| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 6, June 2022, pages 297-301
Infectious Endocarditis Caused by Pseudomona aeruginosa on Bicuspid Aortic Valve
Juan Lacalzada-Almeidaa, Maria Manuela Izquierdo-Gomeza, Amelia Duque-Gonzaleza, Maria del Mar Alonso-Socasb, Rebeca Munoz-Rodrigueza, c
aDepartment of Cardiology, Hospital Universitario de Canarias, Tenerife, Spain
bDepartment of Infectious Diseases, Hospital Universitario de Canarias, Tenerife, Spain
cCorresponding Author: Rebeca Munoz-Rodriguez, Department of Cardiology, Hospital Universitario de Canarias, La Cuesta, 38320, La Laguna, Tenerife, Spain
Manuscript submitted April 12, 2022, accepted May 9, 2022, published online June 11, 2022
Short title: IE by PA on Bicuspid Aortic Valve
doi: https://doi.org/10.14740/jmc3943
| Abstract | ▴Top |
We report the case of a 53-year-old man with psoriatic arthritis, suffering from a malignant and recidivant myoepithelioma in his right axilla and arm, and undergoing two surgeries, with the last one being performed a month prior to actual admission. After the last surgery, he was admitted to hospital with fever without a source. After physical examination, laboratory tests, blood cultures and transthoracic and transesophageal echocardiography, he was diagnosed with infectious endocarditis (IE) on a bicuspid aortic valve (BAV) caused by Pseudomona aeruginosa (PA). Antibiogram-guided antibiotic therapy with meropenem and tobramicin was initiated. However, in the presence of repetitive spleen infarctions and a large vegetation, 12 days after admission, a bioprosthesis aortic valve implantation was performed. The postsurgical evolution was favorable and prolonged antibiotic course with meropenem and tobramicin was completed. The pathological anatomy and the native valve cultured confirmed an IE caused by PA. Gram-negative non-HACEK IE cases are infrequent, accounting for 1.8% of the total IE cases. PA is the second most frequent bacillus in this group, causing endocarditis more prevalently when associated with healthcare procedures rather than injectable drug use. No prior case study has identified IE caused by PA related to a BAV in the last years.
Keywords: Infectious endocarditis; Pseudomona aeruginosa; Bicuspid aortic valve; Axillary myoepithelioma
| Introduction | ▴Top |
Infectious endocarditis (IE) caused by non-HACEK Gram-negative (GN) (organism other than Hemophilus species, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species) is extraordinarily infrequent, accounting for 1.8% of the total IE cases. Escherichia coli and Pseudomona aeruginosa (PA) are the most frequent bacilli of this group, with a prevalence of 29% and 22%, respectively [1]. During the 80s and 90s, intravenous drug use (IDU) was the main cause of non-healthcare-associated right heart IE, mostly related to Staphylococcus aureus and PA [2-4]. During the last decade, the increase in the number of invasive medical procedures performed has constituted a rising and prevalent risk factor to develop IE caused by PA, being the origin of 55.6% of the IE cases analyzed by Lin et al [5] and 57% in Morpeth et al’s study [1]. The latter only reports 4% of cases associated with IDU. We present the case of a patient with a bicuspid aortic valve (BAV) who developed an IE caused by PA on this valve after a surgical resection of a malignant axillary myoepithelioma. In spite of optimal antibiotic treatment, secondary and repetitive septic emboli precipitated an urgent aortic valve substitution surgery. Notwithstanding the high mortality associated with this type of IE, caused by PA on a BAV [1, 6], the postsurgical recovery was uneventful.
| Case Report | ▴Top |
Investigations
A 53-year-old man, consumer of moderate doses of alcohol up to the year prior to admission and affected by psoriatic arthritis under treatment with monthly subcutaneous secukinumab, suffered from a malignant and recidivant myoepithelioma in his right axilla and arm, and underwent two surgeries, the first one 2 years ago and the latter a month prior to the current admission. After the last intervention, while admitted at the general surgery unit, he had some feverish peaks without any other associated symptomatology. Three sets of blood cultures were drawn and empiric antibiotic therapy with ciprofloxacin was initiated. The physical examination exhibited absence of heart failure signs, rhythmic heart sounds and an ejective aortic murmur II/VI along with a protomesodiastolic aortic murmur. No abnormal neurological signs or skin lesions were objectified.
