| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 4, April 2022, pages 178-182
Pneumoperitoneum Post Esophageal Stent Insertion Managed With Paracentesis
Priyanthi Widana Pathiranaa, b, c, Chandika Liyanagea
aDepartment of General Surgery, Dubbo Base Hospital, Dubbo, NSW, Australia
bDepartment of General Surgery, Prince of Wales Hospital, Randwick, NSW, Australia
cCorresponding Author: Priyanthi Widana Pathirana, Department of General Surgery, Prince of Wales Hospital, Randwick, NSW 2031, Australia
Manuscript submitted February 26, 2022, accepted March 18, 2022, published online March 22, 2022
Short title: Pneumoperitoneum Managed by Paracentesis
doi: https://doi.org/10.14740/jmc3920
| Abstract | ▴Top |
Gastrointestinal tract perforation is a known complication of endoscopy and may present itself as a pneumoperitoneum, pneumomediastinum or less commonly subcutaneous emphysema. Due to high insufflation pressures, barotrauma or mechanical trauma may result in a large pneumoperitoneum; however, the leak may spontaneously seal once insufflation has ceased. While unwell and peritonitic patients require prompt surgical intervention, in many cases patients may be clinically stable and respond appropriately to conservative management. We present the case of pneumoperitoneum post esophageal stent insertion for management of malignant dysphagia in a 74-year-old female patient. She experienced severe epigastric pain immediately post procedure and on image confirmation of a pneumoperitoneum underwent a paracentesis with significant pain relief and was then successfully managed conservatively. This case highlights that paracentesis may provide significant symptomatic relief from decompression of intra-abdominal free gas and facilitate non-operative management of pneumoperitoneum post upper gastrointestinal tract endoscopy.
Keywords: Pneumoperitoneum; Endoscopy; Esophageal stent; Paracentesis
| Introduction | ▴Top |
Gastrointestinal tract perforation is a known complication of endoscopy and may present itself as a pneumoperitoneum, pneumomediastinum or less commonly subcutaneous emphysema [1]. Risk of esophageal perforation following diagnostic endoscopy is estimated at 0.03-0.11% and this incidence increases with intervention [2]. In particular, the incidence following esophageal stenting for treatment of malignant dysphagia has been documented at 2% [3].
Due to high insufflation pressures, barotrauma or mechanical trauma may result in a large pneumoperitoneum; however, the leak may spontaneously seal once insufflation has ceased [4]. Additionally, there may be minimal peritoneal contamination with enteric contents, particularly in the setting of upper gastrointestinal endoscopy. Clinically well patients may be trialled with conservative management; however, there is a low threshold for operative intervention in the setting of severe pain, hemodynamic instability or peritonitis [5]. Paracentesis for management of a pneumoperitoneum is not well described in the literature, however may serve to resolve pain from abdominal distension. We present the case of a 74-year-old female patient who developed pneumoperitoneum following esophageal stent insertion for management of extrinsic esophageal compression from non-small cell lung cancer. She underwent an initial paracentesis with significant improvement in pain and was then successfully managed conservatively.
| Case Report | ▴Top |
Investigations
A 74-year-old female patient with locally advanced non-small cell lung cancer presented to hospital with 2 weeks of progressive dysphagia to solids and fluids. On review, she was able to tolerate thin liquids after having recently commenced dexamethasone, however remained dysphagic to solids. Her background included inoperable, left hilar squamous cell carcinoma of the lung with disease progression despite previous chemo-radiotherapy. The patient declined consolidative therapy with durvalumab and had remained on surveillance since late 2021. Her other comorbidities included chronic obstructive airways disease on home oxygen, gastro-esophageal reflux on pantoprazole and osteoporosis. On review she was cachectic and only weighed 39 kg. She appeared comfortable and on examination had a soft, non-tender abdomen.
Diagnosis
Laboratory results noted a normal albumin level of 36 g/L, potassium of 3.6 mmol/L and slightly reduced phosphate of 0.64 mmol/L. Her hemoglobin was normal at 121 g/L.
