| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 1, January 2022, pages 15-20
Intestinal T-Cell Lymphoma With Lung and Lymph Node Involvement at Relapse
Yasuhiro Tanakaa, c, Tatsuzo Mishinab, Hiroaki Miyoshib, Koichi Ohshimab, Masaharu Nohgawaa
aDepartment of Hematology, Japanese Red Cross Wakayama Medical Center, Komatsubaradori 4-20, Wakayama City, Wakayama 640-8558, Japan
bDepartment of Pathology, School of Medicine, Kurume University, Asashimachi 67, Kurume City, Fukuoka 830-0011, Japan
cCorresponding Author: Yasuhiro Tanaka, Department of Hematology, Japanese Red Cross Wakayama Medical Center, 4-20 Komatsubaradori, Wakayama City, Wakayama 640-8558, Japan
Manuscript submitted November 2, 2021, accepted December 13, 2021, published online January 17, 2022
Short title: ITL With Lung Involvement
doi: https://doi.org/10.14740/jmc3830
| Abstract | ▴Top |
Patients with intestinal T-cell lymphomas (ITLs) usually present with perforation of the small intestine and colon at diagnosis. At relapse or in the advanced stage, ITLs involve in other extranodal sites, but biopsy-proven lung involvement has been rarely reported. A 76-year-old male presented with sudden-onset abdominal pain, which was found to be caused by the perforation of colon. Emergency operation was carried out, and histopathological examination of the resected colon led to the diagnosis of ITL, not otherwise specified (NOS). He achieved complete metabolic remission (CMR) after eight courses of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemotherapy. Two months later, computed tomography showed infiltration and ground-glass opacity in the left pulmonary area in addition to the enlargement of mediastinal and left subclavian lymph nodes, although he did not complain of any pulmonary symptoms. Histopathological findings of the biopsied samples from the pulmonary area were consistent with relapsed ITL, NOS. He achieved CMR after three courses of GDP (gemcitabine, dexamethasone, and cisplatin) chemotherapy; but 1 month after the completion of GDP chemotherapy, he relapsed again with involvement of multiple lymph nodes, not in the pulmonary area. He died owing to the progression of disease. This is the third case of ITLs with lung involvement. Active biopsy should be performed when pulmonary nodules, infiltration, or ground-glass opacity are found in ITLs. A regimen for salvage chemotherapy specifically for ITLs is not yet established, and GDP chemotherapy may be an alternative option for relapsed ITLs in addition to new agents, such as romidepsin and pralatrexate.
Keywords: Intestinal T-cell lymphoma; Lung involvement; GDP chemotherapy; Romidepsin; Pralatrexate
| Introduction | ▴Top |
Intestinal T-cell lymphomas (ITLs) usually involve in the small intestine and colon. Patients with ITLs often complain of abdominal pain, diarrhea, nausea, and abdominal distention, and they present with perforation of the small intestine and colon at diagnosis [1]. At relapse or in the advanced stage, ITLs involve in other extranodal sites, such as the stomach, skin, and brain [2]. However, lung involvement in ITLs has rarely been reported. Chemotherapy is the main strategy to treat ITLs, but standard regimens for first-line or salvage chemotherapy are not yet established because ITLs are rare hematological neoplasms, and the survival of ITL patients is very poor [1, 2].
We report the case of a male patient with ITL with lung and lymph node involvement at relapse. Our patient presented with the perforation of colon at diagnosis; but at relapse, he presented with pulmonary infiltration and multiple lymphadenopathy, not perforation. Salvage chemotherapy was temporarily effective for his relapsed ITL. This is the third case report of ITL with lung and lymph node involvement.
| Case Report | ▴Top |
Investigations
A 76-year-old male was admitted to our hospital for the evaluation of diarrhea in March 2019. He had a medical history of bladder cancer 3 years previously. He had no family history of celiac disease or any allergy against gluten. Colonoscopy showed multiple ulcers only in the rectum, and computed tomography (CT) showed no abnormalities. His symptom continued despite medical treatment. Four months later, he complained of sudden-onset abdominal pain. CT showed bilateral pleural effusion and free air around colon, suggesting perforation. Emergency operation by subtotal colectomy was performed.
