.3 mg/dL), sFlt-1 of 12,600 pg/mL, and PlGF of 22.0 pg/mL, and the decision was made to deliver the baby.
Treatment
After hydrocortisone (100 mg) administration, the patient underwent cesarean section under combined spinal-epidural anesthesia. A single, 1,084 g (appropriate for gestational age infant) male neonate with Appearance, Pulse, Grimace, Activity, and Respiration (APGAR) score of 6/8 and an umbilical artery blood pH of 7.22 was delivered.
Follow-up and outcomes
After delivery, the patient’s BP was maintained at approximately 150/80 mm Hg with oral nifedipine (80 mg/day), amlodipine (2.5 mg/day), and candesartan (8 mg/day). Postoperatively, she tolerated the procedure well and she was discharged on the sixth postoperative day.
The pathology of the placenta showed fibrin deposition of the intervillous space and fibrinoid necrosis of the terminal villus, indicating placental circulatory failure with ischemic changes. There was fibrinoid necrosis around the small maternal arteries in the decidua of the placental site (Figs. 1, 2).
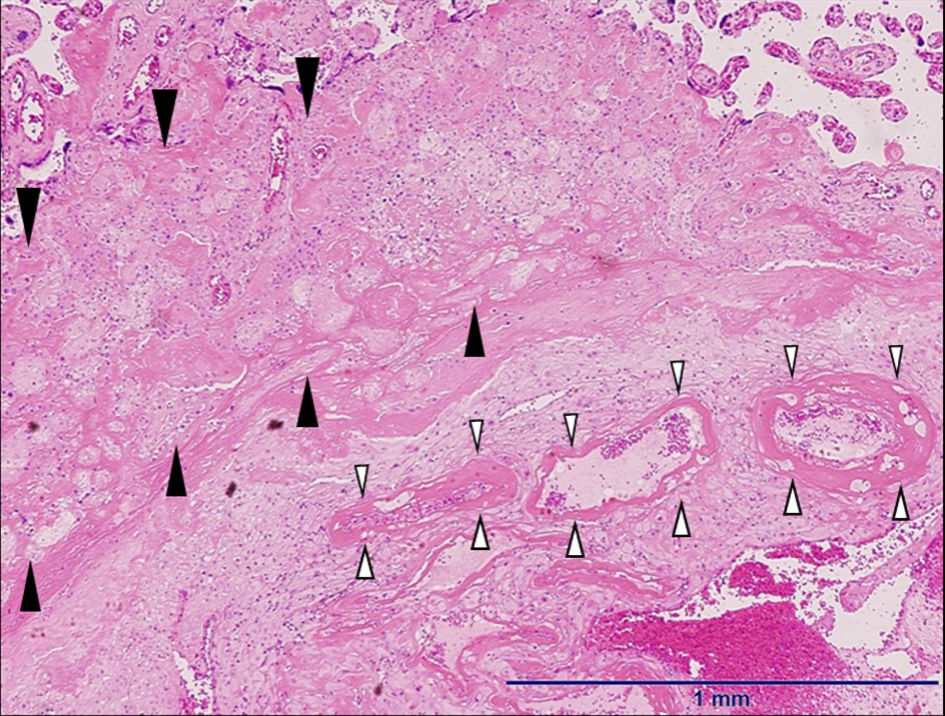 Click for large image | Figure 1. The microscopic findings of the placenta (hematoxylin and eosin stain, × 4). The pathology of the placenta shows fibrin deposition of intervillous space and fibrinoid necrosis of terminal villus called placental circulatory failure with ischemic changes (black arrow heads). Fibrinoid necrosis was observed around the small maternal arteries in the decidua of the placental site (white arrow heads). |
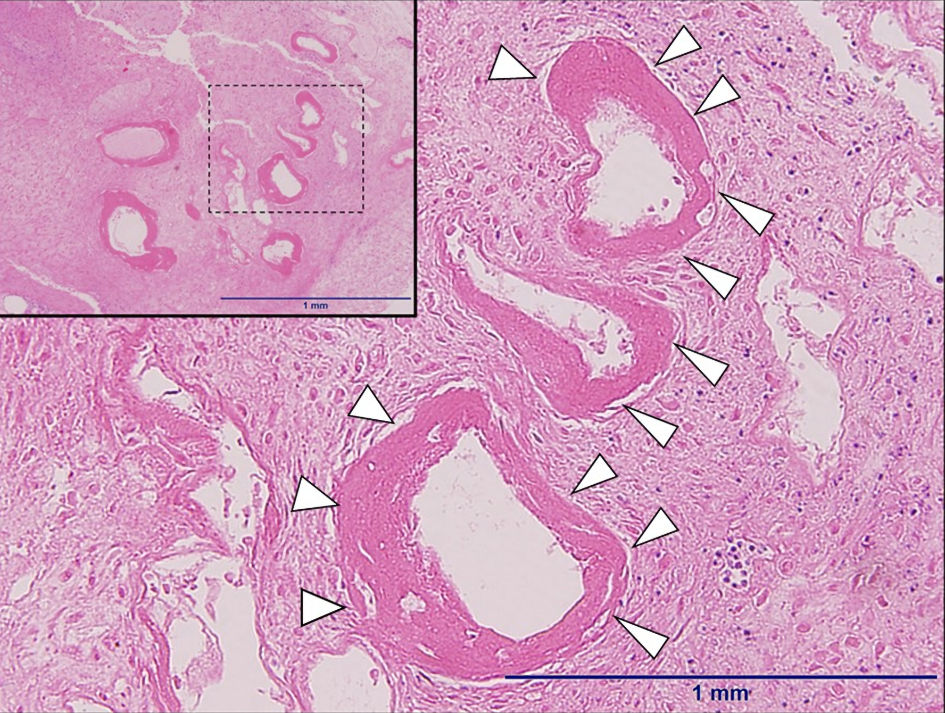 Click for large image | Figure 2. The microscopic findings of the decidua of the non-placental site (hematoxylin and eosin stain, × 4). Fibrinoid necrosis was observed around small maternal arteries in decidua (white arrow heads). The upper left part is scaled-back version (× 4), and the lower part is expanded version (× 10). |