She was investigated with an intravenous (IV) and oral contrast computed tomography (CT) of neck, chest and abdomen which noted focal subcarinal extrinsic esophageal obstruction from her known left hilar mass infiltrating the mediastinum and resulting in complete occlusion of the left lower lobe bronchus. CT also confirmed the passage of oral contrast through to the stomach (Fig. 1). Subsequent gastrografin swallow showed a 4-cm region of subcarinal esophageal stenosis with luminal narrowing approximated at 80-90% (Fig. 2).
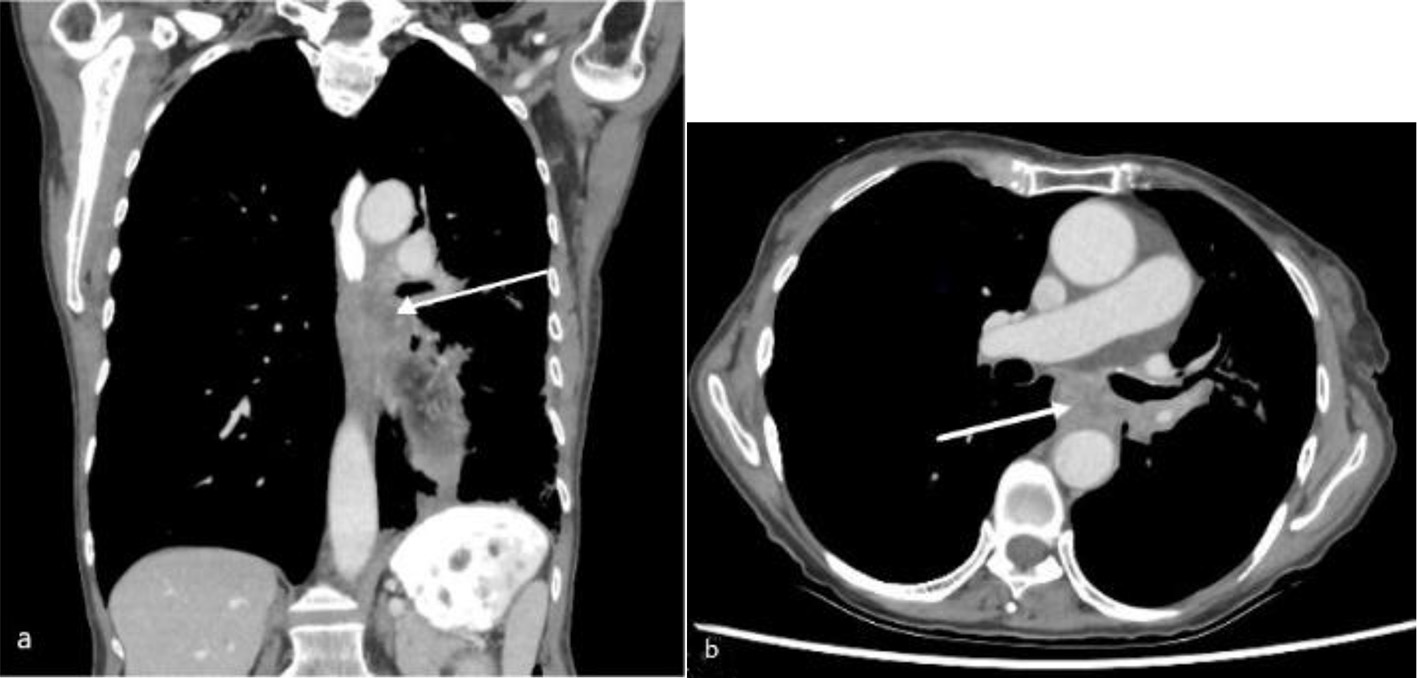 Click for large image | Figure 1. Computed tomography of chest with oral and intravenous contrast