| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 8, August 2022, pages 402-407
Uterine Cervical Adenosarcoma Showing an Endophytic Growth Pattern
Eri Lin-Satoia, d, Tadashi Kaneshirob, Rieko Kandaa, Miho Matsudaa, Yuko Sasajimac, Shun-ichi Ikedaa, d, e
aDepartment of Obstetrics and Gynecology, Kohseichuo General Hospital, Tokyo, Japan
bDepartment of Radiology, Kohseichuo General Hospital, Tokyo, Japan
cDepartment of Pathology, Teikyo University Hospital, Tokyo, Japan
dThese authors contributed equally to this manuscript.
eCorresponding Author: Shun-ichi Ikeda, Department of Obstetrics and Gynecology, Kohseichuo General Hospital, Tokyo 153-8581, Japan
Manuscript submitted June 19, 2022, accepted August 5, 2022, published online August 19, 2022
Short title: Endophytic Growth of Cervical Adenosarcoma
doi: https://doi.org/10.14740/jmc3952
| Abstract | ▴Top |
Adenosarcomas are biphasic neoplasms that usually originate in the uterine corpus and comprise a benign epithelial component and a malignant stromal component. Uterine adenosarcomas typically present with abnormal genital bleeding, an enlarged uterus, and a tumor that protrudes into the endometrial cavity. These tumors rarely protrude through the cervical os and are often misdiagnosed as cervical polyps. We present the case of a patient with cervical adenosarcoma with characteristics different from those reported in previous cases. This tumor showed endophytic growth, which is rare in cervical adenosarcomas. No watery discharge or obvious genital bleeding was noted. Although the tumor measured 4 cm, vaginal bleeding was noted only once at 6 months before diagnosis and was in the form of faint brown discharge.
Keywords: Adenosarcoma; Uterine cervix; Endophytic growth
| Introduction | ▴Top |
Uterine adenosarcomas are mixed tumors comprising benign Mullerian epithelium and malignant mesenchymal cells. Uterine sarcomas account for 7-9% of all malignant tumors of the uterus [1, 2], and uterine adenosarcomas account for only 8% of these uterine sarcomas [3]. Primary adenosarcomas of the cervix are extremely rare and account for only 2% of all uterine adenosarcomas [4].
Typical clinical presentation of uterine adenosarcomas includes vaginal bleeding from a protruding mass, which can be observed during pelvic examination. Adenosarcomas rarely show an endophytic growth pattern. Moreover, cervical adenosarcomas usually present with abnormal discharge or evident genital bleeding [5].
Herein, we report a case of cervical adenosarcoma showing an endophytic growth pattern wherein faint brown discharge appeared only once despite the tumor reaching 4 cm in diameter.
| Case Report | ▴Top |
Investigations
A 51-year-old woman, who was 4 years postmenopausal, underwent radiation therapy after bilateral breast-conserving surgery for breast ductal carcinoma in situ at 41 years old. She had no history of tamoxifen use during breast cancer treatment. She underwent fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) as part of the routine follow-up for breast cancer, and results revealed increased uptake in the cervix (Fig. 1). She subsequently presented to our hospital for further gynecologic evaluation.
