| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 3, March 2022, pages 119-124
A Rare Case of Severe Hemolytic Anemia and Pulmonary Embolism Secondary to Mycoplasma pneumoniae Infection
Aravind Sunderavel Kumaravel Kanagavelua, c, Sateesh K. Nagumantryb, Satyanarayana V. Sagia, Samson O. Oyiboa
aDepartment of General Medicine, Peterborough City Hospital, Bretton Gate, Peterborough, PE3 9GZ, UK
bDepartment of Haematology, Peterborough City Hospital, Bretton Gate, Peterborough, PE3 9GZ, UK
cCorresponding Author: Aravind Sunderavel Kumaravel Kanagavelu, Department of General Medicine, Peterborough City Hospital, Bretton Gate, Peterborough, PE3 9GZ, UK
Manuscript submitted December 2, 2021, accepted February 14, 2022, published online March 5, 2022
Short title: Hemolytic Anemia Secondary to M. pneumoniae
doi: https://doi.org/10.14740/jmc3866
| Abstract | ▴Top |
A 27-year-old woman was admitted with a history of dry cough, breathlessness, fever, lethargy, and nausea and vomiting. On examination, she was febrile, jaundiced, and hypoxic. Blood tests revealed severe leucocytosis and severe hemolytic anemia. The chest imaging demonstrated coexisting pneumonia and pulmonary embolism. An initial blood transfusion worsened the hemolytic anemia to the point that critical care review was required. Subsequent blood tests revealed cold agglutinin hemolytic anemia due to Mycoplasma pneumoniae infection. The patient’s condition improved after receiving a warm-blood transfusion, antibiotics, and steroid therapy. The patient also received anticoagulant therapy for 6 months. Our case is unique in that the patient had very severe anemia and very severe leucocytosis, making us suspect a hematologic malignancy at initial presentation. This case emphasizes the importance of prompt evaluation of hemolytic anemia and the use of warm blood transfusion for cold agglutinin disease.
Keywords: Hemolytic anemia; Mycoplasma pneumoniae; Pulmonary embolism; Blood transfusion; Cold agglutinin disease
| Introduction | ▴Top |
Mycoplasma pneumoniae (M. pneumoniae) is a spindle-shaped mucosal bacterium [1]. It lacks a cell wall and requires a network of proteins that acts as a cytoskeleton to sustain its cell membrane. It is an exclusive human pathogen and spreads from person to person through respiratory droplets [2].
M. pneumoniae produces respiratory manifestations such as tracheobronchitis, pharyngitis, and pneumonia. In most cases, the infection is self-limiting, but on rare occasions, it can cause extrapulmonary hemolytic manifestations such as autoimmune hemolytic anemia (AIHA), hemophagocytic syndrome, thrombocytopenic purpura, disseminated intravascular coagulation and splenic infarction [3, 4]. Hepatitis, central and peripheral nervous system disease, myocarditis, pericarditis, arthritis and erythema multiforme are some of the other extrapulmonary complications of M. pneumoniae infection [3].
Secondary cold agglutinin syndrome is a form of AIHA in which the autoantibody is a cold agglutinin caused by M. pneumoniae [5]. There is the production of cross-reactive autoantibodies to human erythrocytes [6]. These autoantibodies are generally surface antigens that cause agglutination of red blood cells at low temperatures of 0 - 4 °C [7, 8].
There are several case reports of M. pneumoniae complicated by pulmonary embolism in the literature, especially in the pediatric age group [9-12]. However, cases complicated by severe hemolytic anemia are rare. We report a case of a young woman who had M. pneumoniae infection complicated by pulmonary embolism and severe AIHA. This case emphasises the importance of heightened awareness and clinical suspicion when faced with a similar clinical scenario and the importance of warmed blood transfusion for AIHA due to cold agglutinin disease.
| Case Report | ▴Top |
Investigations
A 27-year-old woman presented to the emergency department with a history of an unproductive cough for a period of 10 days, which had worsened in the last 2 days. She felt lethargic, feverish and had nausea and vomiting. She noticed that her urine was dark in color. She was taking a cough syrup for symptomatic control. She was fit and well before admission without significant medical history. She lived with her mother and three children, who were all well. She was a smoker and occasionally drank alcohol. She had no history of drug allergies.