in soft tissue window (a: coronal view; b: axial view) showing a subcarinal soft tissue mass (arrow) at the left lung hilum with obstruction of the mid esophagus. |
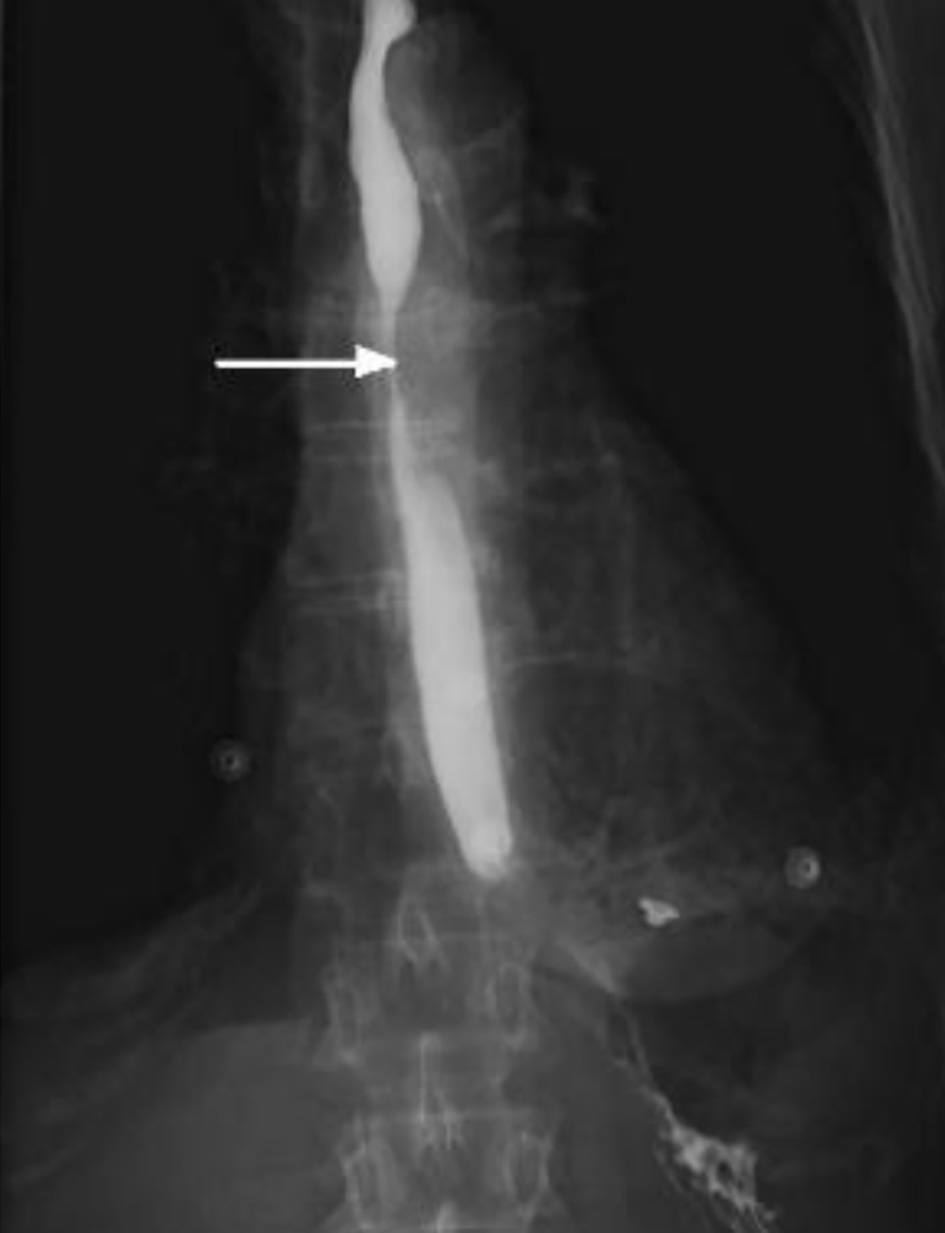 Click for large image | Figure 2. Gastrografin swallow study demonstrating tight stenosis of a 4-cm segment of the mid esophagus (arrow). |
Treatment
She was discussed by oncology at a multidisciplinary team meeting and referred to the upper gastro-intestinal team for further management. She underwent a gastroscopy and insertion of a 23-mm diameter, 105-mm long partially covered Boston scientific stent with appropriate position confirmed fluoroscopically.
