| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 7, July 2022, pages 313-317
Two Patients With Difficulty in Swallowing due to Dysphagia Lusoria
Onyinye Ugonaboa, b, Mujtaba Mohameda, Wesam Frandaha, Ahmed Sherifa
aDivision of Gastroenterology, Department of Medicine, Joan C. Edwards School of Medicine, Marshall University, Huntington, WV 25701, USA
bCorresponding Author: Onyinye Ugonabo, Internal Medicine Residency Program, Joan C. Edwards School of Medicine, Marshall University, Huntington, WV 25701, USA
Manuscript submitted April 5, 2022, accepted May 28, 2022, published online June 16, 2022
Short title: Difficulty in Swallowing due to Dysphagia Lusoria
doi: https://doi.org/10.14740/jmc3930
| Abstract | ▴Top |
Dysphagia lusoria (DL) is a rare clinical entity caused by compression of the esophagus by an aberrant right subclavian artery. It is coined from the Latin word meaning freak or jest of nature, with an estimated prevalence of approximately 0.5%. Before the term DL was known, the artery abnormality was referred to as luxus nature. Most patients are asymptomatic. In 30-40% of cases, DL results in tracheoesophageal symptoms like dysphagia to solid foods, chest pain, cough, and Horner’s syndrome. Symptoms presenting later in life have been linked to arteriosclerosis and diminishing esophageal compliance resulting in compression. Another reason why people become symptomatic is due to Kommerell’s diverticulum, a disorder that was first described by Kommerell, a German radiologist in 1936. It is also known as lusoria diverticulum, remnant diverticulum or lusoria root. This disorder represents a remnant of the left dorsal arch which forms a vascular ring behind the esophagus, leading to external compression. The key to diagnosis of DL is a barium esophagogram which may show extrinsic compression. Computed tomography or magnetic resonance imaging can be used for definite delineation of the vascular anatomy. Treatment approach is dietary modification or surgical intervention for unresponsive cases. Here, we present cases of dysphagia in two middle-aged women caused by compression effect on the esophagus by an aberrant right subclavian artery who did not respond to dietary modification.
Keywords: Dysphagia lusoria; Difficulty in swallowing; Aberrant right subclavian artery; CT; MRI
| Introduction | ▴Top |
Dysphagia lusoria (DL), also known as Bayford-Autenrieth dysphagia, was described by David Bayford in 1787 [1]. It is caused by a congenital vascular anomaly that occurs during aortic arch formation which causes extrinsic compression of the esophagus and surrounding structures. The vessel involved is known as the aberrant right subclavian artery (ARSA), or arterial lusoria. The prevalence is estimated to be between 0.5% and 0.7% of the population [2]. It usually presents in the fourth or fifth decade [3]. About 20-40% of ARSA produces symptoms in patients [2]. ARSA has a female predominance [4], with a gender distribution of about 44.7% and 55.3% in males and females, respectively [1]. The most important clinical hallmark in adults is dysphagia, especially to solids with globus sensation. The majority of patients would report one-sided dysphagia; however, most are asymptomatic and are usually incidentally found on imaging. Other symptoms include chest pain, hoarseness (due to compression of the recurrent laryngeal nerve), chronic cough, Horner’s syndrome (due to oculosympathetic pathway compression), and weight loss due to reduced intake. Symptoms may worsen with advanced age due to atherosclerosis hardening of the arteries and increased esophageal rigidity. Treatment depends on multiple factors such as age, corresponding vascular anomalies, as well as comorbidities, and involves both medical and surgical approaches.