On general examination, she was conscious and alert with a Glasgow coma score of 15. She had conjunctival pallor and generalized jaundice. She was hypoxic with an oxygen saturation of 84% and febrile with a temperature of 38.5 °C. She had tachycardia with a blood pressure of 130/60 mm Hg and a respiratory rate of 22/min. Further cardiovascular examination was unremarkable. Respiratory examination revealed fine crepitation at both lung bases. Abdominal examination revealed tenderness in the right upper quadrant with palpable liver.
Diagnosis
The initial blood results demonstrated severe anemia (hemoglobin: 48 g/L), a relatively low reticulocyte count of 12 × 109/L (percentage of 2.96%), severe leukocytosis (white cell count: 66.5 × 109/L) and thrombocytosis (platelet cell count: 819 × 109/L). The coagulation profile was normal, except for a slightly elevated fibrinogen level of 8.9 g/L. Her C-reactive protein was elevated at 197 mg/L. Renal function tests were normal. The serum lactate level was elevated to 3.1 mmol/L. Liver function showed elevated serum bilirubin (105 µmol/L) with normal alkaline phosphatase.
Electrocardiography showed sinus tachycardia. The chest X-ray revealed opacification in the right and left middle zone, worse on the right (Fig. 1). A chest computed tomography (CT) confirmed bilateral pneumonia with suspicion of pulmonary embolism. A CT pulmonary angiogram (CTPA) confirmed filling defects in the right middle lobe pulmonary arteries, in the segmental branches of the right upper lobe and the right lower lobe pulmonary arteries, consistent with pulmonary embolism (Fig. 2).
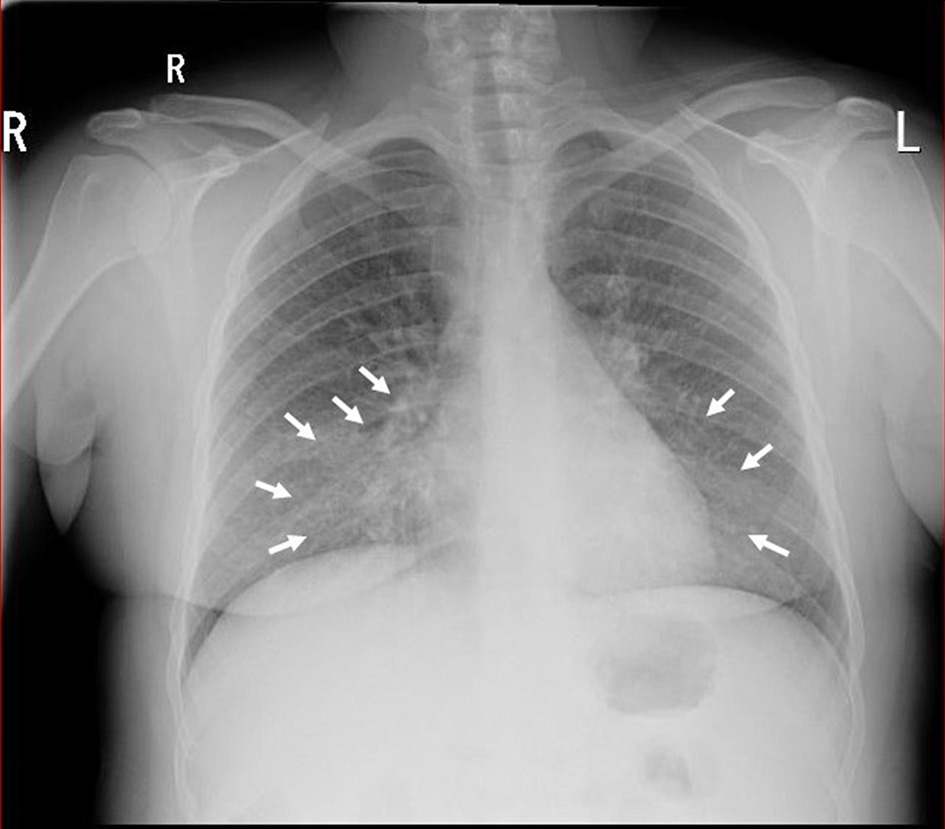 Click for large image | Figure 1. Chest X-ray demonstrating right and left mid-zone opacification consistent with bilateral pneumonia (white arrows). |
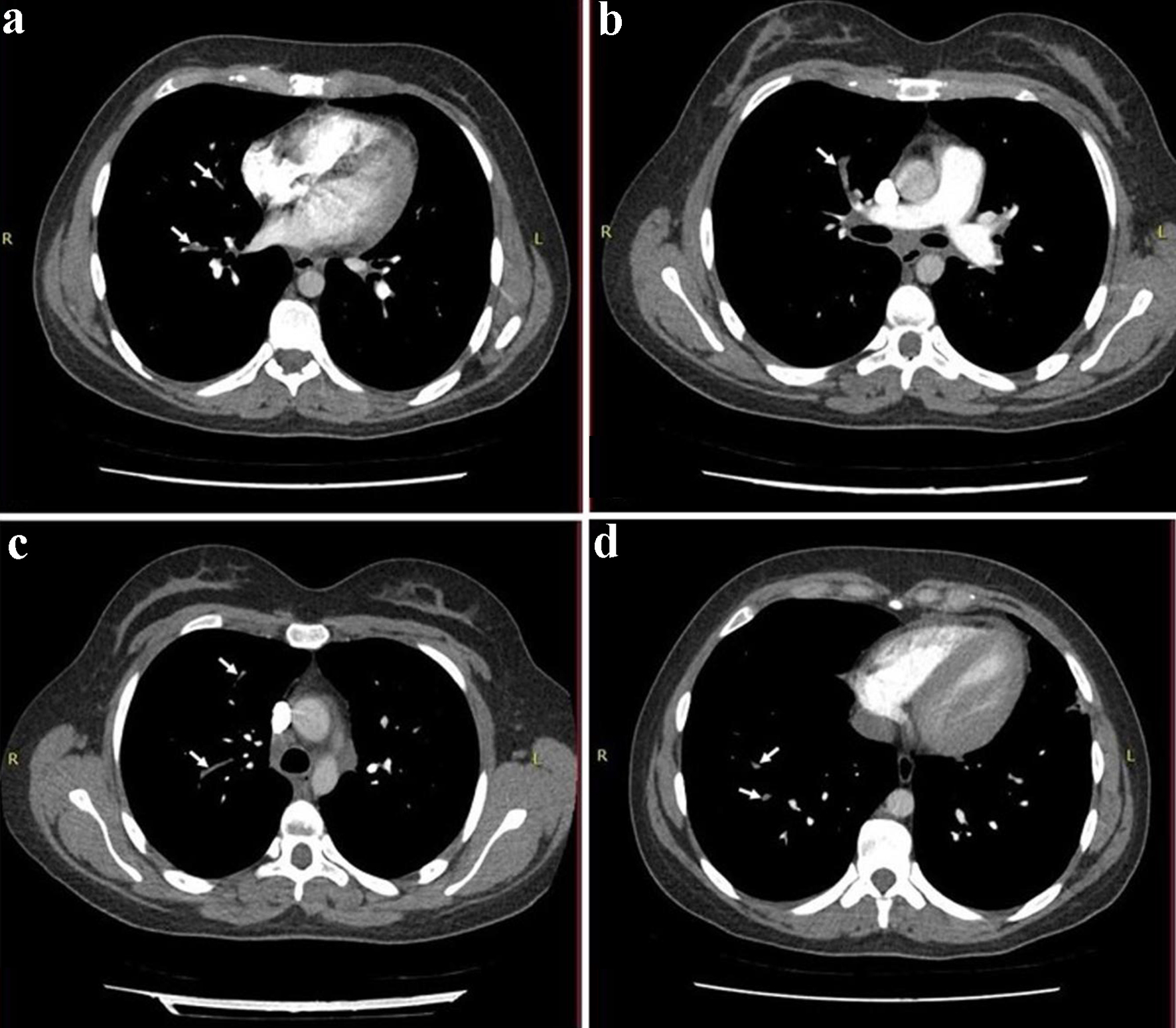 Click for large image | Figure 2. A CT pulmonary angiogram (CTPA) demonstrating filling defects in the right middle lobe pulmonary arteries (a, b), in the segmental branches of right upper lobe (c) and the right lower lobe pulmonary arteries (d), which were consistent with pulmonary embolism (white arrows). |
The peripheral blood smear showed significant red cell agglutination, thrombocytosis, and platelet anisocytosis, but there was no malignant lymphoid population (Fig. 3). The direct Coomb test was positive and the haptoglobin level was low (< 0.1), confirming intravascular hemolysis. The lactate dehydrogenase (LDH) assay was not reportable due to the hemolyzed sample. The direct antiglobulin test (DAT) was positive (1 +) for C3b and C3d, negative for IgG antibodies. The level