Post-procedurally, she immediately reported severe upper abdominal pain that radiated to the shoulder tips and was found to have a large volume pneumoperitoneum on chest X-ray (Fig. 3a). Further investigation with an IV contrast CT of chest, abdomen and pelvis showed a large pneumoperitoneum displacing abdominal organs to the right and small inferior pneumomediastinum just distal to the esophageal stent (Fig. 3b). On CT, there were no concerning features such as free fluid or fat stranding. Reassuringly, she remained afebrile with normal observations and on examination had a distended but soft abdomen with focal guarding in the epigastrium only. It was thought that given her clinical stability her pneumoperitoneum was likely secondary to intra-operative insufflation under pressure with a small esophageal or gastric perforation from the introducer and that the perforation had sealed rather than there being an ongoing leak. While there were no signs of tension pneumoperitoneum such as hypotension, hypoxemia and tachycardia, the decision was made to proceed with needle decompression of her pneumoperitoneum given the severity of her pain. A 14-gauge cannula was inserted into the left upper quadrant just lateral to the rectus muscle with release of intra-peritoneal gas and almost immediate pain relief. She was kept fasting and commenced on IV piperacillin/tazobactam, IV fluid resuscitation, total parenteral nutrition (TPN) and IV pantoprazole.
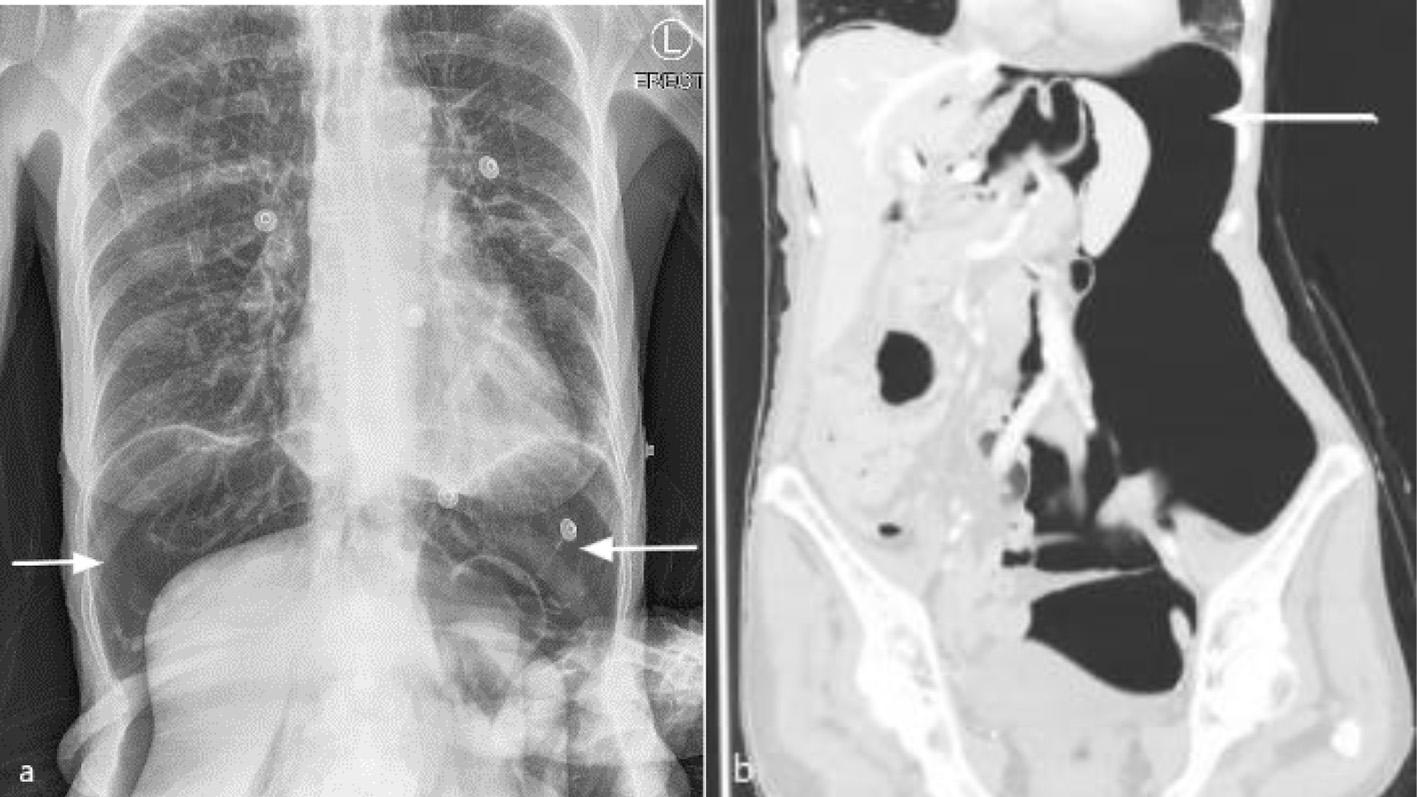 Click for large image | Figure 3. Post-procedure (a) erect chest X-ray and (b) coronal computed tomography of chest and abdomen in left lateral position with intravenous contrast in lung window, showing large pneumoperitoneum (arrow) with organ displacement. |
Follow-up and outcomes
She remained clinically well for the next 72 h and following a gastrografin swallow which demonstrated no esophageal or gastric leak, was commenced on clear fluids (Fig. 4). Her antibiotics were ceased after 7 days and she was gradually progressed onto a puree diet while her TPN was weaned with the aim to progress to solids at 6 weeks.