Diagnosis
Histopathological examinations of the resected colon revealed pleomorphic medium-sized abnormal lymphocytes densely occupying between the lamina propria and the serosa, as well as infiltration of inflammatory cells (Fig. 1a). Immunohistochemical analysis showed that the medium-sized abnormal lymphocytes were positive for cluster of differentiation (CD)3 (Fig. 1b), CD45RO, granzyme B (Fig. 1c), T cell intracellular antigen (TIA)-1, and T cell receptor (TCR)-βF1 (Fig. 1d) and negative for CD4 (Fig. 1e), CD8 (Fig. 1f), CD20, CD30, CD56 (Fig. 1g), and CD103 (Fig. 1h). The Ki-67 labeling index was high. The Epstein-Barr virus-encoded small RNAs (EBERs) in situ hybridization was negative for abnormal lymphocytes. These findings led to the diagnosis of ITL, not otherwise specified (NOS) in accordance with the World Health Organization (WHO) 2017 classification [1]. Chromosomal analysis using G banding and T cell receptor rearrangement analysis were not carried out. Bone marrow examination showed no abnormal cells. He was then transferred to our department. Laboratory examination showed normocytic anemia (hemoglobin 9.1 g/dL), thrombocytopenia (7.7 × 109/L), and elevated levels of lactate dehydrogenase (LDH: 544 U/L; normal range: 124 - 222), C-reactive protein (2.70 mg/dL), and soluble interleukin-2 receptor (sIL-2R: 8,159 U/mL; normally below 496). The serum albumin level decreased to 1.9 g/dL. He was negative for the antibody to the human T-lymphotropic virus type 1 (HTLV-1).
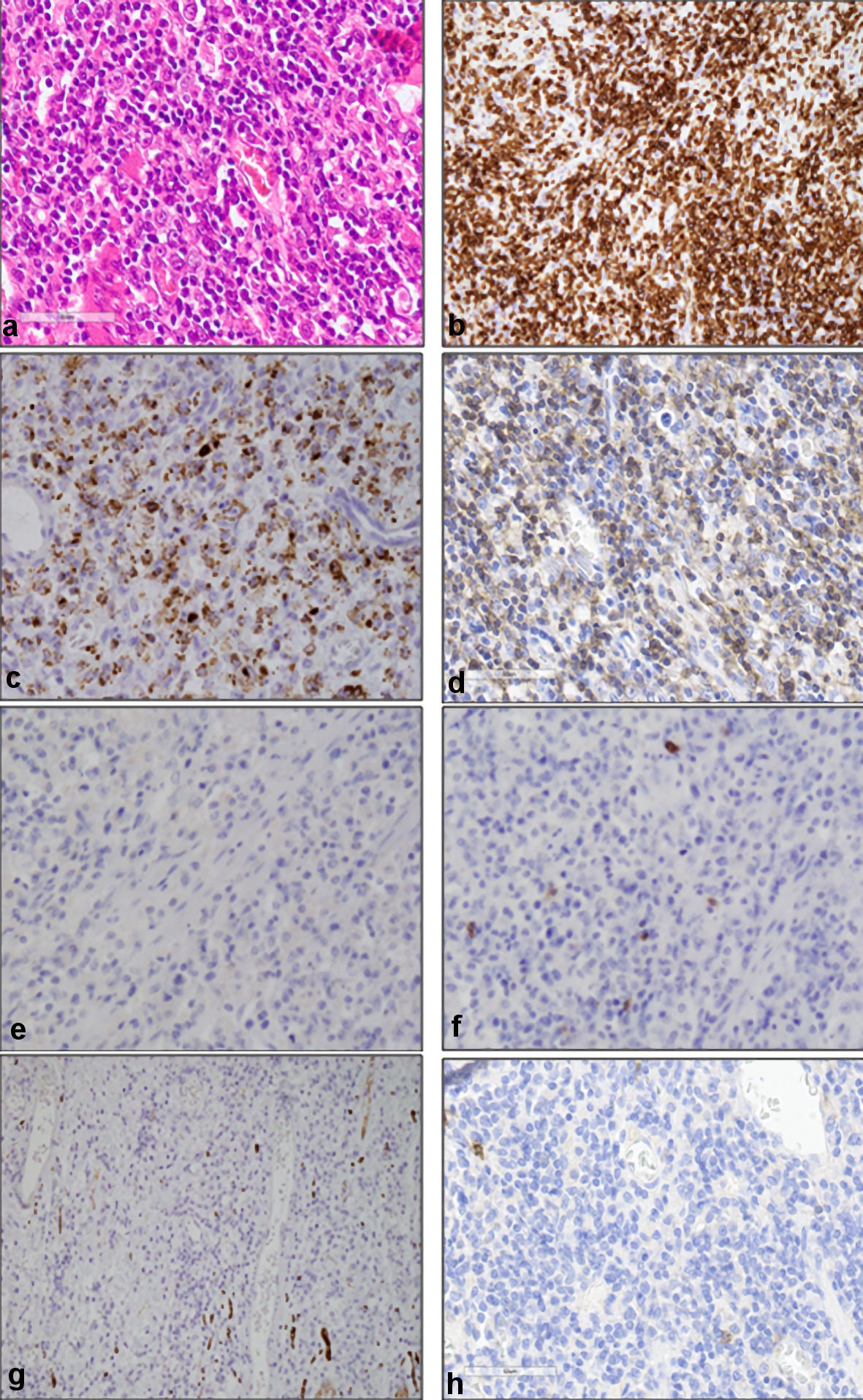 Click for large image | Figure 1. Histopathological findings of resected colon. (a) Dense pleomorphic medium-sized abnormal lymphocytes, as well as infiltrating inflammatory cells (hematoxylin and eosin staining, original magnification, × 400). Immunohistochemical analysis showed that medium-sized abnormal lymphocytes were positive for CD3 (b), granzyme B (c), and TCR-βF1 (d), and negative for CD4 (e), CD8 (f), CD56 (g), and CD103 (h) (original magnification, × 400). CD: cluster of differentiation; TCR: T cell receptor. |
