| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 5, May 2022, pages 197-201
Acquired Hemophilia A After SARS-CoV-2 Infection: A Case Report
Jennifer Nardellaa, Domenico Comitangeloa, Renato Marinob, Giuseppe Malcangib, Marco Damiano Barrattaa, Carlo Sabbaa, Antonio Perronea, c
aDIM - Interdisciplinary Department of Medicine, Internal Medicine Unit “Cesare Frugoni”, University of Bari “Aldo Moro” School of Medicine, Policlinico Hospital, Bari, Italy
bHemophilia and Transfusion Center, University of Bari “Aldo Moro” School of Medicine, Policlinico Hospital, Bari, Italy
cCorresponding Author: Antonio Perrone, DIM - Interdisciplinary Department of Medicine, Internal Medicine Unit “Cesare Frugoni”, University of Bari “Aldo Moro” School of Medicine, Policlinico Hospital, 70124 Bari (BA), Italy
Manuscript submitted March 1, 2022, accepted March 14, 2022, published online April 12, 2022
Short title: Acquired Hemophilia A After SARS-CoV-2
doi: https://doi.org/10.14740/jmc3921
| Abstract | ▴Top |
Acquired hemophilia A is a rare autoimmune coagulation disorder associated to the development of neutralizing antibodies directed towards coagulation factor VIII, known as factor VIII inhibitors, in subjects with previous normal clotting system homeostasis and personal and family history negative for bleeding episodes. This condition, although variable in severity and clinical presentation, may lead to severe and life-threatening hemorrhages which can be either spontaneous or associated with traumatic events and invasive procedures. Here we report the case of a 53-year-old woman who was admitted to our Internal Medicine Unit “Cesare Frugoni”, Policlinico di Bari, in July 2021, and diagnosed with acquired hemophilia A. We aim to raise awareness about this rare condition, its clinical presentation and therapeutic management options in order to obtain a quick diagnosis and an effective therapeutic intervention.
Keywords: Acquired hemophilia A; SARS-CoV-2; Rituximab; Recombinant porcine factor VIII; Activated prothrombin complex concentrate
| Introduction | ▴Top |
Acquired hemophilia A (AHA) is a rare disorder mostly idiopathic in its nature, although it may also be related to autoimmune disorders, malignancy, pregnancy, drugs, and infections [1, 2]. Among the recognized associated infectious agents, viral pathogens play a major role. Recently, cases of AHA have been described following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or the administration of coronavirus disease 2019 (COVID-19) vaccines [3-5]. The pathophysiology between SARS-CoV-2 infection and the development of autoimmune disorders is yet to be fully determined. Evidences address it to a mechanism of molecular mimicry between the virus and human proteins sharing peptide sequences [6]. This cross-reactivity reactions involving self-antigens might be as well at the base of COVID-19 vaccine-related autoimmune episodes [7, 8].
In AHA, the localization of the bleeding site may vary from case to case, although it mainly involves the skin, soft tissues, and muscles; joint bleeding, a typical feature of inherited hemophilia A, is uncommon in the acquired form of the disease. The development of antibodies directed towards coagulation factor VIII (FVIII) induces an alteration of the intrinsic pathway of the coagulation cascade leading to a characteristic isolated prolongation of the activated partial thromboplastin time (aPTT) due to FVIII deficiency.
The therapeutic strategy approaching these patients is directed at bleeding control and inhibitor eradication to prevent subsequent bleeding episodes, as well as detecting and eventually treating any underlying diseases. First-line hemostatic drugs are the so-called “bypassing agents” (activated recombinant factor VII (rFVII) concentrate and activated prothrombin complex concentrate) and recombinant porcine factor VIII (rpFVIII) concentrate. The eradication of the inhibitors is achieved through the administration of corticosteroids, cyclophosphamide, and other immunosuppressive drugs [9-11].