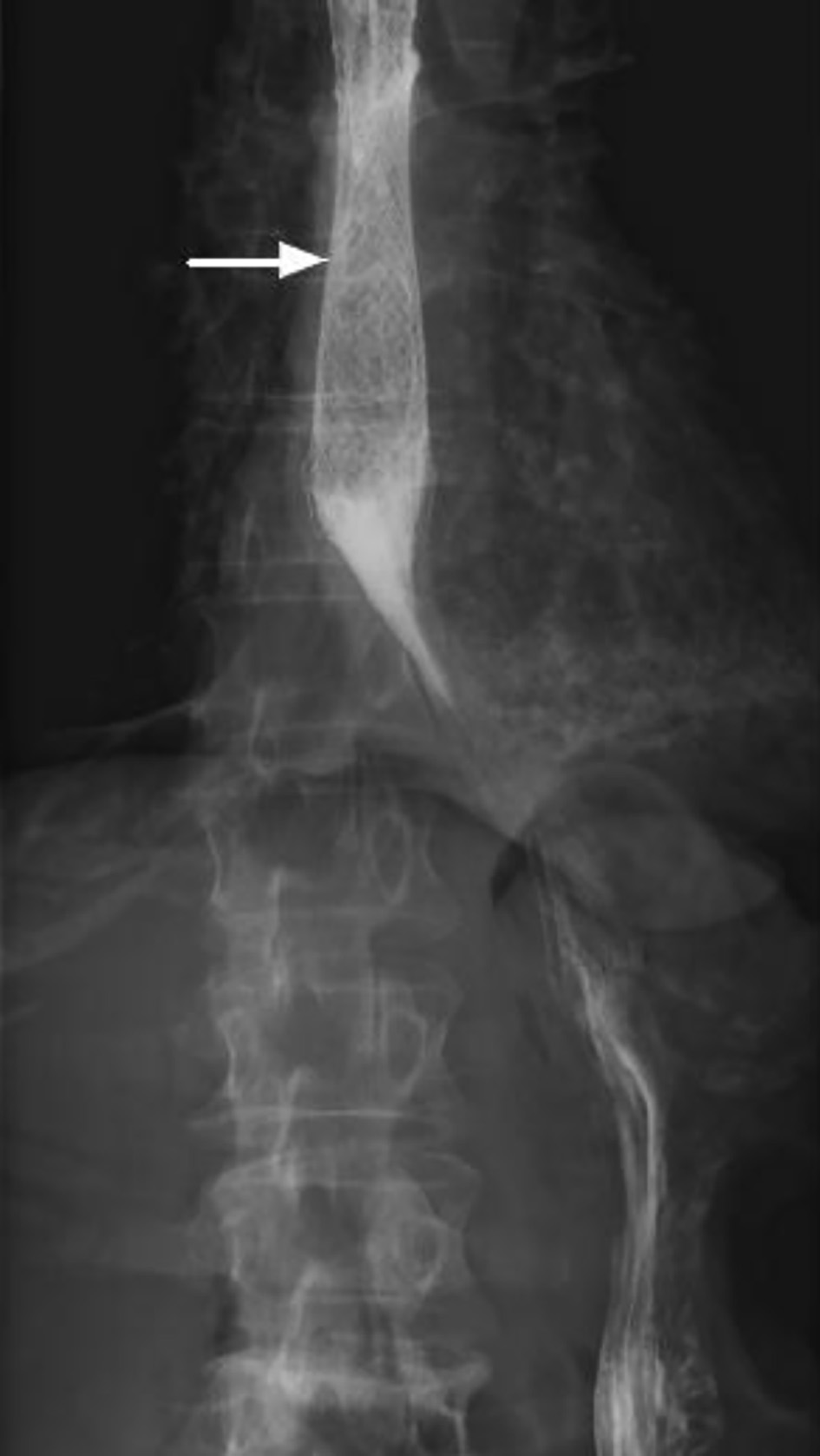 Click for large image | Figure 4. Gastrografin swallow study demonstrating appropriately positioned mid esophageal stent (arrow), normal transit of contrast and no evidence of a leak. |
| Discussion | ▴Top |
The role of esophageal stenting in malignant esophageal obstruction is well established, and aims to improve dysphagia, reduce aspiration risk and overall improve patients’ quality of life [6]. The rate of complication from esophageal stenting is approximately 30-35% and as expected, increases in frequency with longer follow-up [6]. As aforementioned, the rate of perforation during stenting of malignant esophageal obstruction is reported at 2% and may be secondary to high-pressure insufflation and barotrauma; mechanical trauma from the scope, guidewire or stent; or a combination of both [2, 4].
A history of previous chemoradiotherapy has been inconsistently associated with a greater risk of major complications after palliative esophageal stent insertion [7, 8]. A retrospective study by Lecleire et al, comparing 116 patients who underwent esophageal stent insertion for palliative management of dysphagia, found that previous chemoradiotherapy was independently predictive of major post-operative complication with an odds ratio of 5.59 (95% confidence interval: 1.73 - 18.1). Additionally, they noted that patients in the previous chemoradiotherapy group who had developed a major complication had received a significantly greater dose of radiation than those who did not at 55.9 vs. 45.9 Grays (P = 0.007) [7]. Interestingly, the median total dose of radiation was greater in the study by Lecleire et al, at 53 Grays compared to 30 Grays in the study by Homs et al which did not find an increased risk of major complication, perhaps accounting for the discrepancy in findings [7, 8]. While these studies evaluated stenting for palliative esophageal cancer, their results may be extrapolated to other mediastinal tumors which include the esophagus in the radiotherapy field as in the case of our patient with hilar non-small cell lung cancer.