Treatment
Two weeks after the operation, CHOP (cyclophosphamide, 1,000 mg per body; doxorubicin, 65 mg per body; vincristine, 1.8 mg per body; prednisolone, 100 mg per body for 5 days) chemotherapy was administered every 3 weeks. After eight courses of CHOP chemotherapy, he achieved complete metabolic remission (CMR) as shown by positron emission tomography-computed tomography (PET-CT) in June 2020 (Fig. 2a). However, 2 months after achieving CMR, the level of C-reactive protein increased and CT showed infiltration and ground-glass opacity in the left pulmonary area (Fig. 2b, c), in addition to the enlargement of mediastinal and left subclavian lymph nodes (Fig. 2d, e). He did not complain of any pulmonary symptoms. Histopathological examination of the biopsied samples from the pulmonary area showed medium-sized abnormal lymphocytes densely occupying the alveolar septum, as well as fibrosis and localized thickening of the alveolar septum (Fig. 3a). Immunohistochemical analysis showed that the medium-sized abnormal lymphocytes were positive for CD3 (Fig. 3b) and granzyme B (Fig. 3c) and negative for CD8 (Fig. 3d), consistent with relapsed ITL, NOS. Thus, we considered ITL, NOS involving the lung and lymph nodes at relapse. Laboratory examination showed elevated levels of C-reactive protein (1.28 mg/dL) and sIL-2R (2,703 U/mL; normally below 496). GDP (gemcitabine, 1,600 mg per body, day 1 and 8; cisplatin, 100 mg per body, day 1; dexamethasone, 30 mg per body for 4 days) chemotherapy was administered every 3 weeks. After three courses of GDP chemotherapy, CT showed the disappearance of pulmonary shadows and lymphadenopathy (Fig. 2f, g), and he achieved CMR as shown by PET-CT in November 2020 (Fig. 2h). He completed total six courses of GDP chemotherapy in January 2021.