In the following report we present a case of AHA after infection with SARS-CoV-2 in a patient with no history of bleeding or autoimmune disorders.
| Case Report | ▴Top |
Investigations
Here we report the case of a 53-year-old woman admitted to the emergency ward in June 2021 for the sudden development of spontaneous ecchymosis first bilaterally in the deltoid area, followed by more extended subcutaneous hematomas in the lower limbs (Fig. 1). Besides these hemorrhagic manifestations, at the time of hospital admission the patient presented with normal vital signs and the physical examination was unremarkable. The patient’s medical history did not reveal any relevant chronic illnesses, previous bleeding episodes and other prior disease states or medical therapies other than a few days of low-grade fever (37.4 °C) and flu-like symptoms such as cough, sore throat, generalized malaise and ageusia a few weeks prior to the development of the hematomas.
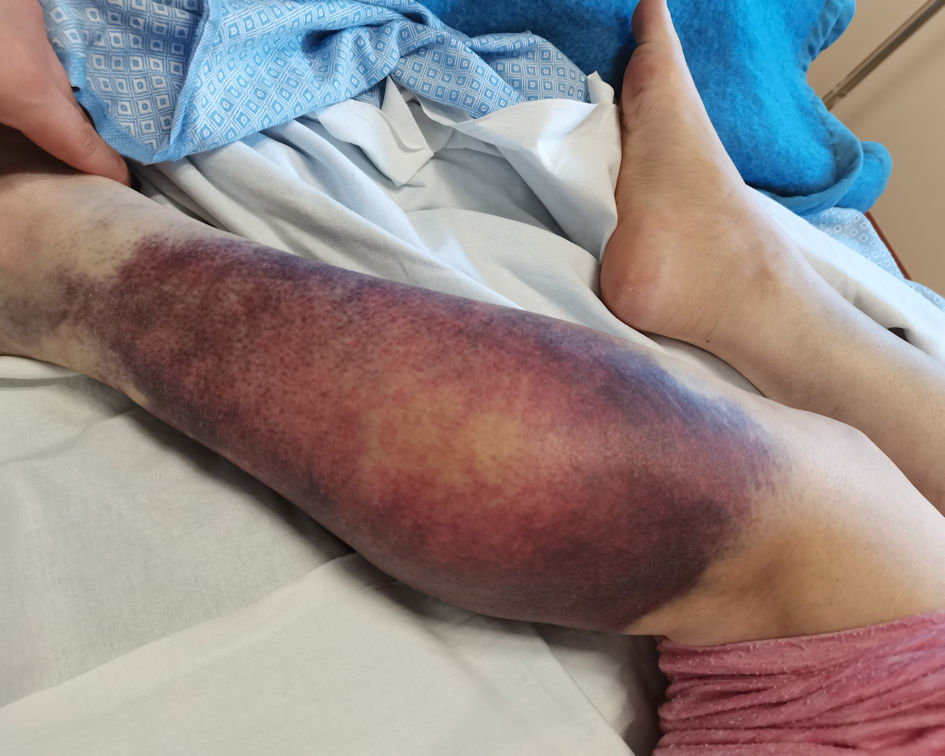 Click for large image | Figure 1. Spontaneous left lower limb hematoma which was already present at the time of hospital admission, prior to the start of any therapeutic interventions. |
Diagnosis
Laboratory tests were carried out which revealed the presence of anemia (hemoglobin (Hb) 7.4 g/dL, normal range (NR): 12 - 15 g/dL) and alteration of the coagulation profile showing an abnormal aPTT of 2.9 (NR: < 1.2) not corrected by the aPTT mixing test, with normal prothrombin time; platelet count was also in the normal range. The clinical picture and the laboratory findings raised the suspicion of a coagulation-related disorder which leads to detection of very low FVIII plasma levels of < 1% (NR: 60-170%) and the presence of FVIII inhibitor activity (142 BU/mL, NR: 0 - 0.5 BU/mL) leading to the diagnosis of AHA. Among the laboratory tests carried out during the hospital stay (QuantiFERON-TB Gold, lymphocytes subset distribution test, hepatitis and other major viral infections, blood and urine cultures) no relevant alterations were detected besides abnormal thyroid homeostasis (thyroid-stimulating hormone (TSH) 4.37 mU/L, NR: 0.36 - 3.74 mU/L; free triiodothyronine (FT3) 1.8 pg/mL, NR: 2.3 - 4.1 pg/mL; free thyroxine (FT4) 0.96 pg/mL, NR: 0.88 - 1.48 pg/mL), the presence of anti-thyroglobulin (1,276 U/mL, NR: 0 - 115 U/mL) and anti-thyroid peroxidase antibodies (210 U/mL, NR: 0 - 34 U/mL). Based on the flu-like symptoms described by the patient the presence of SARS-CoV-2 anti-spike protein antibodies were investigated and detected (818 U/mL, NR: < 80) in the absence of any vaccinations against the virus suggesting a previous infection with this pathogen. Imaging studies (computed tomography (CT) scan, magnetic resonance imaging (MRI) and positron emission tomography (PET) scan) did not show any relevant clinical findings.