| Case Reports | ▴Top |
Case 1
Investigations
A 56-year-old woman with a past medical history of peptic ulcer disease presented with a chief complaint of dysphagia to solid foods, globus sensation felt mostly on the right side of the chest, and occasional chest pain for several years. Chest pain was retrosternal, dull, 6/10 with no radiation, associated with shortness of breath, especially on moderate exertion, cough, and easy fatigability. On examination, the patient appeared frail. Vitals were stable. Body mass index (BMI) was 19 kg/m2 (18.5 - 24.9 kg/m2). Other examination findings were unremarkable. Laboratory data showed hemoglobin of 13 mg/dL (12 - 16 mg/dL), white blood cell (WBC) of 7.5 × 103/µL (4 - 11 × 103/µL), serum creatinine of 0.8 mg/dL (0.4 - 1.0 mg/dL), and blood urea nitrogen (BUN) of 8 mg/dL (5 - 25 mg/dL). Troponins and liver function test were unremarkable. The patient had a chest radiograph which was unremarkable. Due to persistent chest pain, a left heart catheterization (LHC) was performed, which was negative for coronary artery disease. Autoimmune antibodies like antinuclear antibody, anti-Jo-1, anticentromere, anti-scl-70, and anti-ribonucleoprotein were all negative.
Diagnosis
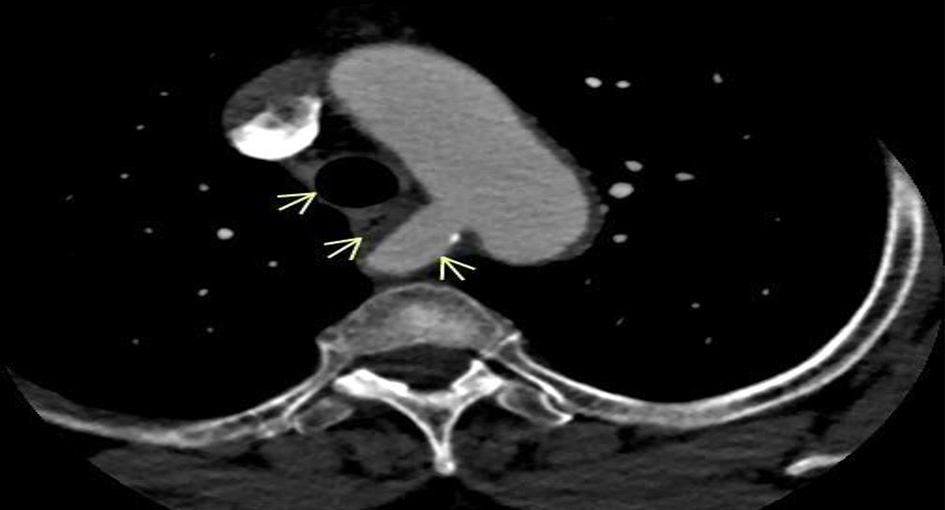
Esophagogastroduodenoscopy (EGD) done was unrevealing of any abnormality. The patient eventually had a barium esophagogram which came back positive for esophageal stricture (Fig. 1). A subsequent computed tomography (CT) angiography of the chest revealed an abnormal right subclavian artery around the trachea and esophagus (Fig. 2).