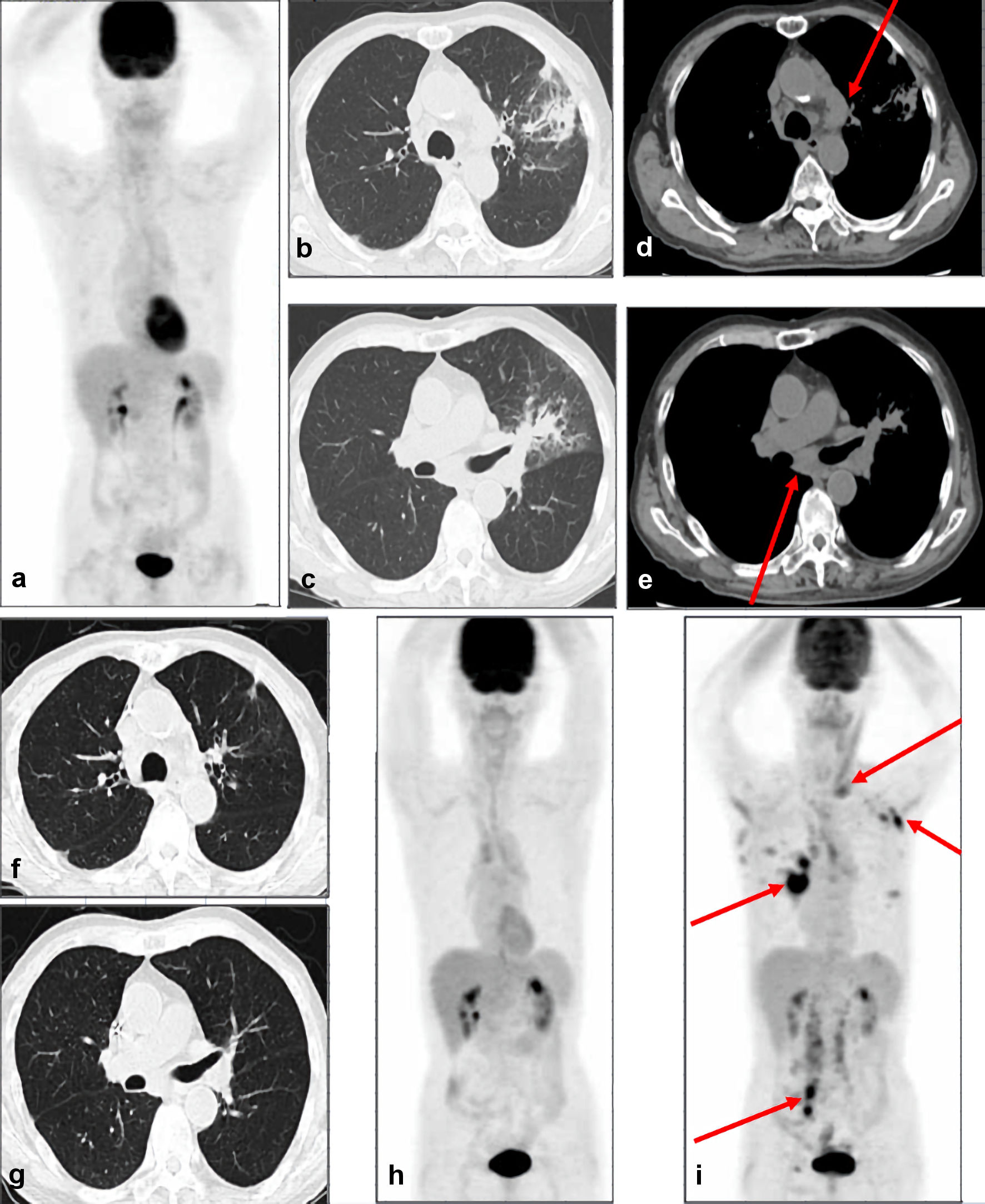 Click for large image | Figure 2. CT and PET-CT images. (a) PET-CT images show no abnormal accumulation of fluorodeoxyglucose in the whole body, suggesting CMR. CT images at relapse show infiltration and ground-glass opacity in the left pulmonary area (b, c), in addition to the enlargement of mediastinal and left subclavian lymph nodes (d, e). Arrows indicate enlarged lymph nodes. CT images after three courses of GDP chemotherapy show the disappearance of pulmonary shadows and lymphadenopathy (f, g). (h) PET-CT images after three courses of GDP chemotherapy show no abnormal accumulation of fluorodeoxyglucose in the whole body, suggesting CMR. (i) PET-CT images at second relapse show the accumulation of fluorodeoxyglucose in multiple lymph nodes in left supraclavicular fossa, left axilla, bilateral hilum, mesentery, para-aorta, right iliac area, and right inguinal area. Arrows indicate the abnormal accumulation in lymph nodes. PET-CT: positron emission tomography-computed tomography; GDP: gemcitabine, dexamethasone, and cisplatin; CMR: complete metabolic remission. |
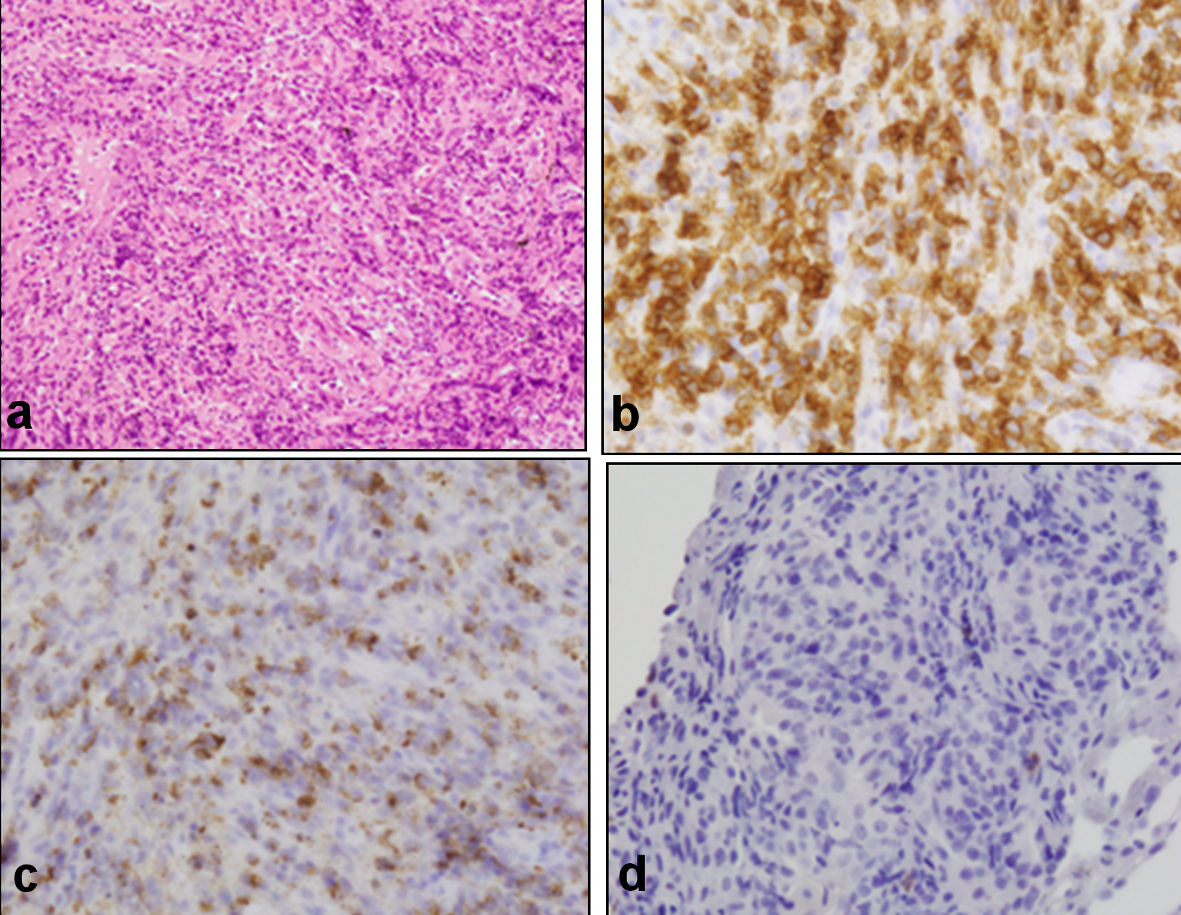 Click for large image | Figure 3. Histopathological findings of the biopsied samples from lung. (a) Medium-sized abnormal lymphocytes densely occupying alveolar septum, as well as fibrosis and localized thickening of the alveolar septum (hematoxylin and eosin staining, original magnification, ×200). Immunohistochemical analysis showed that medium-sized abnormal lymphocytes were positive for CD3 (b) and granzyme B (c) and negative for CD8 (d), consistent with relapsed ITL, NOS (original magnification, ×400). CD: cluster of differentiation; NOS: not otherwise specified. |