Treatment
Given the clinical picture and the severity of the hematomas, in collaboration with the Hemophilia and Thrombosis Centre of our hospital, immediate treatment with activated rFVII concentrate (rFVIIa, NOVOSEVEN, Novo Nordisk) at the dose of 100 µg/kg every 2 h was initiated associated with the administration of methylprednisolone (1 mg/kg/day) and oral cyclophosphamide (1 mg/kg/day). After a few days of treatment, the patient developed a severe oropharyngeal mucositis due to the fact that she was chewing onto the administered drugs, therefore cyclophosphamide was discontinued due to the patient’s inability to properly follow the instructions for a correct oral administration. Within the first 2 days of treatment with rFVIIa, Hb levels were stable enough to progressively elongate the time between each administration of rFVIIa, and it was finally discontinued 7 days after the start of the therapy. On the following days of hospital stay the patient experienced an episode of high-grade fever (39 °C) and generalized malaise. Blood cultures were found positive for Klebsiella pneumoniae infection, which showed multidrug resistance. Given this evidence ceftazidime-avibactam (Zavicefta, Pfizer) was introduced as antibiotic regimen (2 g/0.5 g, every 8 h) in combination with amikacin (0.5 g, twice a day), with a successful clinical outcome showing that blood cultures taken 4 days after the initiation of the antimicrobial therapy yielded negative results. A few days after this acute event, the patient started complaining about lower back pain especially radiated to the right limb and to develop new ecchymosis associated with a drop in Hb levels. An abdominal ultrasound was performed and showed a right iliopsoas hematoma. This finding was confirmed with an abdominal CT scan (Fig. 2). Treatment with rFVII was then reintroduced, but unfortunately was not leading to the same results as in the previous cycle of administration. Hb levels kept decreasing and new hematomas developed on the contralateral arm and leg (Fig. 3). For these reasons, rFVIIa administration was discontinued and treatment with rpFVIII (Obizur, Takeda) at the dosage of 100 IU/kg every 6 h was started. This new therapy resulted in a partial clinical and laboratory response, being the detected FVIII plasma levels lower than expected. A midline venous catheter was positioned 30 min after Obizur infusion, due to the excessive leakage of blood in the subcutaneous tissues occurring while drawing blood to check for laboratory testing. After 4 days of rpFVIII treatment, once Hb levels were stable enough, a treatment course with activated prothrombin complex concentrate (Feiba, Takeda) was carried out, at the initial dosage of 100 IU/kg every 12 h on the first day, gradually adjusted up to 50 IU every 12 h for 13 days with a successful control of the bleeding episodes. Considering that the patient presented high-titer FVIII inhibitor and the very low FVIII level, a more intensive immunosuppressive strategy with rituximab (375 mg/m2) was necessary to control the underling autoimmune process once a week for 4 weeks.