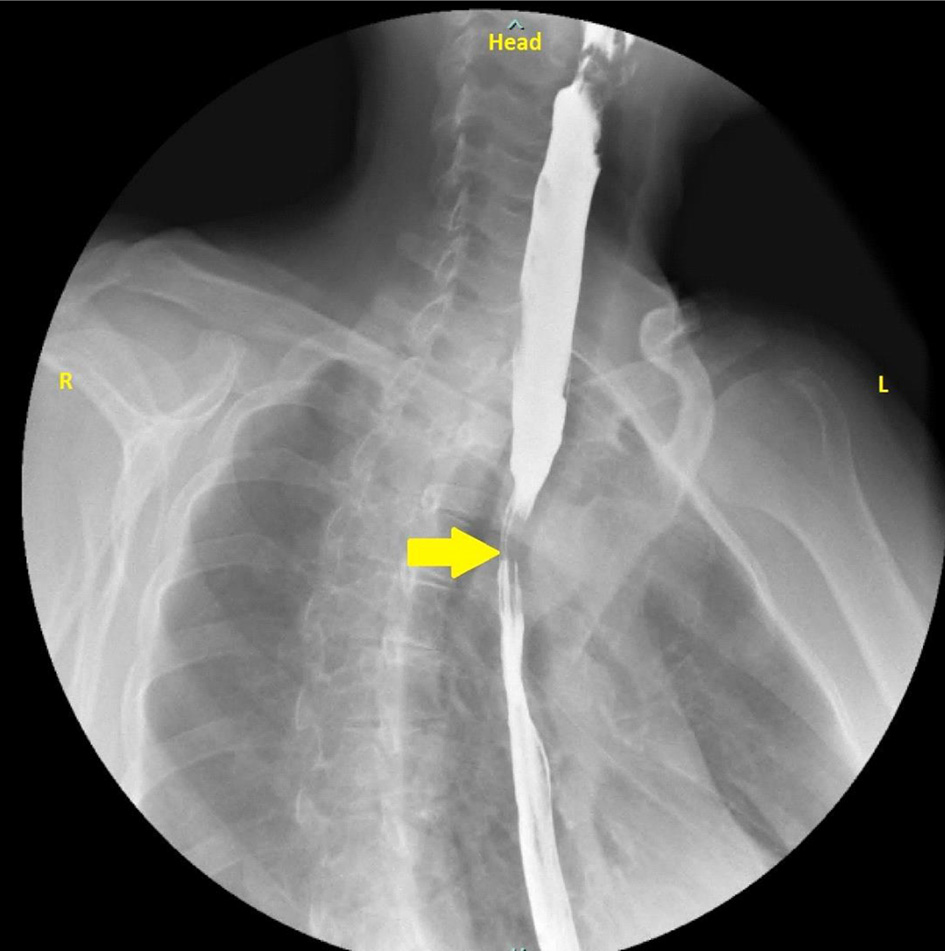 Click for large image | Figure 1. Barium swallow with esophageal narrowing (yellow arrow). |
 Click for large image | Figure 2. CT angiography of the chest showing arrow (blue) pointing at the aberrant right subclavian artery compressing the esophagus and the trachea. CT: computed tomography. |
Treatment
Dietary modification was recommended and was unsuccessful. The patient eventually opted for surgical management. Surgical incision was through cervical approach. The aberrant artery was ligated near its root and connected with the right carotid artery.
Follow-up and outcomes
Patient recovered well from surgery. On follow-up by the 4th month, she reported being able to tolerate solids without difficulties. She denied any further chest pain. We carried out a follow-up barium esophagogram which came out normal with no external compression of the esophagus.
Case 2
Investigations
A 60-year-old woman with a past medical history of coronary artery disease status post LHC with percutaneous coronary intervention presented to the clinic complaining about mild to moderate intermittent dysphagia to solids for many years, unresponsive to acid suppressants. She denied any burning chest pain, non-steroidal anti-inflammatory drug use, laryngeal or esophageal surgeries. Physical examination revealed stable vitals with a body mass index of 18 kg/m2. Other examination findings were unremarkable. Significant laboratory findings showed hemoglobin of 11.5 mg/dL (12 - 16 mg/dL), sodium of 135mmol/L (136 - 145 mmol/L), and potassium of 3.6 mmol/L (3.6 - 5.1 mmol/L). Troponins were negative. Chest radiography was unrevealing of any acute abnormality. Electrocardiography was negative for any ischemic changes.