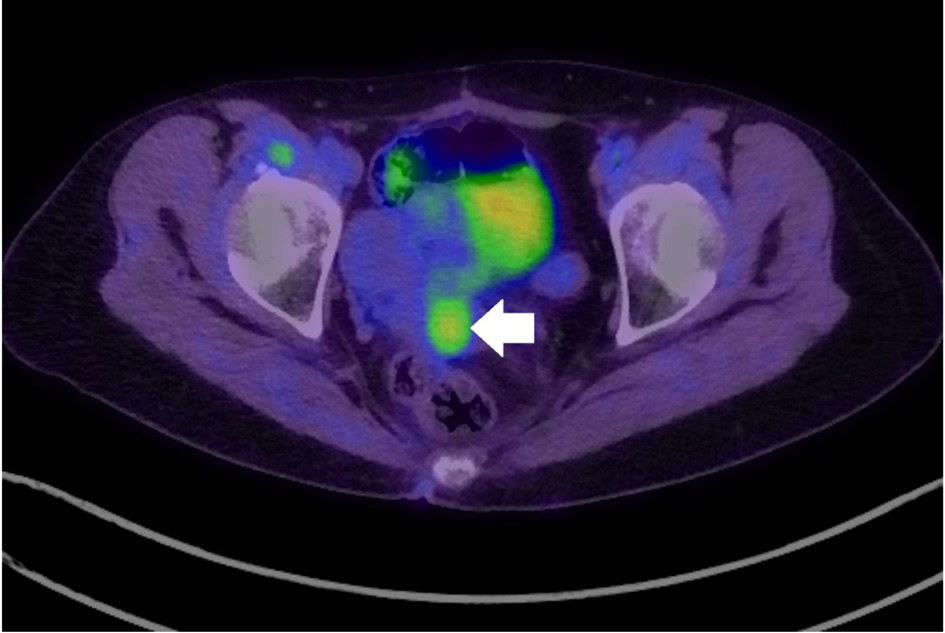 Click for large image | Figure 1. Fluorodeoxyglucose positron emission tomography-computed tomography indicating increased uptake in the cervix (arrow: cervical tumor). |
Diagnosis
Gynecological examination revealed that the 4-cm long cervix had been replaced by a gray-white tumor with no vaginal or bilateral parametrial involvement. Magnetic resonance imaging (MRI) indicated that the tumor was 4 cm in size, contained small cysts, and had entirely replaced the cervix, similar to gross findings. The cervical mass exhibited heterogeneously high attenuation on diffusion-weighted imaging (DWI) and mild diffusion restriction on apparent diffusion coefficient (ADC) (Fig. 2a, b). These MRI findings suggested a degenerated adenomyoma; however, the possibility of a rare invasive tumor could not be ruled out. Multiple hyperintense small cysts were found inside the tumor on T2-weighted imaging (T2WI) (Fig. 3). There was no evidence of ascites or pelvic lymphadenopathy. Contrast-enhanced chest and abdominal computed tomography showed no evidence of distant metastases. Cervical biopsy revealed a mesenchymal tumor; however, the biopsy result was inconclusive as the amount of biopsied tissue was small.