of complement C4 was very low (< 0.03 g/L) suggesting complement consumption. Antibody screening for blood transfusion showed positive Lutheran antibodies due to previous pregnancy. There was only a slight increase in immunoglobulin M (IgM) at 4.68 g/L, but IgG and immunoglobulin A (IgA) levels were normal. Autoimmune tests including anti-nuclear antibodies, antineutrophil cytoplasmic antibody, myeloperoxidase antibodies, proteinase 3 antibodies, and antiglomerular basement membrane antibodies were all negative. There were no monoclonal bands in protein electrophoresis.
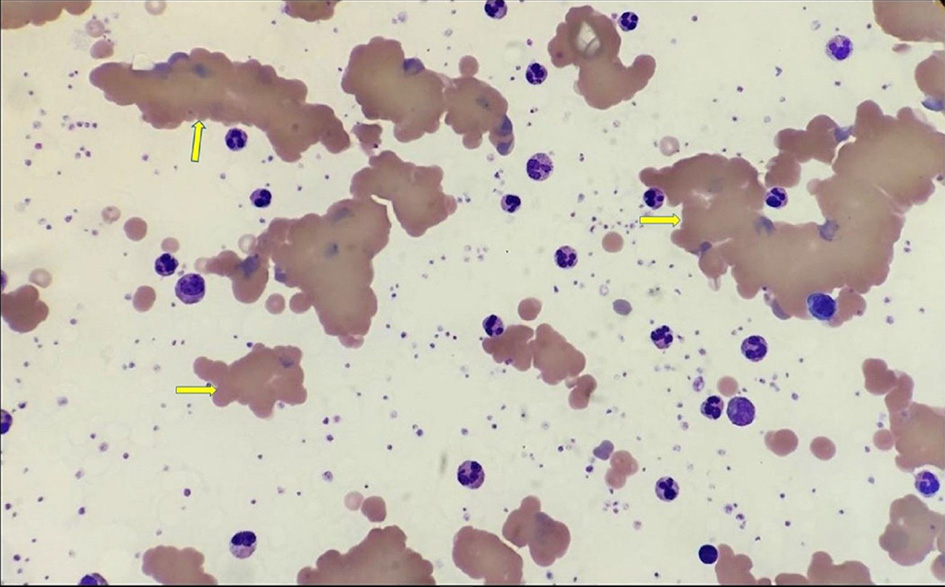 Click for large image | Figure 3. Blood film demonstrating significant red cell agglutination and fragmentation (yellow arrows). |
The coronavirus disease 2019 (COVID-19) screen (2019-nCoV RNA) was also negative. Urine and blood cultures were negative. Viral screens that included hepatitis A, B, C, and human immunodeficiency virus (HIV) were negative. The routine community-acquired pneumonia screen including the urine antigen of Streptococcus pneumonia and the Legionella urine antigen were negative. Further atypical pneumonia screening demonstrated a complement fixation test (CFT) for mycoplasma titer > 1:128, IgM titer > 4 and IgA titer < 4, all confirming recent infection with M. pneumoniae.