Diagnosis
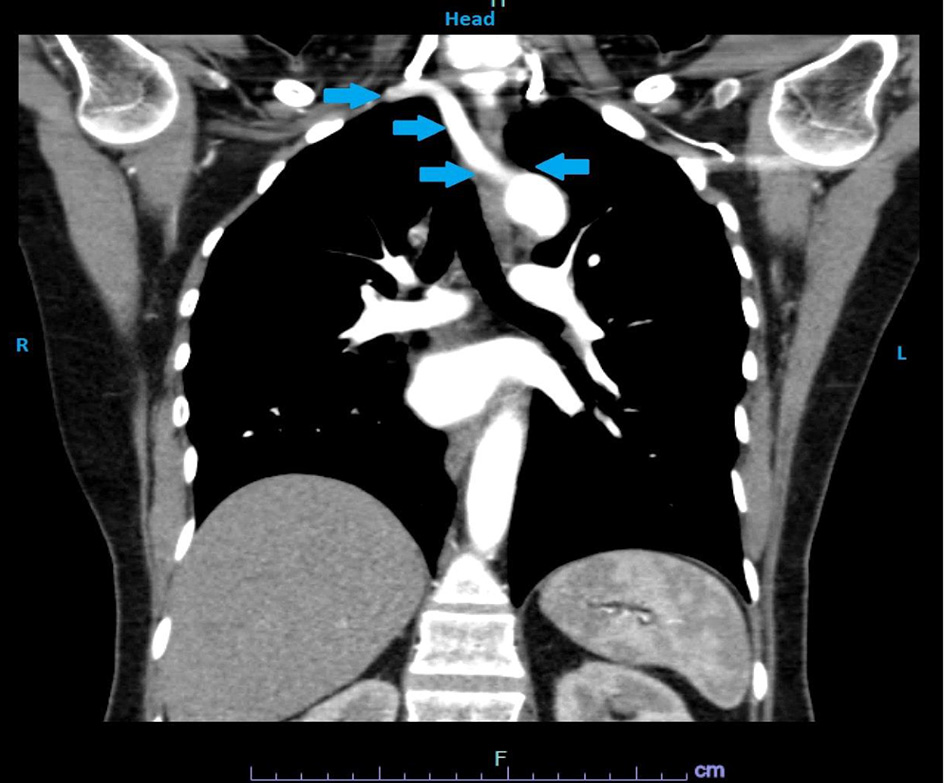
EGD did not show intra-luminal stricture (Fig. 3). Esophageal manometry was normal. Barium esophagogram obtained showed esophageal narrowing. Due to high suspicion of a possible vascular anomaly, CT angiography was obtained which showed an ARSA compressing on the esophagus (Fig. 4). Considering her cardiac history, patient also had an LHC which showed multivessel coronary artery disease.
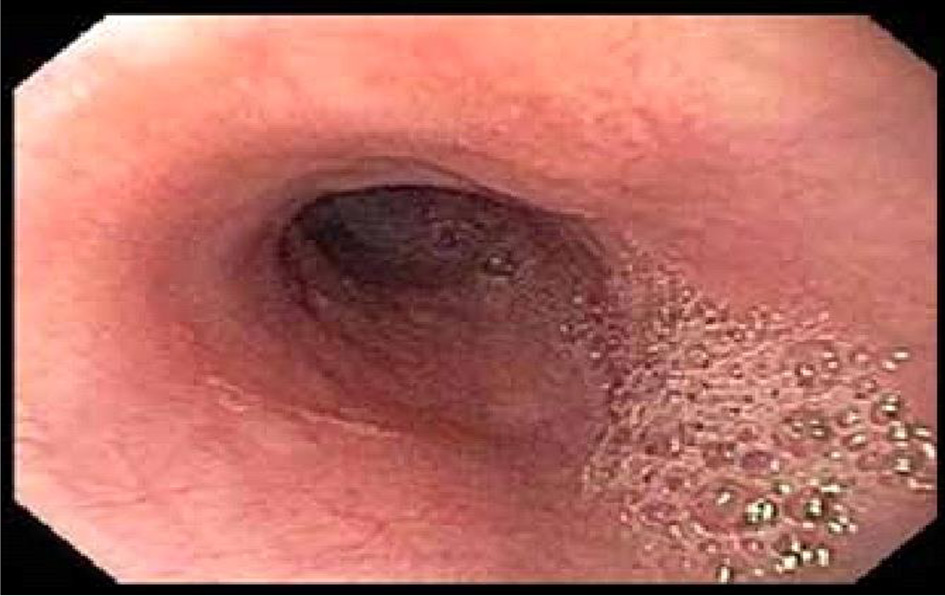 Click for large image | Figure 3. EGD with a normal esophagus and no pulsatile indentation. EGD: esophagogastroduodenoscopy. |
 Click for large image | Figure 4. CT angiography showing an ARSA causing external compression of the esophagus with no tracheal compression (arrows). CT: computed tomography; ARSA: aberrant right subclavian artery. |
Treatment
The patient chose to have a surgical procedure after an explanation of the risks and benefits. Patient underwent coronary artery bypass surgery for concomitant coronary artery disease. The aberrant artery was divided at its origin through a median sternotomy and the distal subclavian artery was anastomosed to the aortic arch using a short interposition graft.