Diagnosis
Laboratory studies showed a normocytic and normochromic anemia with high levels of ferritin, leukocytosis with neutrophilia, thrombopenia and high levels of C-reactive protein, quantified in 226 mg/dL (normal values < 3 mg/dL). Troponin T was elevated to 161 pg/mL (normal values < 14 pg/mL). Urine culture was negative. An initial pair of blood culture and another control 1 week later were obtained, and in all of them, PA was isolated. The electrocardiogram showed a 70 bpm sinus rhythm without any alterations. The transthoracic echocardiogram exhibited a non-dilated left ventricle, with moderate hypertrophy, with preserved ejection fraction. BAV was calcified, with double moderate lesions (Fig. 1 and Supplementary Video 1, www.journalmc.org). Given the poor ultrasound acoustic window as well as the high suspicious of endocarditis, a transesophageal echocardiography (TEE) was performed, confirming the presence of BAV with ecodense and mobile images adhered to both cusps, suggestive of vegetations, measuring the largest 11 mm (Fig. 2 left image and Supplementary Video 2, www.journalmc.org). Double moderate lesion of the BAV was confirmed (Figs. 3 and 4 and Supplementary Videos 2 and 3, www.journalmc.org). A total body computed tomography was performed, showing multiple spleen infarctions as a fundamental finding and dilatation of the aorta was ruled out. Once the surgical valvular substitution was performed, the native valve culture confirmed an acute IE caused by PA and the pathological anatomy showed neutrophil infiltration, fibrolymphocyte exudate and chondroid metaplasia (Fig. 5).
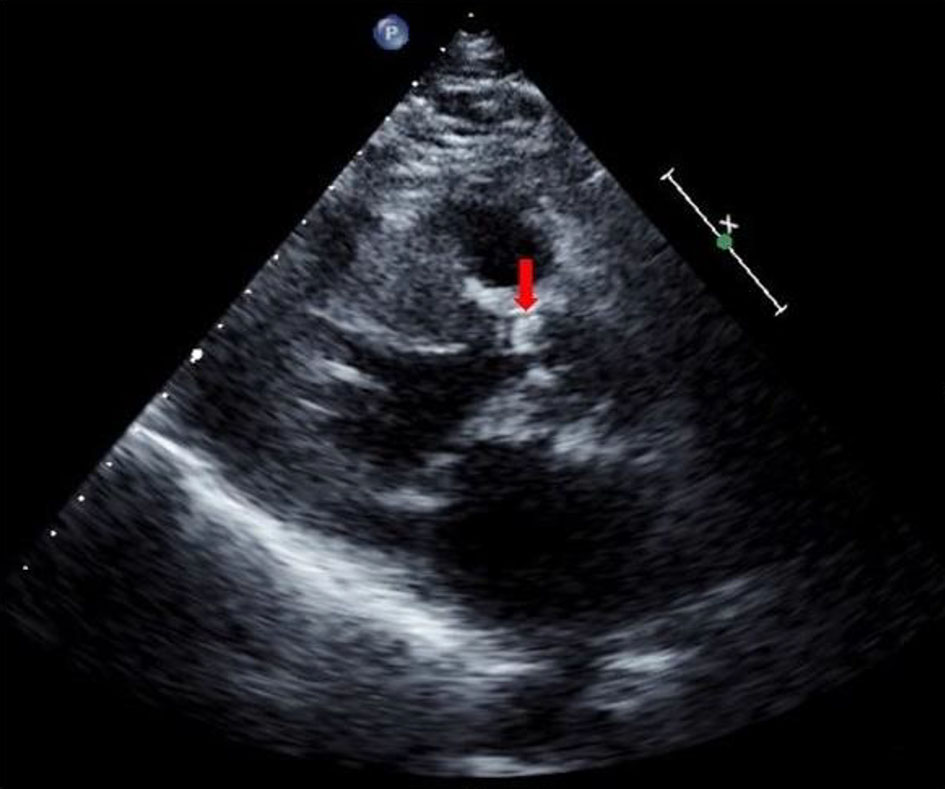 Click for large image | Figure 1. Trans-thoracic paraesternal long-axis view showing a bicuspid, calcified aortic valve without a definitive vegetation image (arrow). |
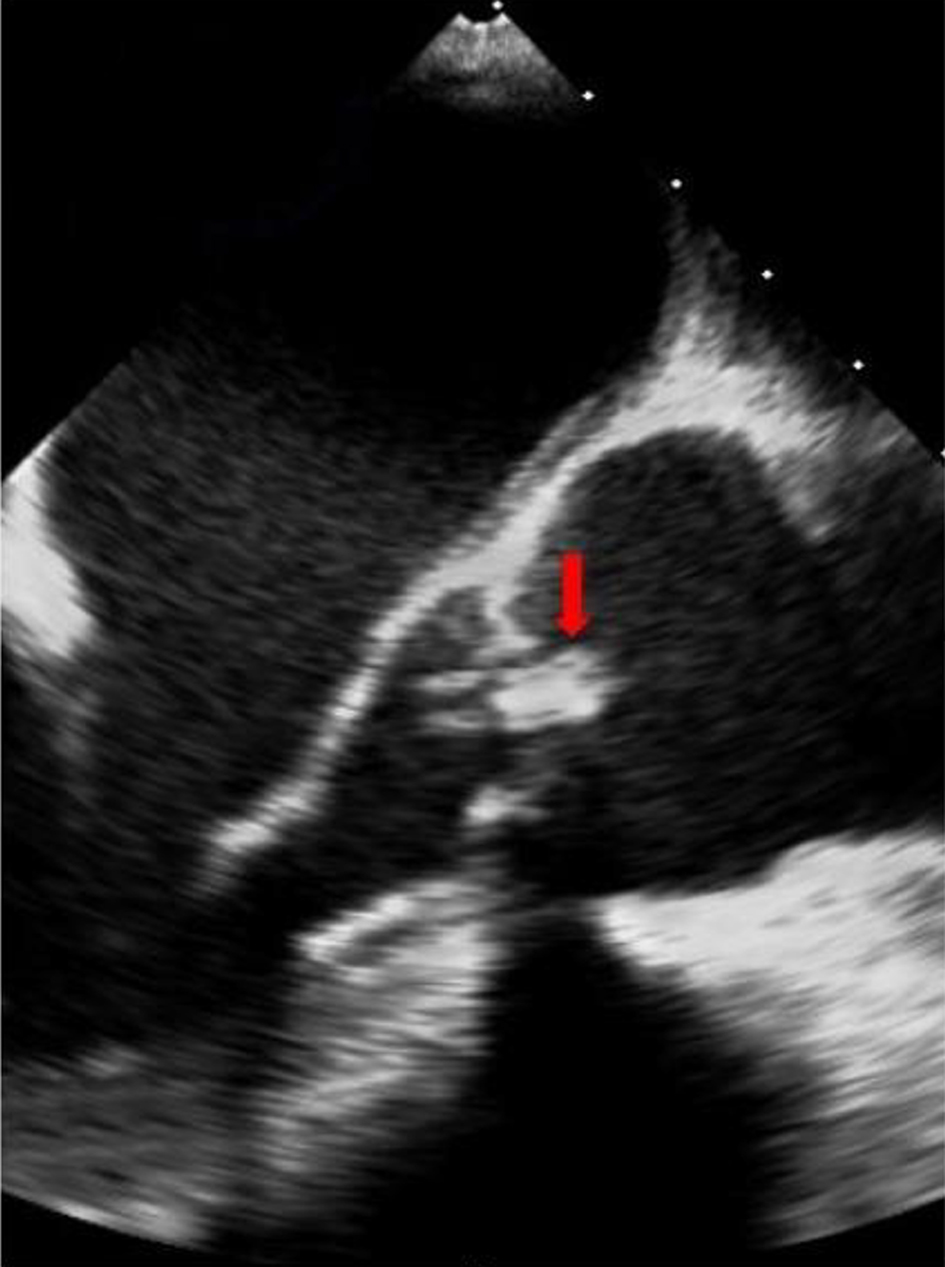 Click for large image | Figure 2. Transesophageal long-axis view exhibiting an image compatible to a vegetation on a bicuspid aortic valve (arrow). |
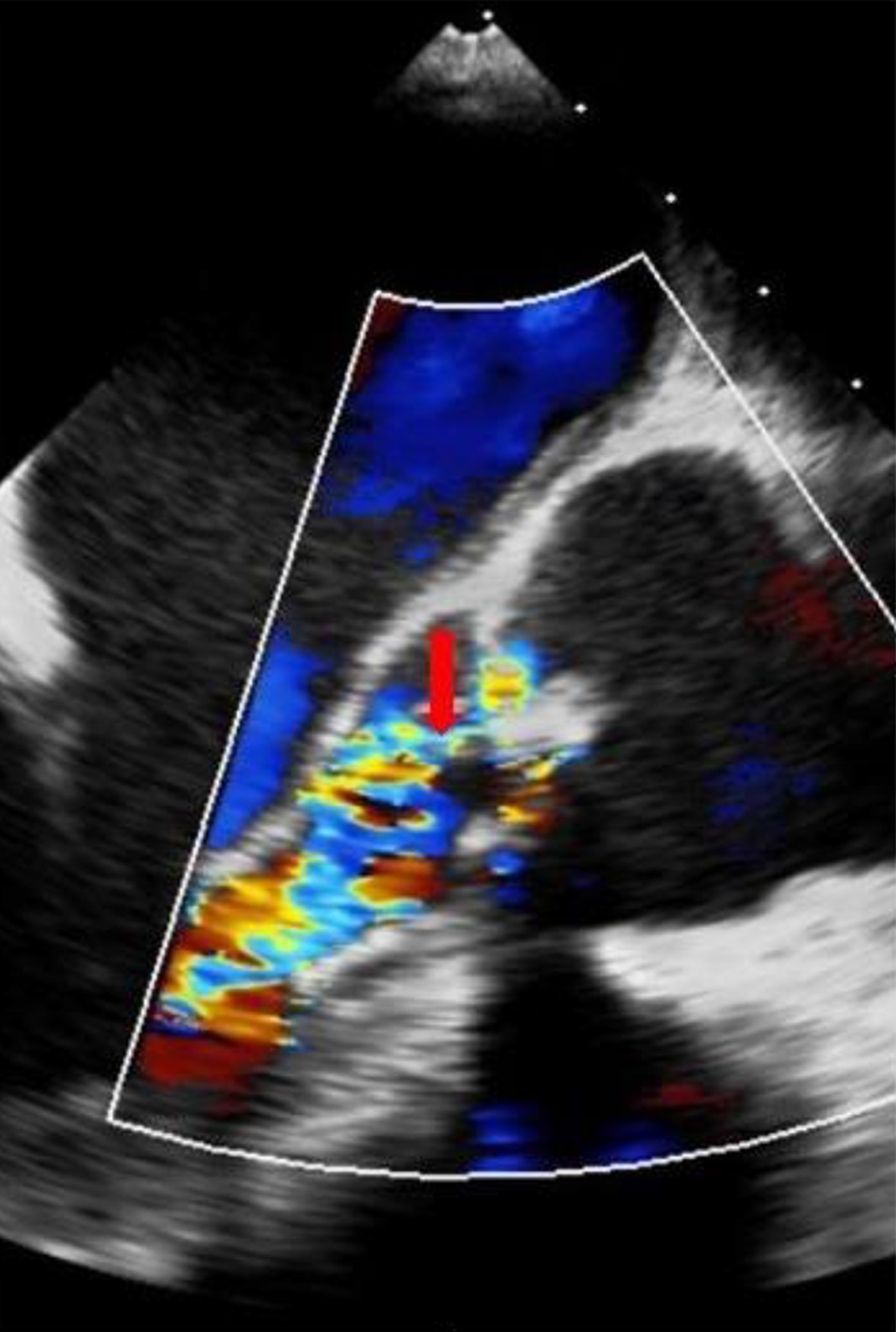 Click for large image | Figure 3. Transesophageal long-axis view confirming the presence of double aortic lesion (arrow). |
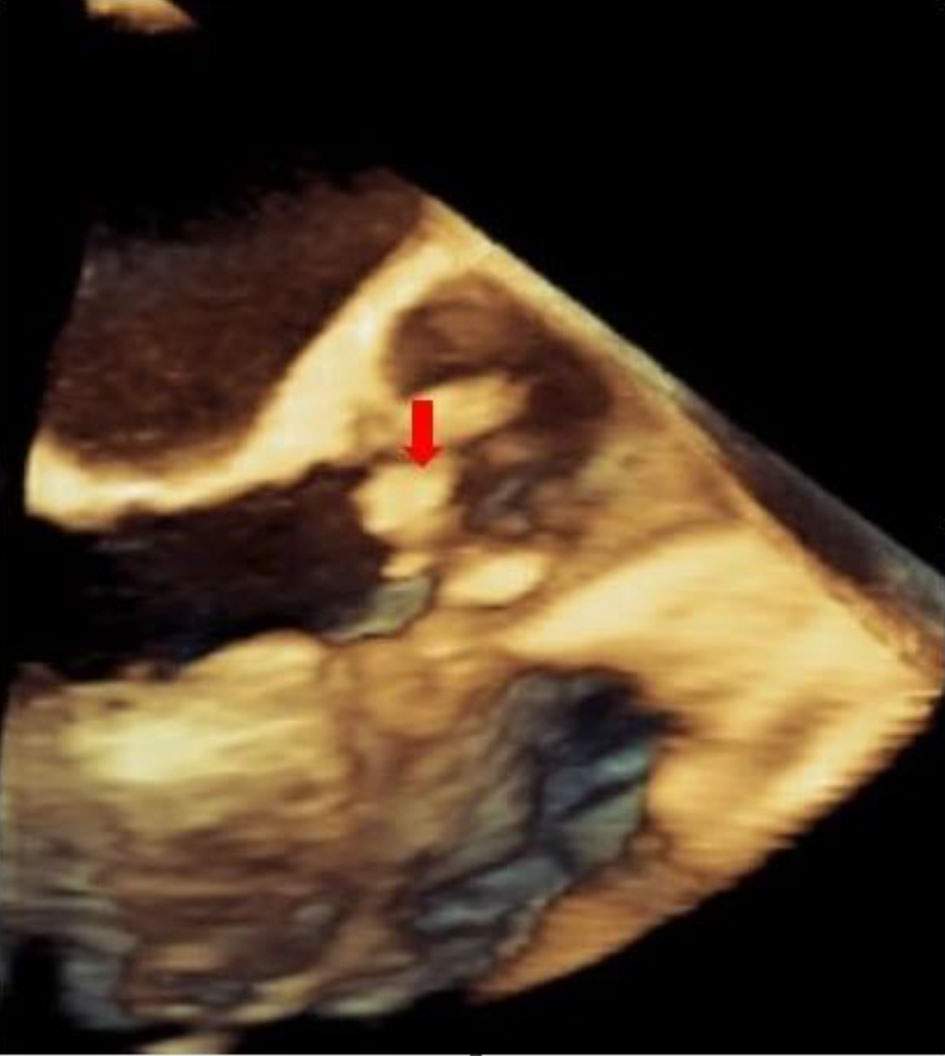 Click for large image | Figure 4. 3D transesophageal long-axis view showing the vegetation volume and its spacial relationship with the aortic valve (arrow). |