Based on the initial history and examination, sepsis secondary to cholecystitis or community-acquired pneumonia was suspected. Further blood results and scans ruled out any active liver or gall bladder disease. Low oxygen saturation, elevated temperature, increased infection markers and ground-glass appearance on CT raised suspicion of COVID-19 and other atypical respiratory infections.
A combination of severe anemia, severe leukocytosis, and thrombocytosis opened more differentials, such as hematologic malignancies (e.g., leukemia). However, the peripheral smear showed reactive neutrophilia and no evidence of malignant lymphoid cells.
Treatment
This patient required 4 L/min of oxygen to maintain oxygen saturation above 94%. She had a transfusion of 3 units of packed red blood cells and started with an intravenous combination of tazobactam and piperacillin (4.5 g 8-hourly) for suspected community-acquired pneumonia. Despite the blood transfusion, the hemoglobin level did not increase from baseline: in fact, it dropped to 40 g/L. Once the cold agglutinin disease was discovered, the patient received warm blood transfusion. Following 4 units of warm blood transfusion, her hemoglobin levels improved (Fig. 4). She also received 5 mg of folic acid daily and 30 mg of oral prednisolone daily to inhibit further red cell hemolysis, and her antibiotic regimen changed to oral ciprofloxacin 500 mg twice a day and clarithromycin 500 mg twice a day to cover atypical pneumonia. Once stable she was commenced on low molecular weight heparin (dalteparin) for the pulmonary embolism. This was later converted to a direct oral anticoagulant (rivaroxaban). The patient’s condition improved rapidly, and she was discharged with prednisolone, folic acid, rivaroxaban, and ciprofloxacin.
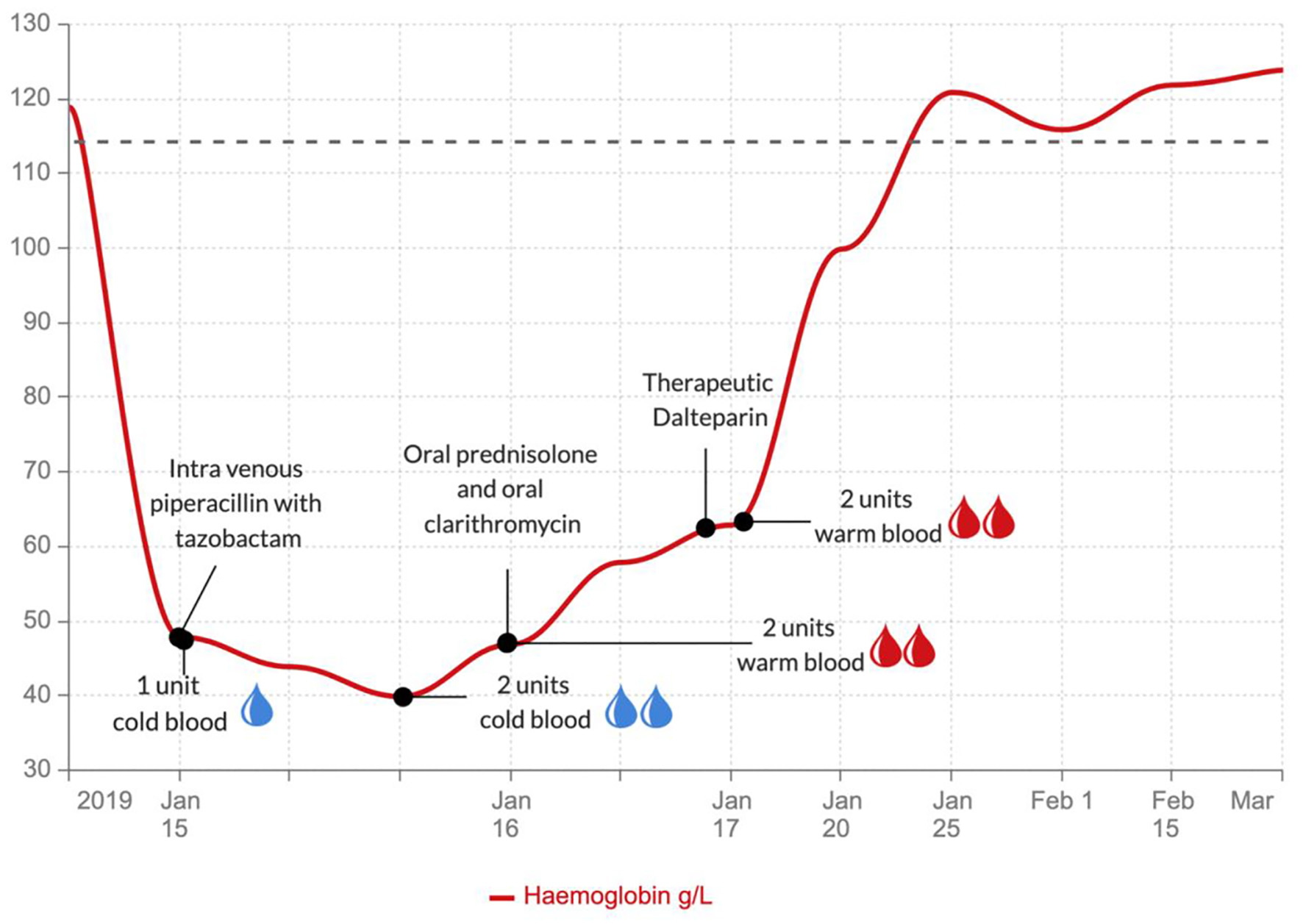 Click for large image | Figure 4. Graph showing the hemoglobin levels during the various treatments. |
Follow-up and outcomes