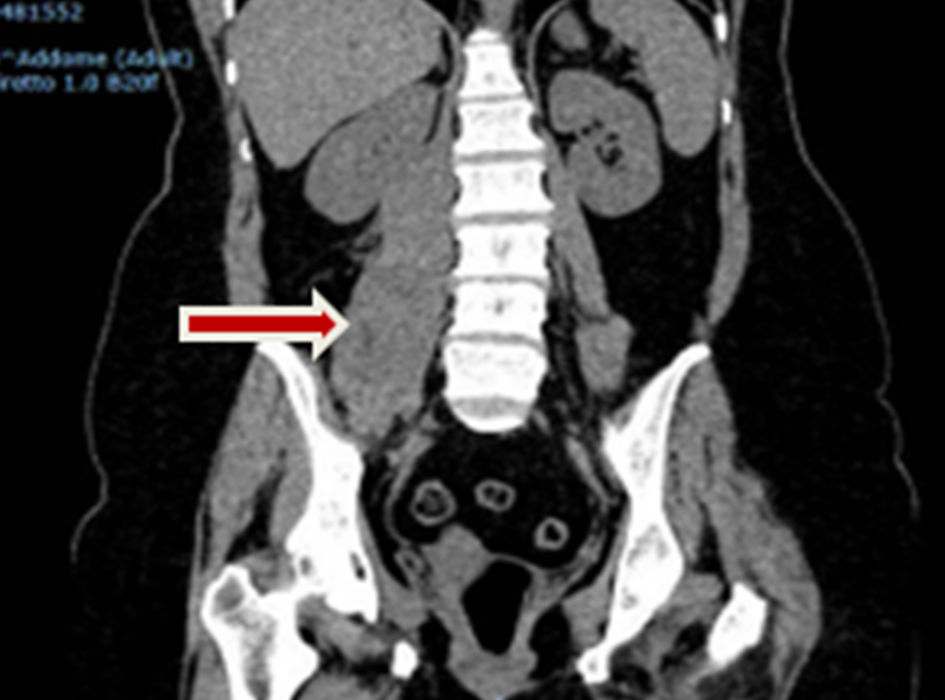 Click for large image | Figure 2. Abdominal CT scan image, with arrow indicating the presence of a right iliopsoas hemorrhagic focus which the patient developed during the treatment with corticosteroids only. CT: computed tomography. |
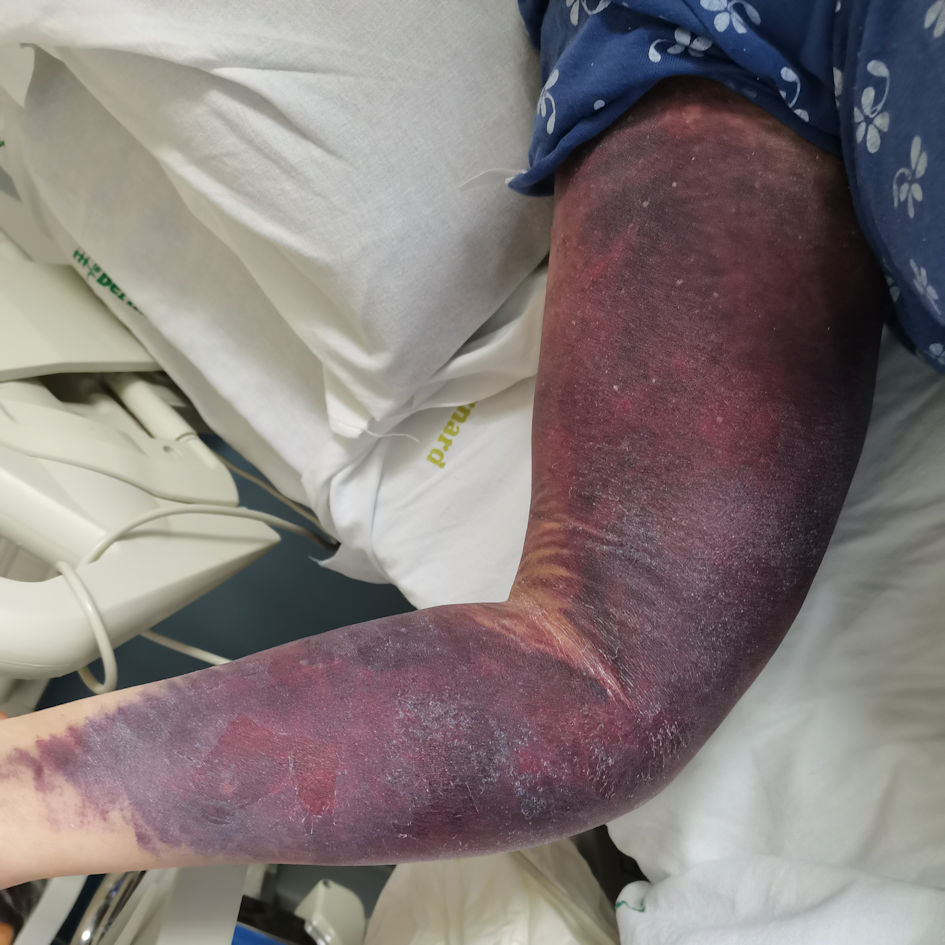 Click for large image | Figure 3. Right upper limb hematoma due to blood sampling, occurred during therapy with recombinant porcine factor VIII. |
Follow-up and outcomes
The 4 weeks’ cycle of rituximab was well tolerated by the patient who was then able to return home after a short rehabilitation cycle (Fig. 4). She was prescribed to continue corticosteroid therapy with dexamethasone 10 mg a day and scheduled for monthly ambulatory follow-up. After 6 months of clinical and laboratory follow-up, FVIII inhibitor was completely eradicated, and corticosteroid treatment was discontinued.
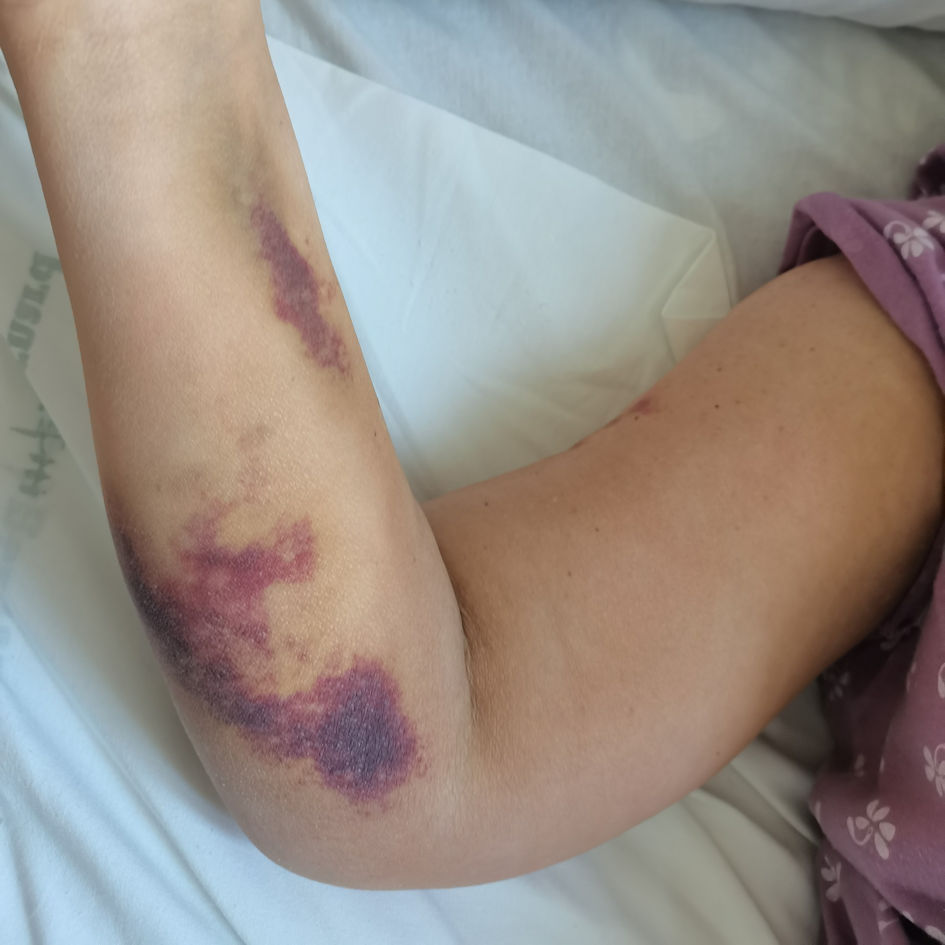 Click for large image | Figure 4. Resolution of the right upper limb hematoma after complete rituximab cycle, 4 weeks from the initiation of the treatment. |
| Discussion | ▴Top |
Our case highlights the complex management of bleeding episodes of these patients, which often requires the administration of multiple therapeutic agents. As far as our patient is concerned rituximab treatment associated with corticosteroids demonstrated efficacy, leading to a gradual reduction of FVIII inhibitor titer until its disappearance. Rituximab is mainly used as second-line therapeutic option in acquired hemophilia, alone or in association with other immunosuppressive agents [12-16]. The first bleeding episode was successfully treated with rFVIIa, the most widely used drug in this disorder which yielded an initial accurate control of the bleeding, but on the following days the patient became refractory to this therapy