| Discussion | ▴Top |
PAN in pregnancy is associated with worsened vasculitis postpartum, which can lead to maternal death caused by acute renal failure and embolization or necrosis-induced acute myocardial infarction [13].
Since 1944, 17 cases of PAN during pregnancy have been reported (Table 2 [8-10, 13-17]). Until 1964, PAN was not diagnosed during pregnancy; hence all pregnancies resulted in postpartum maternal death. In 1973, a pregnant woman was diagnosed with PAN, which necessitated prednisolone administration, and resulted in the patient’s survival [18]. Currently, among the 18 reported cases (including the case reported herein) of PAN during pregnancy, seven have resulted in maternal death. Among the seven mortalities, six (85.7%) were due to the development of HDP, which was untreated. The maternal prognosis is poor if PAN is diagnosed during the pregnancy or postpartum period [19]. Additionally, recurrence may occur despite maintenance therapy [14]. PAN may cause vascular inflammation in various systemic organs, often resulting in severe hypertension and renal damage (elevated serum creatinine, proteinuria) [13]. HDP occurs in 40% of PAN in pregnancy [12]. Until 1980, six of eight cases (75%) were complicated with HDP, which was higher than its prevalence in 1989 (five of 11 cases, 45.5%). This decrease in incidence may be attributed to the high number of treated PAN patients during pregnancy (eight of 11 cases, 72.7%), leading to improved maternal survival and favorable prognosis. A limitation in the case review data is that the fatal cases are biased toward older publications when the recognition and treatment of preeclampsia (PE) and PAN may have been far from current standards.