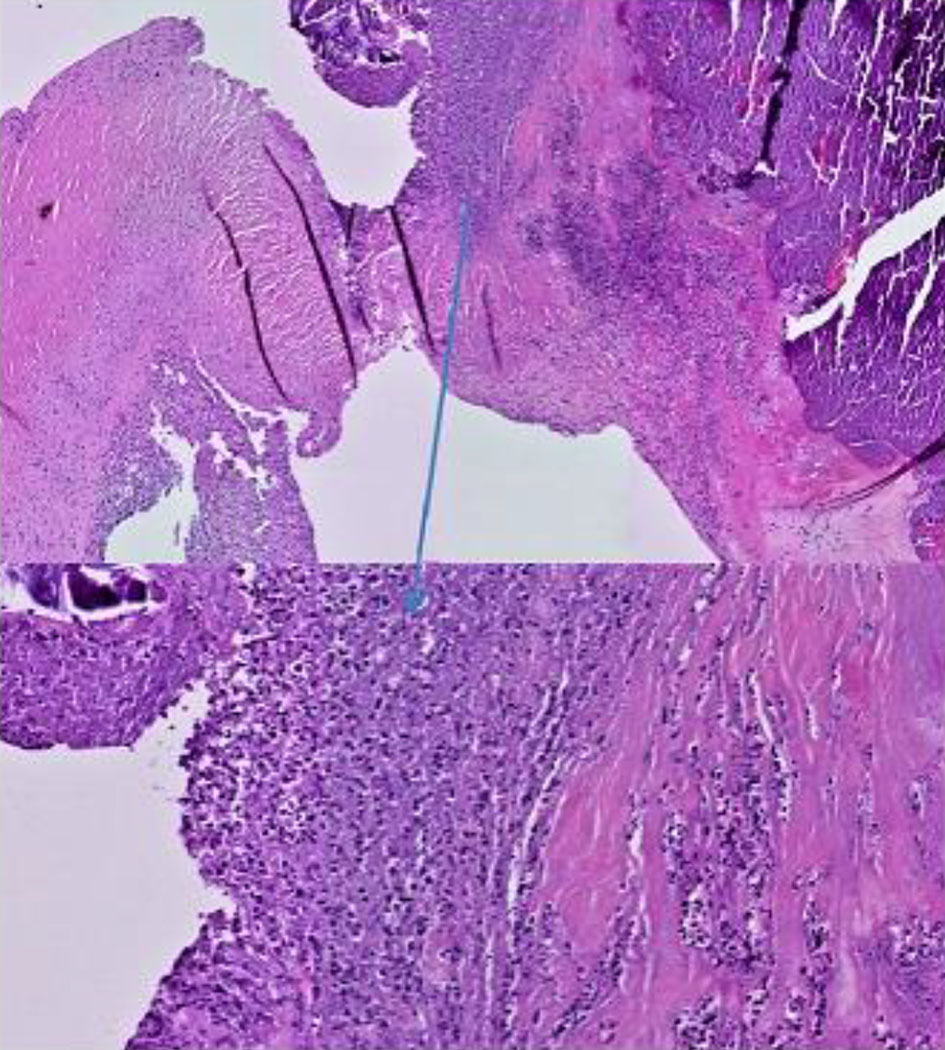 Click for large image | Figure 5. Histology of the native aortic valve (hematoxylin and eosin, ×20 and ×40), showing a thickened and fibrosed valve structure with histological signs of acute endocarditis. Foci of ulceration and fibrinoleukocyte infiltrate with predominance of neutrophils can be observed. |
Treatment
Antibiogram-directed antibiotic therapy with meropenem and tobramicin was initiated. However, in the presence of repetitive spleen infarctions and the vegetation size, an aortic valve substitution surgery was performed 12 days after admission and 6 days after the IE diagnosis was established, following the current clinical practice guidelines of IE [7, 8]. A bioprosthesis aortic valve Mosaic no. 23 was implanted and a 6-week antibiogram-guided antibiotic regimen was completed.
Follow-up and outcomes
The postsurgical evolution was favorable and the patient was discharged after a month of admission with ambulatory follow-up and a home hospitalization regime for the antibiotic administration. Cultures prior to discharge were negative for PA. A screening transthoracic -echocardiography to look for BAV or dilatation of aortic sinuses and ascendant aorta would be suggested to his first-grade relatives [9].
| Discussion | ▴Top |