with the subsequent development of new ecchymosis. For this reason, rpFVIII treatment was started with a sufficient stabilization of the clinical picture although associated with a partial laboratory response, probably related to the presence of cross-reacting anti-rpFVIII inhibitor, which has been evidenced in more than 40% of patients with acquired hemophilia translating into a reduced efficacy of the drug [17, 18]. Subsequently a short-term prophylaxis course with activated prothrombin complex concentrate, was carried out, which successfully prevented the onset of further bleeding episodes. At the moment of diagnosis, the patient presented with a clinical history free of major comorbidities prior to the development of the hematomas. The patient did not receive any COVID-19 vaccinations and reported close contact with a SARS-CoV-2 infected individual (caregiver to an infected patient). Although we do not have a positive test for SARS-CoV-2, the presence of SARS-CoV-2 anti-spike protein antibodies and the symptoms described allow us to address a previous infection with SARS-CoV-2. This leads us to point out the role of this infection in establishing an alteration of the immune profile in our patient, who besides the presence of AHA, was diagnosed with an autoimmune thyroiditis. There are reported cases of COVID-19-mediated alteration of the thyroid profile through the activation of inflammatory response triggered by the angiotensin-converting enzyme (ACE)-2-mediated viral tropism in the thyroid gland [19]. This process triggers both a process of viral-mediated organ damage and molecular mimicry with subsequent autoimmune response [20].
Learning points