Follow-up and outcomes
However, 1 month after the completion of GDP chemotherapy, he developed a fever above 38 °C. PET-CT showed the accumulation of fluorodeoxyglucose in multiple lymph nodes, not in the pulmonary area, suggesting a second relapse (Fig. 2i). His symptom temporarily resolved after one course of romidepsin, but he died owing to the disease progression in May 2021. His family did not permit an autopsy.
| Discussion | ▴Top |
Here, we reported a rare case of ITL, NOS with lung and lymph node involvement at relapse. The 2017 WHO classification includes four subtypes of ITL: enteropathy-associated T-cell lymphoma (EATL), monomorphic epitheliotropic T-cell lymphoma (MEITL), ITL, NOS, and indolent T-cell lymphoproliferative disorder of the gastrointestinal tract [1]. ITL, NOS was exclusively diagnosed on the basis of the absence of conformation to either EATL or MEITL. In EATL, there is a clear association with celiac disease. Abnormal lymphocytes are pleomorphic medium- to large-sized cells, and they are often accompanied by a component of inflammatory cells. Immunophenotyping of abnormal lymphocytes were positive for CD3, CD7, CD103, alpha/beta-type of TCR, and cytotoxic molecules such as TIA-1 and granzyme B. On the other hand, abnormal lymphocytes in MEITL are monomorphic medium-sized cells and show prominent epitheliotropism. Immunophenotyping of abnormal lymphocytes were positive for CD3, CD8, CD56, gamma/delta-type of TCR, and TIA-1. Unlike EATL, our patient did not have a past history or family history of celiac disease or gluten sensitivity. In our case, pleomorphic medium-sized abnormal lymphocytes densely infiltrated into the lamina propria