Follow-up and outcomes
The patient showed successful recovery from surgery with no further complaints about difficulty swallowing.
| Discussion | ▴Top |
DL is caused by external esophageal compression by any of several congenital vascular anomalies such as ARSA, double aortic arch (caused by persistent right dorsal aorta), or an arterial lusoria aneurysm. The word aberrant is used when describing DL because the right subclavian artery (RSA) does not arise from the brachiocephalic trunk as it should physiologically but from the aortic arch. Developmental anomalies of the aortic arch are found in about 3% of people during autopsy. Three vessels that arise from the aortic arch during embryogenesis include the left common carotid artery, the left subclavian artery, and the innominate artery which gives rise to the right subclavian and common carotid artery. In the embryonic period, during the development of the heart and the aorta, there are a total of six aortic arches. The first and second arches usually degenerate. The third arch forms the internal carotid artery. The right aortic arch while still connected to the right posterior aorta gives rise to the brachiocephalic trunk which gives rise to the RSA. The right posterior aorta eventually obliterates leaving just the RSA. Occasionally, impaired degeneration of the right dorsal aorta results in a double aortic arch which can cause dysphagia. However, in a majority of DL, the RSA originates just inferior to the left subclavian artery and courses towards the right causing external compression of the esophagus with or without tracheal involvement. In 80% of cases, the aberrant artery crosses between the esophagus and the vertebral column. In 15% of cases, it runs between the esophagus and the trachea, and in 5% of cases, it passes anterior to both the trachea and esophagus. In both patients, we reported that the aberrant artery was between the esophagus and vertebral column. Some studies suggest this anomaly can only produce symptoms in the setting of a concomitant congenital anomaly of the carotid arteries, either a common trunk to both carotid arteries (“bicarotid truncus”) or a close origin of the carotid arteries, leading to decreased distensibility of the esophagus as it is compressed posteriorly by the aberrant vessel. However, this specific anomaly is absent in many reported cases of DL [5].
Vascular abnormalities are more prevalent in children with Down syndrome. Up to 37% specifically have an aberrant RSA and congenital heart disease. The abnormality may be seen in up to 2% of patients with tetrology of Fallot, pulmonary atresia, and major aortopulmonary collateral arteries. Other vascular anomalies that may cause dysphagia include persistent right aortic arch with aberrant left subclavian artery, a tortuous or aneurysmal thoracic aorta (dysphagia aortica), and left atrial enlargement [6]. This anomaly is often asymptomatic and may be discovered incidentally on imaging or postmortem analysis. The majority of patients, 60-80%, remain symptom-free in their lifetime. Symptomatic patients usually present with dysphagia (91% of cases) consistent with mechanical obstruction. Symptoms are mostly for solids and are associated with regurgitation of food, chest pain (20%), and symptoms that frequently change with position. Other complaints include coughing, thoracic pain, or Horner’s syndrome. Rarely, do patients present with rupture of an aneurysmal aberrant artery or Kommerell’s diverticulum [7]. In infants, respiratory symptoms are most common. This is due to the absence of tracheal rigidity, allowing for its compression and leading to stridor, wheezing, cyanosis, or recurrent pneumonia. However, patients who usually do not present in childhood, develop dysphagia later in life probably due to increased esophageal rigidity, development of atherosclerosis, elongation of the aorta, and aneurysm formation [8-10].