An interesting aspect of this case of AHA is that it turned out to be refractory to the use of the first-line therapeutic interventions. The standard oral therapy with corticosteroids and orally administered cyclophosphamide was not administered due to the inability of the patient to swallow. This might have contributed to the initial incomplete and delayed success of the therapy. To effectively control the risk of bleeding it was necessary to introduce therapy with bypassing agents (rpFVIII, activated prothrombin complex concentrate). In order to fully control the underling autoimmune process, reflected by high-titer FVIII inhibitor and the very low FVIII level, treatment with rituximab was necessary.
Acknowledgments
We would like to thank the medical staff of the Internal Medicine Unit Cesare Frugoni and the Hemophilia and Transfusion Center who were involved in the care of this patient.
Financial Disclosure
All authors have no financial disclosure to declare.
Conflict of Interest
All authors declare no conflict of interest.
Informed Consent
We obtained the informed consent from the patient for the publication of this case report and accompanying images.
Author Contributions
Antonio Perrone, Jennifer Nardella, Domenico Comitangelo and Marco Damiano Barratta all contributed to the diagnosis and management of the patient, discussion, writing and drafting of this case report. Renato Marino and Giuseppe Malcangi contributed to the discussion, designs, acquisition and interpretation of the data. Carlo Sabba contributed to the revision and final approval of the case report.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Ogawa Y, Yanagisawa K, Uchiumi H, Ishizaki T, Mitsui T, Gouda F, Ieko M, et al. Clinical characteristics and outcomes of acquired hemophilia A: experience at a single center in Japan. Int J Hematol. 2017;106(1):82-89.
doi pubmed - Knoebl P, Marco P, Baudo F, Collins P, Huth-Kuhne A, Nemes L, Pellegrini F, et al. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost. 2012;10(4):622-631.