and serosal, as well as inflammatory cells, and they did not show epitheliotropism. Abnormal lymphocytes were positive for CD3, CD45RO, granzyme B, TIA-1, and TCR-βF1 and negative for CD4, CD8, CD20, CD30, CD56, and CD103. We consider that ITL, NOS may be a reasonable diagnosis for our patient.
ITLs mainly involve in the small intestine and colon; but in the advanced stage, they also involve in other extranodal sites, such as the stomach, skin, and brain [2]. Only two cases of MEITL with lung involvement have been reported [3, 4]. The International Peripheral T-Cell Lymphoma Project reported that about 5% of 62 cases of EATL show lung involvement; however, they did not describe the details of any cases [2]. To the best of our knowledge, this is the third case of ITL with lung involvement. Bhatlapenumarthi et al reported the case of a 64-year-old male diagnosed as having MEITL with small intestine and lung involvement at diagnosis. He complained of shortness of breath. PET-CT showed multiple pulmonary nodules accompanied by the accumulation of fluorodeoxyglucose. He died owing to recurrence of perforation of the small intestine after one course of CHOP chemotherapy [3]. Suzuki et al reported the case of a 74-year-old male diagnosed as having MEITL with small intestine, lung, and brain involvement. CT showed multiple nodules with cavitation and thick-walled cysts. He did not undergo chemotherapy owing to his poor performance status. They did not mention his pulmonary symptoms and the outcome of their case [4]. In both cases, lung involvement was confirmed by lung biopsy. Thus, active biopsy should be performed when pulmonary nodules, infiltration, or ground-glass opacity are found in ITLs.