The nonspecific symptoms and unfamiliarity of DL to clinicians have made prompt diagnosis more challenging. Most cases are often misdiagnosed as gastroesophageal reflux disease (GERD), esophageal malignancy, and coronary artery disease. Both patients had a LHC to rule out coronary artery disease. DL may be diagnosed by performing a barium esophagogram which may show some indentation or constriction around the esophagus. In some cases, a pulsating mass or an indented esophagus may be visualized on EGD which was not seen in both of our patients. Confirmatory radiological imaging is CT or magnetic resonance imaging (MRI) angiography, which helps detect the culprit vessel and its anatomy. The management of patients with DL depends on the severity of symptoms and impact to weight and nutrition. Mild to moderate symptoms are often treated symptomatically, with changes in lifestyle and dietary modification, such as avoiding exacerbating foods, eating slower, chewing well, taking smaller bites, sipping liquids, as well as reassurance. As well, acid suppression and prokinetic agents can be used [8]. For those who do not respond to medical management, a surgical approach is considered. The surgical approach largely depends on the specific vascular anatomy as well as the surgeon’s personal preference. The goal of surgery is to remove the aberrant vessel and reconstruct the vessel into an appropriate position. This involves ligation of the aberrant artery, followed by restoration of blood flow to the right arm by joining the ARSA to either the right common carotid artery, aortic arch, or ascending aorta [11]. There does not appear to be a consensus in the surgical literature as to the best surgical approach. The surgical approaches commonly used include median sternotomy, left thoracotomy, right supraclavicular, or right thoracotomy [11]. The traditional approach consists of dividing the aberrant artery at its origin through a median sternotomy and translocating the distal subclavian artery to the aortic arch or right carotid artery. A cervical approach is preferred because there is a decreased rate of complications, as well as better visibility of the subclavian and carotid arteries. Through a cervical approach, the aberrant artery is ligated near its root and connected with the right carotid artery [12]. Reported mortality rates have been as high as 16-25%, largely due to a two-step surgical repair. With newer surgical techniques, more recent reports quote mortality rates as low as 0% [12, 13]. More recent surgical approaches were reported by La Regina et al in which right carotid-subclavian bypass was followed by a robotic-assisted thoracoscopic resection of the aberrant RSA. Advanced robotic technology helped to move the esophagus and aberrant artery minimizing open thoracotomy. According to same report, ligation of vessel was the most challenging step. As well, presence of aneurysm within the aberrant vessel makes this approach more challenging [14]. Choosing surgical approach depends on patient comorbidities, candidacy for surgery. Our second patient had a coronary artery bypass and open thoracotomy was the optimal approach to correct aberrant artery. In poor surgical candidates, endoscopic dilation of the esophageal narrowing may temporarily relieve symptoms of dysphagia [15]. There are not enough cases in the literature to know the success rate of this procedure. DL can be overlooked during endoscopy but a barium esophagogram reveals the disorder most of the time. This disorder should be added to our differential diagnosis especially in patients presenting with chronic dysphagia with atypical chest pain. It is paramount that clinicians acquaint themselves more with this disorder as this would help in prompt diagnosis, treatment, and avoidance of unnecessary procedures not needed by the patient. For patients who cannot undergo surgery, endoscopic dilatation of the esophagus can be done. It is safe and can be repeated safely upon recurrence.
Learning points
The takeaway point from this case is to make clinicians aware of the congenital abnormality, DL, which should be part of differential diagnosis when working on a patient presenting with difficulty swallowing not responding to medical therapy. Even when EGD or barium swallow is negative for any abnormality, CT or MRI angiography should be the next imaging option.
Acknowledgments
We would like to acknowledge our library clerk Mr. Frederick Price for supplying us with articles to complete this manuscript.