doi pubmed - Wang KY, Shah P, Roarke DT, Shakil SA. Severe acquired haemophilia associated with asymptomatic SARS-CoV-2 infection. BMJ Case Rep. 2021;14(7):e242884.
doi pubmed - Franchini M, Glingani C, De Donno G, Casari S, Caruso B, Terenziani I, Perotti C, et al. The first case of acquired hemophilia A associated with SARS-CoV-2 infection. Am J Hematol. 2020;95(8):E197-E198.
doi pubmed - Hafzah H, McGuire C, Hamad A. A case of acquired hemophilia A following SARS-CoV-2 infection. Cureus. 2021;13(7):e16579.
doi pubmed - Ehrenfeld M, Tincani A, Andreoli L, Cattalini M, Greenbaum A, Kanduc D, Alijotas-Reig J, et al. COVID-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597.
doi pubmed - Radwi M, Farsi S. A case report of acquired hemophilia following COVID-19 vaccine. J Thromb Haemost. 2021;19(6):1515-1518.
doi pubmed - Soliman DS, Al Battah A, Al Faridi D, Ibrahim F. Acquired hemophilia A developed post COVID-19 vaccine: an extremely rare complication. J Med Cases. 2022;13(1):1-4.
- Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, Tiede A, et al. Acquired hemophilia A: Updated review of evidence and treatment guidance. Am J Hematol. 2017;92(7):695-705.
doi pubmed - Tiede A, Collins P, Knoebl P, Teitel J, Kessler C, Shima M, Di Minno G et al. International recommendations on the diagnosis and treatment of patients with acquired hemophilia A. Haematologica. 2020;105(7):1791-1801.
doi pubmed - Toschi V, Baudo F. Diagnosis, laboratory aspects and management of acquired hemophilia A. Intern Emerg Med. 2010;5(4):325-333.
doi pubmed - Zanon E, Pasca S, Borchiellini A, Lodigiani C, Molinari AC, Ambaglio C, Valeri F, et al. Susoctocog-alfa (Obizur((R))) in the treatment of nine elderly patients with acquired haemophilia A: an Italian multicentre real world experience. Blood Transfus. 2020;18(4):312-321.
- Campbell S, Mason J, Prasad R, Ambrose H, Hunt S, Tran H. Acquired haemophilia and haemostatic control with recombinant porcine factor VIII: case series. Intern Med J. 2021;51(2):215-219.
doi pubmed - D'Arena G, Grandone E, Di Minno MN, Musto P, Di Minno G. The anti-CD20 monoclonal antibody rituximab to treat acquired haemophilia A. Blood Transfus. 2016;14(2):255-261.
- Mumoli N, Giorgi-Pierfranceschi M, Ferretti A, Vitale J, Cei M. Acquired hemophilia treated using low-dose of rituximab. J Am Geriatr Soc. 2016;64(8):1744-1745.
doi pubmed - Yao Q, Zhu X, Liu Y, Zhang F, Yuan T, Xu J, Wang X. Low-dose rituximab in the treatment of acquired haemophilia. Hematology. 2014;19(8):483-486.
doi pubmed - Arokszallasi A, Razso K, Ilonczai P, Olah Z, Bereczky Z, Boda Z, Schlammadinger A. A decade-long clinical experience on the prophylactic use of activated prothrombin complex concentrate in acquired haemophilia A: a case series from a tertiary care centre. Blood Coagul Fibrinolysis. 2018;29(3):282-287.
doi pubmed - Turkantoz H, Konigs C, Knobl P, Klamroth R, Holstein K, Huth-Kuhne A, Heinz J, et al. Cross-reacting inhibitors against recombinant porcine factor VIII in acquired hemophilia A: Data from the GTH-AH 01/2010 Study. J Thromb Haemost. 2020;18(1):36-43.
doi pubmed - Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, Law CY, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021;106(2):e926-e935.
doi pubmed - Knack RS, Hanada T, Knack RS, Mayr K. Hashimoto's thyroiditis following SARS-CoV-2 infection. BMJ Case Rep. 2021;14(8):e244909.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