 Click to view | Table 2. Reported Cases of Polyarteritis Nodosa in Pregnancy |
No biomarker can determine the progression of PAN in pregnancy to SPE. Our patient developed CH during the non-pregnant remission period, suggesting that the risk of SPE is increased by hypertension, even in remission. Generally, SPE occurs in 20-50% of CH and 75% of secondary hypertension, which necessitates the early detection of SPE [20]. sFlt-1 is a type 1 receptor for soluble vascular endothelial growth factor (VEGF). Increased maternal sFlt-1 leads to PlGF and VEGF inactivation, decreasing free PlGF and VEGF, resulting in vascular endothelial lesions [21]. Before clinical onset of PE, increasing sFlt-1 and decreasing PlGF levels are observed. Notably, a previous study found that sFlt-1/PlGF ratio > 38 is a predictive marker of developing PE within 4 weeks (positive predictive value of 36.7%) [22]. Our patient demonstrated a sFlt-1/PlGF ratio of 57 and 572 at 22 and 30 weeks of gestation, respectively. Vascular endothelial damage may have commenced at 22 weeks of pregnancy; however, sFlt-1 and PlGF levels were insufficient to differentiate between PAN and HDP.
PAN is a necrotizing vasculitis of medium- and small-sized arteries, without glomerulonephritis or arteriolar, capillary, or venular vasculitis [2]. Necrotizing vasculitis is characterized by pathologic neutrophilic infiltration of blood vessels, with nuclear dust of leukocyte origin, resulting in blood vessel destruction, and vessel wall and lumen eosinophilic substance deposition (fibrinoid necrosis or degeneration) [23]. Vasculitis is mainly characterized by pathologic necrotizing vasculitis, necessitating the presence of fibrinoid necrosis. Histologically, PAN is classified into four stages according to Arkin’s staging system: stage I, degenerative stage; stage II, acute inflammatory stage; stage III, granulation stage; and stage IV, scar stage [23]. Clinically, stage I and II lesions show severe blood vessel inflammation throughout the body, and stage III and IV lesions show ischemia in affected organs. In stage I, the tunica media and intima of blood vessels are edematous, with fibrinoid degeneration. In stage II, neutrophil, eosinophil, lymphocyte, and plasma cell infiltration occur in the tunica media and adventitia of blood vessels, with extension of fibrinoid necrosis to all layers [2]. The blood vessel wall has complete or partial fibrinoid degeneration, leading to the tearing and disappearance of the inner elastic plate [23]. These findings may indicate the development of thrombosis [5].
HDP needs to be differentiated from PAN. Histologically, HDP shows damaged vascular endothelium due to edema, thickening, thrombus formation, fibrinoid necrosis, and foam macrophage and lymphocyte infiltration. Additionally, vascular occlusion occurs due to multiple infarctions among the villus (Tenney-Parker change) [24]. There are some similar histological changes observed with both HDP and PAN. Histological examination of our patient showed fibrin deposition in the placental intervillous space and fibrinoid necrosis of terminal villus called placental circulatory failure. Moreover, ischemic changes and fibrinoid necrosis were observed around the small maternal arteries in the decidua (Figs. 1 and 2). Initially, our patient was diagnosed with CH, with no PAN recurrence. However, it is unclear whether PAN worsened the HDP due to the presence of fibrinoid necrosis in the maternal small arteries of the decidua.
Learning points
In this case, we found that PAN in pregnancy was difficult to differentiate from HDP. Our case report highlights the importance of carefully managing HDP as a serious complication of PAN.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
This study was approved by the institutional review board, and written informed consent was obtained from the patient.
Author Contributions
Emi Kondo and Eiji Shibata were responsible for drafting the manuscript. All authors read and approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kussmaul A, Maier K. Uber eine nicht bisher beschriebene eigenthumliche Arterienerkrankung (Periarteritis nodosa), die mit Morbus Brightii und rapid fortschreitender allgemeiner Muskelahmung einhergeht. Deutsche Archiv Klinische Medizin. 1866;1:484-518.