Financial Disclosure
This project was not supported by any grant or funding agency.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Informed Consent
The patients had given informed consents for the case reports to be published.
Author Contributions
Each author has individually been involved and participated in drafting the manuscript and revising it critically for important intellectual content and have given final approval of the version to be published. Each has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. WF and AS encouraged MM and OU to learn about dysphagia lusoria and its management. All authors discussed the medical literature. MM and OU presented the idea, and OU wrote the manuscript with input from all authors.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Polguj M, Chrzanowski L, Kasprzak JD, Stefanczyk L, Topol M, Majos A. The aberrant right subclavian artery (arteria lusoria): the morphological and clinical aspects of one of the most important variations—a systematic study of 141 reports. ScientificWorldJournal. 2014;2014:292734.
doi pubmed - Bennett AL, Cock C, Heddle R, Morcom RK. Dysphagia lusoria: a late onset presentation. World J Gastroenterol. 2013;19(15):2433-2436.
doi pubmed - Ongun N, Tumkaya F, Degirmenci E, Ozcan V. A rare cause of horner syndrome: arteria lusoria. J Neurol Neurophysiol. 2017;8:445.
doi - Jain KK, Braze AJ, Shapiro MA, Perez-Tamayo RA. Aberrant right subclavian artery-esophageal fistula and severe gastrointestinal bleeding after surgical correction of scimitar syndrome. Tex Heart Inst J. 2012;39(4):571-574.
- Nguyen P, Gideon RM, Castell DO. Dysphagia lusoria in the adult: associated esophageal manometric findings and diagnostic use of scanning techniques. Am J Gastroenterol. 1994;89(4):620-623.
- Panebianco V, Anzidei M, Catalano C, Passariello R. Dysphagia lusoria in combination with multiple congenital anomalies of the aortic arch. Eur J Cardiothorac Surg. 2006;29(1):105.
doi pubmed - Knight G C, Codd J E. Anomalous right subclavian aneurysm, report of 3 cases with a review of the literature. Tex Heart Inst J. 1991;18:208-218.
- Janssen M, Baggen MG, Veen HF, Smout AJ, Bekkers JA, Jonkman JG, Ouwendijk RJ. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95(6):1411-1416.
doi pubmed - McNally PR, Rak KM. Dysphagia lusoria caused by persistent right aortic arch with aberrant left subclavian artery and diverticulum of Kommerell. Dig Dis Sci. 1992;37(1):144-149.
doi pubmed - Kantarceken B, Bulbuloglu E, Yuksel M, Cetinkaya A. Dysphagia lusorium in elderly: a case report. World J Gastroenterol. 2004;10(16):2459-2460.
doi pubmed - Rogers AD, Nel M, Eloff EP, Naidoo NG. Dysphagia lusoria: a case of an aberrant right subclavian artery and a bicarotid trunk. ISRN Surg. 2011;2011:819295.
doi pubmed - Kiernan PD, Dearani J, Byrne WD, Ehrlich T, Carter W, Krasicky G, Harshaw W. Aneurysm of an aberrant right subclavian artery: case report and review of the literature. Mayo Clin Proc. 1993;68(5):468-474.
doi - Ota T, Okada K, Takanashi S, Yamamoto S, Okita Y. Surgical treatment for Kommerell's diverticulum. J Thorac Cardiovasc Surg. 2006;131(3):574-578.
doi pubmed - La Regina D, Prouse G, Mongelli F, Pini R. Two-step treatment of dysphagia lusoria: robotic-assisted resection of aberrant right subclavian artery following aortic debranching. Eur J Cardiothorac Surg. 2020;58(5):1093-1094.
doi pubmed - Bogliolo G, Ferrara M, Masoni L, Pietropaolo V, Pizzicannella G, Miscusi G. Dysphagia lusoria: proposal of a new treatment. Surg Endosc. 1987;1(4):225-227.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