Iatrogenic perforation from upper gastrointestinal endoscopy should be suspected in patients with post-procedural worsening retrosternal, abdominal or shoulder tip pain, fevers and hemodynamic instability, particularly in setting of a tension pneumoperitoneum. Diagnosis is readily confirmed on imaging, with an erect chest or abdominal X-ray often sufficient. CT however, provides greater diagnostic clarification due to its superior sensitivity and may be able to localize a perforation through extravasation of ingested water-soluble contrast [5].
Management of post-endoscopy perforation and pneumoperitoneum depends on the clinical and radiological features. Hemodynamic instability from sepsis, generalized peritonism on examination and free fluid or extraluminal contrast extravasation indicating an ongoing leak on imaging, strongly suggest need for surgical intervention [5]. Importantly, size of the pneumoperitoneum does not correspond to need for surgical intervention, particularly with intra-operative perforations where volume of free air may reflect pressure of insufflation rather than perforation size [9]. Endoscopic management is a rapidly developing field with endoscopically applied clips, stents to cover luminal defects or endoscopic suturing all possible treatment options in acute post-endoscopy perforations without evidence of sepsis, particularly for perforations in the thoracic or abdominal esophagus [10].
Clinically well patients may be appropriately managed conservatively with close monitoring for deterioration. A review of post-colonoscopy perforations noted a variable success rate of up to 73% for conservative management, likely dependent on differing patient factors and other clinical factors [5]. Theoretically, perforation of the more sterile foregut may be more amenable to conservative treatment.