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1-11.
doi pubmed - Wigley RD. The aetiology of Polyareritis nodosa: A review. N Z Med J. 1970;71(454):151-158.
- Lightfoot RW, Jr., Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33(8):1088-1093.
doi pubmed - Hernandez-Rodriguez J, Alba MA, Prieto-Gonzalez S, Cid MC. Diagnosis and classification of polyarteritis nodosa. J Autoimmun. 2014;48-49:84-89.
doi pubmed - Phillip R, Luqmani R. Mortality in systemic vasculitis: a systematic review. Clin Exp Rheumatol. 2008;26(5 Suppl 51):S94-104.
- Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, Thibult N, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore). 1996;75(1):17-28.
doi pubmed - Pagnoux C, Le Guern V, Goffinet F, Diot E, Limal N, Pannier E, Warzocha U, et al. Pregnancies in systemic necrotizing vasculitides: report on 12 women and their 20 pregnancies. Rheumatology (Oxford). 2011;50(5):953-961.
doi pubmed - Rash CJ. What lies beneath. A case report on polyarteritis nodosa in pregnancy. The Medical City Journal. 2018;1(1):22-25.
- Damian L, Pamfil C, Fodor M, Rogojan L, Hagau N, Rednic S. Polyarteritis nodosa in pregnancy. Ochsner J. 2018;18(1):94-97.
- Doria A, Bajocchi G, Tonon M, Salvarani C. Pre-pregnancy counselling of patients with vasculitis. Rheumatology (Oxford). 2008;47(Suppl 3):iii13-15.
doi pubmed - Pagnoux C, Mahendira D, Laskin CA. Fertility and pregnancy in vasculitis. Best Pract Res Clin Rheumatol. 2013;27(1):79-94.
doi pubmed - Reed NR, Smith MT. Periarteritis nodosa in pregnancy: report of a case and review of the literature. Obstet Gynecol. 1980;55(3):381-384.
doi - Aya AG, Hoffet M, Mangin R, Balducchi JP, Eledjam JJ. Severe preeclampsia superimposed on polyarteritis nodosa. Am J Obstet Gynecol. 1996;174(5):1659-1660.
doi - Owen J, Hauth JC. Polyarteritis nodosa in pregnancy: a case report and brief literature review. Am J Obstet Gynecol. 1989;160(3):606-607.
doi - Fernandes SR, Cury CP, Samara AM. Pregnancy with a history of treated polyarteritis nodosa. J Rheumatol. 1996;23(6):1119-1120.
- Owada K, Katoh T, Asano K, Watanabe K, Shigetomi S, Watanabe T. Successful pregnancy complicated by microscopic polyarteritis nodosa. Clin Nephrol. 2005;63(6):500-502.
doi pubmed - De Beukelaer MM, Travis LB, Roberts DK. Polyarteritis nodosa and pregnancy: report of a successful outcome. South Med J. 1973;66(5):613-615.
doi pubmed - Gatto M, Iaccarino L, Canova M, Zen M, Nalotto L, Ramonda R, Punzi L, et al. Pregnancy and vasculitis: a systematic review of the literature. Autoimmun Rev. 2012;11(6-7):A447-459.
doi pubmed - American College of Obstetricians and Gynecologists' Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133(1):e26-e50.
doi pubmed - Jena MK, Sharma NR, Petitt M, Maulik D, Nayak NR. Pathogenesis of preeclampsia and therapeutic approaches targeting the placenta. Biomolecules. 2020;10(6):953.
doi pubmed - Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennstrom M, Olovsson M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13-22.
doi pubmed - Arkin A. A clinical and pathological study of periarteritis nodosa: a report of five cases, one histologically healed. Am J Pathol. 1930;6(4):401-426.5.
pubmed - Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649-658.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