The patient attended the outpatient clinic 2 and 4 months after discharge. Her hemoglobin level was 132 g/L. She remained in good health under outpatient hematology surveillance with no chest symptoms, and stopped the oral anticoagulant after a total of 6 months of therapy.
| Discussion | ▴Top |
M. pneumoniae bacteria adhere to the ciliated columnar epithelium of the respiratory tract with the help of adhesin proteins [2, 13]. This stimulates the production of proinflammatory cells and cytokines, which contribute to pulmonary and extra-pulmonary manifestations [1, 2, 14, 15]. Affected patients can also present with community-acquired respiratory distress syndrome [15].
Cold agglutinin-mediated AIHA is divided into primary (idiopathic) and secondary, caused by infection (most often M. pneumoniae, Epstein-Barr virus infection, cytomegalovirus infection, SARS-CoV-2), malignancy, and lymphoproliferative disorders [8]. In both types, antigen-bound IgM-cold agglutinin binds to complement factor C1 and initiates the classic complement pathway, generating C3b. Cells opsonized with C3b are prone to phagocytosis by the mononuclear phagocytic system, mainly in the liver (extravascular hemolysis). C3b can also react to form C5 convertase, which initiates the terminal complement cascade. Terminal complement activation, in turn, leads to the formation of the membrane attack complex and intravascular hemolysis seen in this case with an absent haptoglobin response. This also produces the positive DAT or the direct Coomb’s test with detection of C3. The optimal temperature for this reaction is 0 - 4 °C at which the destruction of red blood cells occurs at the rate of 200 mL of red blood cells per hour [7, 8, 16-18]. Management includes treating the underlying cause and giving warm blood transfusion [19]. Steroids are not considered helpful in cases of cold agglutinin disease but are still used for other types of AIHA [7, 8, 16-18].