We present the case of a patient suffering from IE caused by PA on a BAV, which has rarely been reported [10]. IE caused by non-HACEK germs is a rare disease with high mortality and morbidity rates [1, 11]. Despite gram-positive bacteria being the main IE etiology, the rising prevalence of infections caused by non-HACEK GN germs (-) has attracted attention because of their propensity to develop and spread antibiotic resistance, rising mortality and elevating the health expenditure [6, 12]. In a large multi-centric and international database, including 2,761 patients admitted between 2000 and 2005, only 49 cases (1.8%) were attributable to aerobic GN non-HACEK germs [1]. The 57% of the IE cases of this series were healthcare-associated (HCA-IE) and only 4% were related to IDU. This reduction of the IDU, a known risk factor for the right heart community-acquired IE in the past years, has been related to a decrease of the parenteral drug abuse. In this review, Escherichia coli and PA were the more frequent non-HACEK GN bacilli, representing 29% and 22%, respectively, with 59% isolated on a prosthetic valve [1]. Other authors have demonstrated this rise of the non-HACEK GN IE associated with HCA-IE, as well as the decrease of the IDU related. In a most recent review, carried out between 2005 and 2015, a total amount of 26 IE caused by PA were found: three of them were community-acquired, eight HCA-IE with a nosocomial origin and 10 non-nosocomial but HCA-IE-related. Five cases had not a conclusive origin, being the great majority, as our patient one, linked to a prior medical intervention. Native valves were involved in a 65.4%, not being reported any of them as bicuspid [2]. To this day, the only case reported as BAV and IE causes by PA is the one published in 1991 [10]. Systemic and cardiac complications occurred in a third and a fifth, respectively, of the cases [2]. The non-HACEK GN IE represents 4-10% of the IE over native valves and 2-15% on prosthetic valves [13]. In spite of the HCA-IE caused by PA, the low PA incidence, in comparison to other pathogens, makes the clinical characterization of this infection more difficult [1]. Although our patient was not IDU, he presented some risks predisposing to infection: ex-alcohol consumer, being under immunosuppression therapy for psoriatic arthritis, did not know his BAV and, as previously referred, was twice intervened from malignant myoepithelioma, 2 years and a month prior to admission.