CHOP chemotherapy is usually administered for ITLs as a first-line treatment, but a regimen for salvage chemotherapy is not yet established specifically for ITLs due to rare neoplasms [1, 2]. We chose the GDP chemotherapy as the salvage regimen for relapsed ITL, NOS because only one reported case of EATL had achieved CR after GDP chemotherapy, and GDP chemotherapy could be tolerable for elderly patients like our patient [5, 6]. GDP chemotherapy was temporarily effective for pulmonary and lymph node lesions of ITL, NOS; however, our patient did not remain in CR and eventually died owing to disease progression without pulmonary involvement. On the other hand, Tabata et al [7] reported the case of a patient with relapsed MEITL who achieved durable CR after the treatment with pralatrexate for 9 months, and one case of a patient with EATL who achieved durable partial response, not CR, after the treatment with romidepsin for 8 months [8]. These reports showed that new agents could overcome the poor survival of patients with relapsed ITLs.
Conclusions
We reported the case of ITL, NOS with lung and lymph node involvement at relapse. Our patient temporarily achieved CR after three courses of GDP chemotherapy, but died owing to disease progression without pulmonary lesions. Clinicians should keep in mind that active biopsy should be performed when pulmonary nodules, infiltration, or ground-glass opacity are found.
Acknowledgments
We would like to thank the medical staff of Japanese Red Cross Wakayama Medical Center who were involved in the care of this patient.
Financial Disclosure
All authors have no financial disclosure to declare.
Conflict of Interest
All authors declare no conflict of interest.
Informed Consent
We verbally obtained the informed consent from the patient for the publication of this case report and accompanying images.
Author Contributions
YT treated this patient, designed the study, collected the data, and wrote the paper; TM, HM, and KO made pathological diagnosis and provided all the pathological images; MN supervised this study. All authors contributed to the editing of this manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Jaffe ES, Chott A, Ott G, Chan JKC, Bhagat G, Tan SY, Stein H, et al. Intestinal T-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, et al. eds. WHO classification of tumors of haematopoietic and lymphoid tissues. Revised 4th ed, Lyon: IARC. 2017; p. 372-380.
- Delabie J, Holte H, Vose JM, Ullrich F, Jaffe ES, Savage KJ, Connors JM, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118(1):148-155.
doi pubmed - Bhatlapenumarthi V, Patwari A, Siddiqui AD. An unusual case of enteropathy-associated T-cell lymphoma type 2 with pulmonary metastasis. Cureus. 2019;11(9):e5714.
doi - Suzuki Y, Minemura H, Tomita H, Saito M, Watanabe N, Umeda T, Kawamata T, et al. Monomorphic epitheliotropic intestinal T-cell lymphoma involving the lung and brain: a rare case study. Intern Med. 2020;59(20):2559-2563.
doi pubmed - Arkenau HT, Chong G, Cunningham D, Watkins D, Sirohi B, Chau I, Wotherspoon A, et al. Gemcitabine, cisplatin and methylprednisolone for the treatment of patients with peripheral T-cell lymphoma: the Royal Marsden Hospital experience. Haematologica. 2007;92(2):271-272.
doi pubmed - Park BB, Kim WS, Suh C, Shin DY, Kim JA, Kim HG, Lee WS. Salvage chemotherapy of gemcitabine, dexamethasone, and cisplatin (GDP) for patients with relapsed or refractory peripheral T-cell lymphomas: a consortium for improving survival of lymphoma (CISL) trial. Ann Hematol. 2015;94(11):1845-1851.
doi pubmed - Tabata R, Tabata C, Okamura M, Takei Y, Ohshima K. Successful treatment of monomorphic epitheliotropic intestinal T cell lymphoma with pralatrexate. Ann Hematol. 2019;98(5):1301-1303.
doi pubmed - Piekarz RL, Frye R, Prince HM, Kirschbaum MH, Zain J, Allen SL, Jaffe ES, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117(22):5827-5834.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