Our patient had intravascular hemolysis with severe anemia, as well as a thrombotic event. She had classic laboratory characteristics of intravascular hemolysis, including elevated bilirubin, low levels of haptoglobin, worsened hemolysis after cold blood transfusion, and improvement after warmed blood transfusion. These characteristics indicated cold agglutinin-induced hemolytic anemia. This was confirmed by a positive direct Coomb’s test followed by a positive IgM titer for M. pneumoniae infection. Absolute reticulocytosis supports the presence of hemolysis, but the reticulocyte count can be normal or low [6, 20]. Our patient had a normal percentage of reticulocytes (with low direct count) which indicates a delayed response of the bone marrow to hemolytic stress. Although reticulocytopenia can occur in the acute phase of AIHA, other conditions such as hematinic deficiency, marrow infiltration, aplastic anemia, and parvovirus B19 infection should also be considered [21]. The presence of reticulocytopenia can often represent a clinical emergency with an extremely high transfusion need and poor outcome [16]. During initial blood transfusions with un-warmed blood, our patient continued to have hemolysis due to cold agglutinin disease. After receiving warm blood in conjunction with supportive treatment with steroids, her hemoglobin level improved.
The coexistence of pulmonary embolism with any type of pneumonia is not an uncommon phenomenon: it is probably underdiagnosed. A previous study found that more than 50% of patients with pneumonia had underlying pulmonary embolism. Furthermore, the presence of consolidation on chest radiographs and positive D-dimer predicted this better than just D-dimer itself [22]. A recent study indicated an increased incidence of thrombosis even among patients with cold agglutinin disease, with a relative risk of 1.7 to 2.4 for affected patients compared to the general population [23]. The pathological mechanism remains unclear.
Our case is unique in that the patient had very severe anemia and very severe leukocytosis, making us suspect a hematologic malignancy at initial presentation. In a case report and review of previous cases (1967 to 2020), 17 cases of M. pneumoniae infection complicated by pulmonary embolism were discussed [24]. Thirteen of these were adults. Most of the cases reported normal hemoglobin levels or mild anemia: only one case had moderate anemia. Most cases reported mild leukocytosis: only one case had severe leukocytosis [24]. Although the presence of cold agglutinins is common with M. pneumoniae infection, the appearance of excessive hemolysis or severe anemia is rare [25]. Leucocyte count is generally normal or slightly elevated in mycoplasma infections, but there have been reports of severe leukocytosis accompanying hemolytic anemia, as reported in our case. The association remains unclear, but the coexistence of severe leukocytosis may be an indication of the presence of severe hemolytic anemia [26].
We have reported a case of a woman with mycoplasma pneumonia, complicated by severe hemolytic anemia and pulmonary embolism. The hemolytic anemia was exacerbated by the initial administration of cold blood transfusion before we administered the appropriate warm blood transfusion. This case emphasizes the importance of increasing awareness and clinical suspicion when faced with a similar clinical scenario and the importance of warm blood transfusion for AIHA due to cold agglutinin disease.
Learning points
M. pneumoniae infection can have pulmonary and extrapulmonary manifestations. Severe leucocytosis and severe hemolytic anemia rarely occur in cases of M. pneumoniae infection. The coexistence of severe leucocytosis could be an indication of severe hemolytic anemia. Both pneumonia and pulmonary embolism can coexist in patients with M. pneumoniae infection. A prompt evaluation of hemolytic anemia is required to assess the need for a warm blood transfusion for cold agglutinin disease.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was taken from the patient for publication as a case report including relevant images.
Author Contributions
Acquisition of data, initial drafting of paper and critical revision of the final paper were done by Aravind Sunderavel Kumaravel Kanagavelu, Sateesh K. Nagumantry, Satyanarayana V. Sagi and Samson O. Oyibo. All parties agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work, are appropriately investigated and resolved.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17(4):697-728.
doi pubmed - Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32(6):956-973.
doi pubmed - Waites KB, Atkinson TP. The role of Mycoplasma in upper respiratory infections. Curr Infect Dis Rep. 2009;11(3):198-206.
doi pubmed - Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010;16(3):162-169.
doi pubmed - Hill QA, Hill A, Berentsen S. Defining autoimmune hemolytic anemia: a systematic review of the terminology used for diagnosis and treatment. Blood Adv. 2019;3(12):1897-1906.
doi pubmed - Jager U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, Jilma B, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648.