Non-HACEK GN IE, compared to gram-positive germs, has been related to a higher frequency of intravascular dispositive presence (29% vs. 11%; P < 0.001), a non-oral gastrointestinal or urinary main door (35% vs. 12%; P < 0.001), a non-dental invasive procedure on the 60 days prior to symptom initiation (38% vs. 19%; P = 0.002) and a higher in-hospital mortality rates (24% vs. 17%, although this last difference was not statistically significant, P = 0.190) [1]. A bibliographic review of 40 cases of IE caused by PA in non-IDU people showed a global mortality rate around 64% [14].
A recent published article analyzed 3,028 IE cases from 31 Spanish hospitals (GAMES group), finding 54 patients with IE on BAV [15]. The majority were men (n = 43, 79.6%), with a mean age of 43 years (interquartile range (IQR): 36 - 53 years) and low comorbidity rate. From the BAV patients group, only one had a non-HACEK GN bacillus (1.9%), with Streptococcus viridans being the most frequent IE responsible germ (35.2%) and the oral cavity being the main door (14.8%). The intracardiac complications and heart failure were frequent (50% and 40.7%, respectively). In our case, the initial high troponin T level suggested poor cardiovascular prognosis [16]. Cardiac surgery was indicated in 75.9% and performed in 68% of the cases. The in-hospital mortality was 5.6%. The authors conclude that the patients with IE on BAV have a similar clinical evolution to those considered under high risk for IE prophylaxis, under the current recommendations [7], as well as they have more intracardiac complications compared to the low/intermediate risk and an increased need for surgery [15]. Moreover, the prevalence of IE in the BAV population is 2-5%, significantly higher than the IE prevalence in the general population (1 - 3 per 100,000 people) [17, 18]. According to the indirect data, prophylaxis IE should be considered on this group of patients. In addition, first-grade relatives should undergo a screening transthoracic echocardiography to rule out this pathology given the potential complications associated [9]. Although the microbiology and the ideal treatment for IE caused by non-HACEK GN are relatively unknown, the current recommendations suggest cardiac surgery and combined and prolonged antibiotic therapy as a reasonable therapeutical approach [7]. When this combination is not performed, left-side IE shows higher mortality rates. In a current bibliographic review, Hagiya et al observed a mortality rate of 62% with antibiotic therapy alone, compared to a 31% when a combination of surgery and medical therapy was performed. However, there is certain bias increasing the mortality rate of the non-surgical group as cardiac surgery was denied to the high surgical risk patients [2]. The actual consensus recommends a combined antibiotic therapy with a B-lactam (cephalosporin or carbapenems), and an aminoglycoside or fluoroquinolone for 6 weeks [7, 8]. Despite the combined therapy efficacy is controversial, it could be preferable given the possibility of antibiotic-resistant strains [2] and infectious disease experts should be consulted [7]. Nevertheless, the great variability between the different observational studies design, the lack of long-term follow-up data and the surgical patients’ selection bias makes a limitation for taking definitive conclusions about the optimal treatment [1]. Nowadays, the GAMES group are analyzing the IE caused by GN from a multi-centric, large national series, with data since 2009, which probably will provide some answers about the evolution and therapeutic approach to the patients suffering from PA IE.
As a conclusion of this report, here, we present the case of a 53-year-old man who was a consumer of moderate doses of alcohol up to the year prior to admission and affected by psoriatic arthritis under treatment with monthly secukinumab. He was not known to have a BAV and he developed an IE, caused by PA on this valve, after a surgical resection of a malignant axillary myoepithelioma. Antibiogram-directed antibiotic therapy was initiated. However, in the presence of repetitive spleen infarctions and the vegetation size, an aortic valve substitution surgery was performed with a bioprosthesis valve implantation 12 days after admission. Despite the high morbimortality of IE caused by this GN bacillus, the combination of an adequate medical and surgical approach resulted in a satisfactory evolution of the patient and he could be discharged from our hospital.
Learning points
HCA-IE, and specially those caused by non-HACEK GN germs, constitutes a growing entity, given the exponential increase on the performance of invasive procedures in the contemporaneous medical practice, even in patients with broad comorbidities and advanced age. This case pretends to draw attention to the prevalence of IE after invasive procedures and the need to consider a combined medical and surgical therapeutical approach given the high mortality rates and the prevalence of antibiotic-resistant strains on this group. In addition, patients with BAV show a similar clinical evolution to those considered under high risk for IE prophylaxis and higher rate of intracardiac complications, supporting the need to consider the candidacy of this patient to antibiotic profilaxis when undergoing an invasive procedure.
| Supplementary Material | ▴Top |
Suppl 1. Trans-thoracic paraesternal long-axis view showing a bicuspid, calcified aortic valve with an oscillating and echodense mass, suggestive of vegetation.