While surgical and increasingly endoscopic interventions for treatment of iatrogenic pneumoperitoneum are well recognized options, paracentesis is a largely unreported option. Description in the literature is limited to case studies and has primarily been used in the setting of tension pneumoperitoneum for immediate correction of resulting hemodynamic compromise or for the management of non-resolving pneumoperitoneum in clinically well patients [11-14]. In reporting this case, we aim to highlight the utility of paracentesis as a minimally invasive option in the acute setting for treatment of abdominal and referred pain from a pneumoperitoneum, potentially preventing unnecessary surgical intervention.
Learning points
Perforation is a known complication of esophageal stent insertions and if located in the distal esophagus may result in a pneumoperitoneum. While unwell and peritonitic patients require prompt surgical intervention, in many cases patients may be clinically stable and respond appropriately to conservative management. Paracentesis may provide significant symptomatic relief from decompression of intra-abdominal free gas and facilitate non-operative management in borderline patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was gained prior to completion of the case report.
Author Contributions
PWP contributed to conception, writing and editing of the case report. CL contributed to the conception and editing of the case report.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CT: computed tomography; IV: intravenous; TPN: total parenteral nutrition
| References | ▴Top |
- Falidas E, Anyfantakis G, Vlachos K, Goudeli C, Stavros B, Villias C. Pneumoperitoneum, retropneumoperitoneum, pneumomediastinum, and diffuse subcutaneous emphysema following diagnostic colonoscopy. Case Rep Surg. 2012;2012:108791.
doi pubmed - Lampridis S, Mitsos S, Hayward M, Lawrence D, Panagiotopoulos N. The insidious presentation and challenging management of esophageal perforation following diagnostic and therapeutic interventions. J Thorac Dis. 2020;12(5):2724-2734.
doi pubmed - Vermeulen BD, Siersema PD. Esophageal stenting in clinical practice: an overview. Curr Treat Options Gastroenterol. 2018;16(2):260-273.
doi pubmed - Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95(12):3418-3422.
doi pubmed - Lohsiriwat V. Colonoscopic perforation: incidence, risk factors, management and outcome. World J Gastroenterol. 2010;16(4):425-430.
doi pubmed - Sharma P, Kozarek R, Practice Parameters Committee of American College of Gastroenterology. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol. 2010;105(2):258-273.
doi pubmed - Lecleire S, Di Fiore F, Ben-Soussan E, Antonietti M, Hellot MF, Paillot B, Lerebours E, et al. Prior chemoradiotherapy is associated with a higher life-threatening complication rate after palliative insertion of metal stents in patients with oesophageal cancer. Aliment Pharmacol Ther. 2006;23(12):1693-1702.
doi pubmed - Homs MY, Hansen BE, van Blankenstein M, Haringsma J, Kuipers EJ, Siersema PD. Prior radiation and/or chemotherapy has no effect on the outcome of metal stent placement for oesophagogastric carcinoma. Eur J Gastroenterol Hepatol. 2004;16(2):163-170.
doi pubmed - Yang S, Zeng MS, Zhang ZY, Zhang HL, Liang L, Zhang XW. Pneumomediastinum and pneumoperitoneum on computed tomography after peroral endoscopic myotomy (POEM): postoperative changes or complications? Acta Radiol. 2015;56(10):1216-1221.
doi pubmed - Al-Asiry J, Lord R, Mohammed N. Management of spontaneous and iatrogenic perforations, leaks and fistulae of the upper gastrointestinal tract. Ther Adv Gastrointest Endosc. 2019;12:2631774519895845.
doi pubmed - Siboni S, Bona D, Abate E, Bonavina L. Tension pneumoperitoneum following endoscopic submucosal dissection of leiomyoma of the cardia. Endoscopy. 2010;42(Suppl 2):E152.
doi pubmed - Fu K, Ishikawa T, Yamamoto T, Kaji Y. Paracentesis for successful treatment of tension pneumoperitoneum related to endoscopic submucosal dissection. Endoscopy. 2009;41(Suppl 2):E245.
doi pubmed - Vieira A, Fernandes V, Freitas J. Post-colonoscopic polypectomy pneumoperitoneum successfully treated by paracentesis. Endoscopy. 2005;37(8):782.
doi pubmed - Brotherton T, Chhaparia A, Presti M, Sayuk G, Elwing J. Symptomatic pneumoperitoneum after gastrostomy tube placement managed by pneumocentesis. ACG Case Rep J. 2021;8(11):e00700.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