doi pubmed - Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69(4):258-271.
doi pubmed - Berentsen S. New insights in the pathogenesis and therapy of cold agglutinin-mediated autoimmune hemolytic anemia. Front Immunol. 2020;11:590.
doi pubmed - Graw-Panzer KD, Verma S, Rao S, Miller ST, Lee H. Venous thrombosis and pulmonary embolism in a child with pneumonia due to Mycoplasma pneumoniae. J Natl Med Assoc. 2009;101(9):956-958.
doi - Sheng CQ, Yang CF, Ao Y, Zhao ZY, Li YM. Mycoplasma pneumoniae pneumonia with pulmonary embolism: A study on pediatric cases in Jilin province of China. Exp Ther Med. 2021;21(3):201.
doi pubmed - Su HY, Jin WJ, Zhang HL, Li CC. [Clinical analysis of pulmonary embolism in a child with Mycoplasma pneumoniae pneumonia]. Zhonghua Er Ke Za Zhi. 2012;50(2):151-154.
- Brown SM, Padley S, Bush A, Cummins D, Davidson S, Buchdahl R. Mycoplasma pneumonia and pulmonary embolism in a child due to acquired prothrombotic factors. Pediatr Pulmonol. 2008;43(2):200-202.
doi pubmed - Waldo RH, 3rd, Krause DC. Synthesis, stability, and function of cytadhesin P1 and accessory protein B/C complex of Mycoplasma pneumoniae. J Bacteriol. 2006;188(2):569-575.
doi pubmed - Becker A, Kannan TR, Taylor AB, Pakhomova ON, Zhang Y, Somarajan SR, Galaleldeen A, et al. Structure of CARDS toxin, a unique ADP-ribosylating and vacuolating cytotoxin from Mycoplasma pneumoniae. Proc Natl Acad Sci U S A. 2015;112(16):5165-5170.
doi pubmed - Chaudhry R, Ghosh A, Chandolia A. Pathogenesis of Mycoplasma pneumoniae: An update. Indian J Med Microbiol. 2016;34(1):7-16.
doi pubmed - Barcellini W, Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis Markers. 2015;2015:635670.
doi pubmed - Dhaliwal G, Cornett PA, Tierney LM, Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599-2606.
- Jefferies LC. Transfusion therapy in autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 1994;8(6):1087-1104.
doi - Berentsen S, Barcellini W. Autoimmune hemolytic anemias. N Engl J Med. 2021;385(15):1407-1419.
doi pubmed - Barcellini W, Giannotta J, Fattizzo B. Autoimmune hemolytic anemia in adults: primary risk factors and diagnostic procedures. Expert Rev Hematol. 2020;13(6):585-597.
doi pubmed - Hill QA, Stamps R, Massey E, Grainger JD, Provan D, Hill A, British Society for H. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol. 2017;176(3):395-411.
doi pubmed - Paparoupa M, Spineli L, Framke T, Ho H, Schuppert F, Gillissen A. Pulmonary embolism in pneumonia: still a diagnostic challenge? Results of a case-control study in 100 patients. Dis Markers. 2016;2016:8682506.
doi pubmed - Broome CM, Cunningham JM, Mullins M, Jiang X, Bylsma LC, Fryzek JP, Rosenthal A. Increased risk of thrombotic events in cold agglutinin disease: A 10-year retrospective analysis. Res Pract Thromb Haemost. 2020;4(4):628-635.
doi pubmed - Mirijello A, La Marca A, D'Errico MM, Curci S, Vendemiale G, Grandone E, De Cosmo S. Venous thromboembolism during mycoplasma pneumoniae infection: case report and review of the literature. Eur Rev Med Pharmacol Sci. 2020;24(19):10061-10068.
- Defilippi A, Silvestri M, Tacchella A, Giacchino R, Melioli G, Di Marco E, Cirillo C, et al. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir Med. 2008;102(12):1762-1768.
doi pubmed - Rehman H. Hemolytic anaemia following mycoplasma infection. The Internet Journal of Hematology. 2007;4:1.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