Suppl 2. Transesophageal long-axis view showing double moderate aortic lesion.
Suppl 3. 3D transesophageal long-axis view confirming the presence of the vegetation and showing its volume and position.
Acknowledgments
We would like to thank and acknowledge all the healthcare workers that helped taking care of this patient.
Financial Disclosure
This research received no external funding.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was taken from the patient for publication as a case report including relevant images.
Author Contribution
JLA and RMR were involved in the data collection, literature review and writing. Critical revision of the paper was performed by MMIG, ADG and MAS.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Morpeth S, Murdoch D, Cabell CH, Karchmer AW, Pappas P, Levine D, Nacinovich F, et al. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147(12):829-835.
doi pubmed - Hagiya H, Tanaka T, Takimoto K, Yoshida H, Yamamoto N, Akeda Y, Tomono K. Non-nosocomial healthcare-associated left-sided Pseudomonas aeruginosa endocarditis: a case report and literature review. BMC Infect Dis. 2016;16(1):431.
doi pubmed - Reyes MP, Ali A, Mendes RE, Biedenbach DJ. Resurgence of pseudomonas endocarditis in Detroit, 2006-2008. Medicine (Baltimore). 2009;88(5):294-301.
doi pubmed - Cohen PS, Maguire JH, Weinstein L. Infective endocarditis caused by gram-negative bacteria: a review of the literature, 1945-1977. Prog Cardiovasc Dis. 1980;22(4):205-242.
doi - Lin TI, Huang YF, Liu PY, Chou CA, Chen YS, Chen YY, Hsieh KS, et al. Pseudomonas aeruginosa infective endocarditis in patients who do not use intravenous drugs: Analysis of risk factors and treatment outcomes. J Microbiol Immunol Infect. 2016;49(4):516-522.
doi pubmed - Kaye KS, Pogue JM. Infections caused by resistant gram-negative bacteria: epidemiology and management. Pharmacotherapy. 2015;35(10):949-962.
doi pubmed - Baddour L, Wilson W, Bayer A, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation. 2015;132(15):1435-1486.
doi pubmed - Habib G, Lancellotti P, Iung B. 2015 ESC Guidelines on the management of infective endocarditis: a big step forward for an old disease. Heart. 2016;102(13):992-994.
doi pubmed - Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, Jneid H, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2021;143(5):e35-e71.
doi - Finkelstein R, Boulus M, Markievicz M. Hospital-acquired Pseudomonas aeruginosa endocarditis. J Hosp Infect. 1991;18(2):161-163.
doi - Falcone M, Tiseo G, Durante-Mangoni E, Ravasio V, Barbaro F, Ursi MP, Pasticci MB, et al. Risk factors and outcomes of endocarditis due to non-HACEK gram-negative bacilli: data from the prospective multicenter italian endocarditis study cohort. Antimicrob Agents Chemother. 2018;62(4):e02208-17.
doi pubmed - Noureddine M, de la Torre J, Ivanova R, Martinez FJ, Lomas JM, Plata A, Galvez J, et al. [Left-sided endocarditis due to gram-negative bacilli: epidemiology and clinical characteristics]. Enferm Infecc Microbiol Clin. 2011;29(4):276-281.
doi pubmed - Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345(18):1318-1330.
doi pubmed - Dawson NL, Brumble LM, Pritt BS, Yao JD, Echols JD, Alvarez S. Left-sided Pseudomonas aeruginosa endocarditis in patients without injection drug use. Medicine (Baltimore). 2011;90(4):250-255.
doi pubmed - Zegri-Reiriz I, de Alarcon A, Munoz P, Martinez Selles M, Gonzalez-Ramallo V, Miro JM, Falces C, et al. Infective Endocarditis in Patients With Bicuspid Aortic Valve or Mitral Valve Prolapse. J Am Coll Cardiol. 2018;71(24):2731-2740.
doi pubmed - Postigo A, Vernooij RWM, Fernandez-Aviles F, Martinez-Selles M. Cardiac troponin and infective endocarditis prognosis: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2021;10(3):356-366.
doi pubmed - Masri A, Svensson LG, Griffin BP, Desai MY. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart. 2017;103(17):1323-1330.
doi pubmed - Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