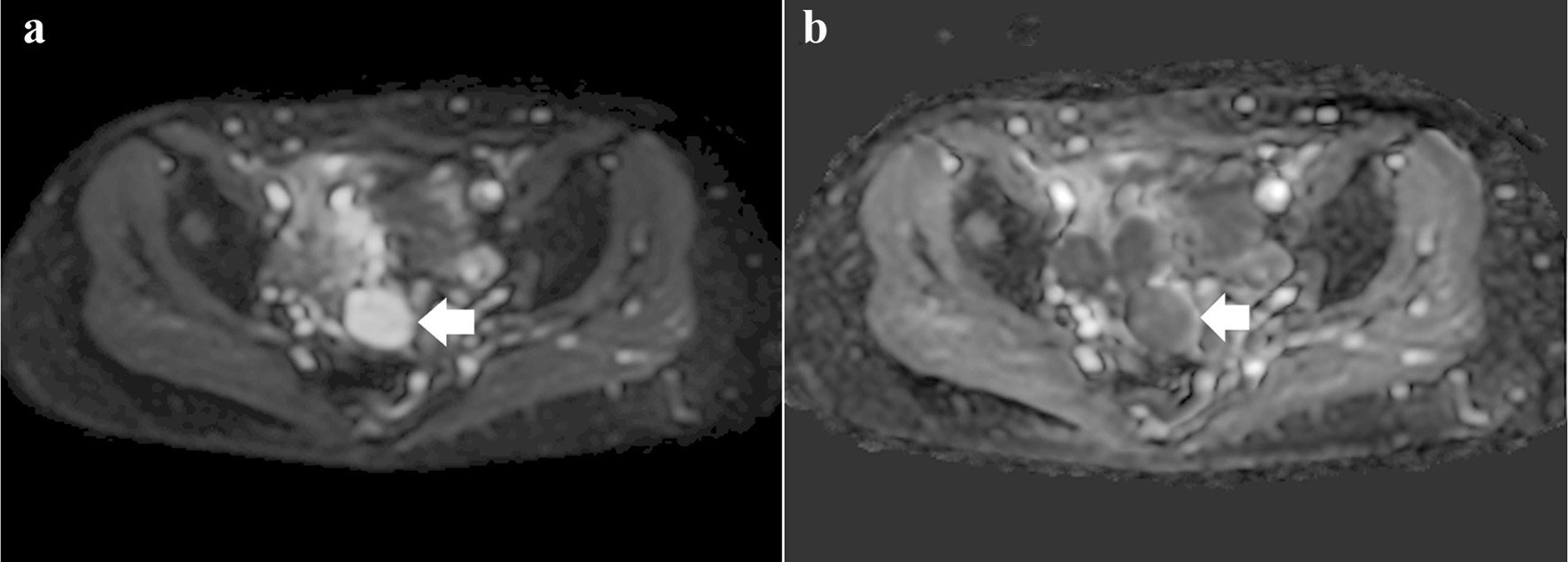 Click for large image | Figure 2. (a) Diffusion-weighted image showing a heterogeneous high-intensity tumor (arrow). (b) Apparent diffusion coefficient image showing a tumor with mild diffusion restriction (arrow). |
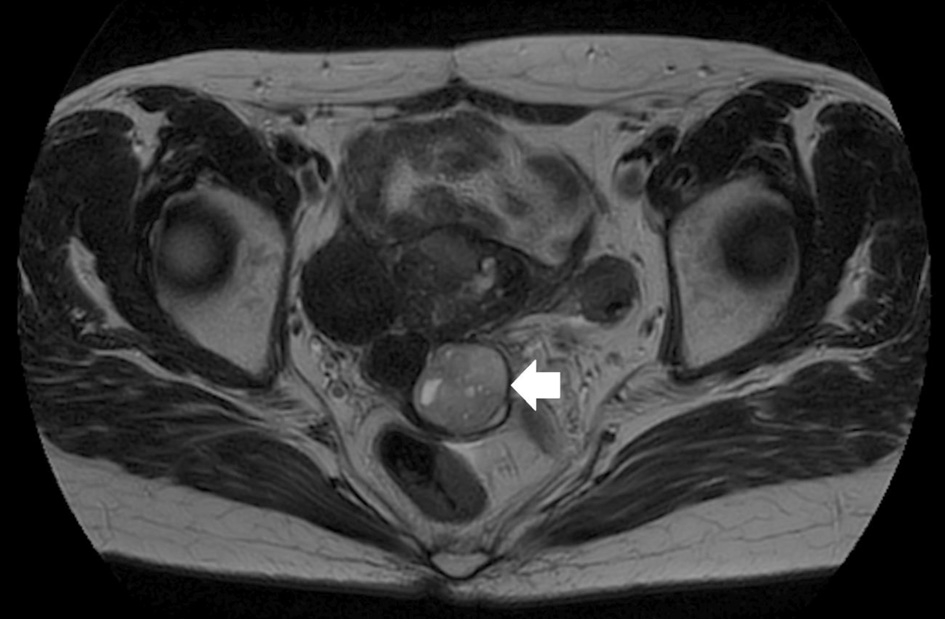 Click for large image | Figure 3. Multiple hyperintense small cysts inside the tumor on T2-weighted imaging (arrow). |
Treatment
We decided to further evaluate all lesions in the cervix through hysterectomy and accordingly performed total abdominal hysterectomy with bilateral salpingo-oophorectomy. Gross examination of the cervix revealed that the cut surface of the tumor was white and that the cervix had been completely replaced by the tumor (Fig. 4). Histologically, the surface of the tumor had a leaf-like papillary appearance, with a benign-appearing glandular epithelium (Fig. 5a, b). There was also a distinctive band of hypercellular stroma surrounding the glandular epithelium, known as periglandular cuffing (Fig. 6a, b). The mean mitotic count of the tumor was 2 per 10 high-power fields (Fig. 7). These findings confirmed cervical adenosarcoma from the removed uterus. Adenosarcoma has been associated with endometriosis, although only few reports support this association [6, 7]; however, there were no findings of endometriosis in the excised uterus and bilateral adnexa.
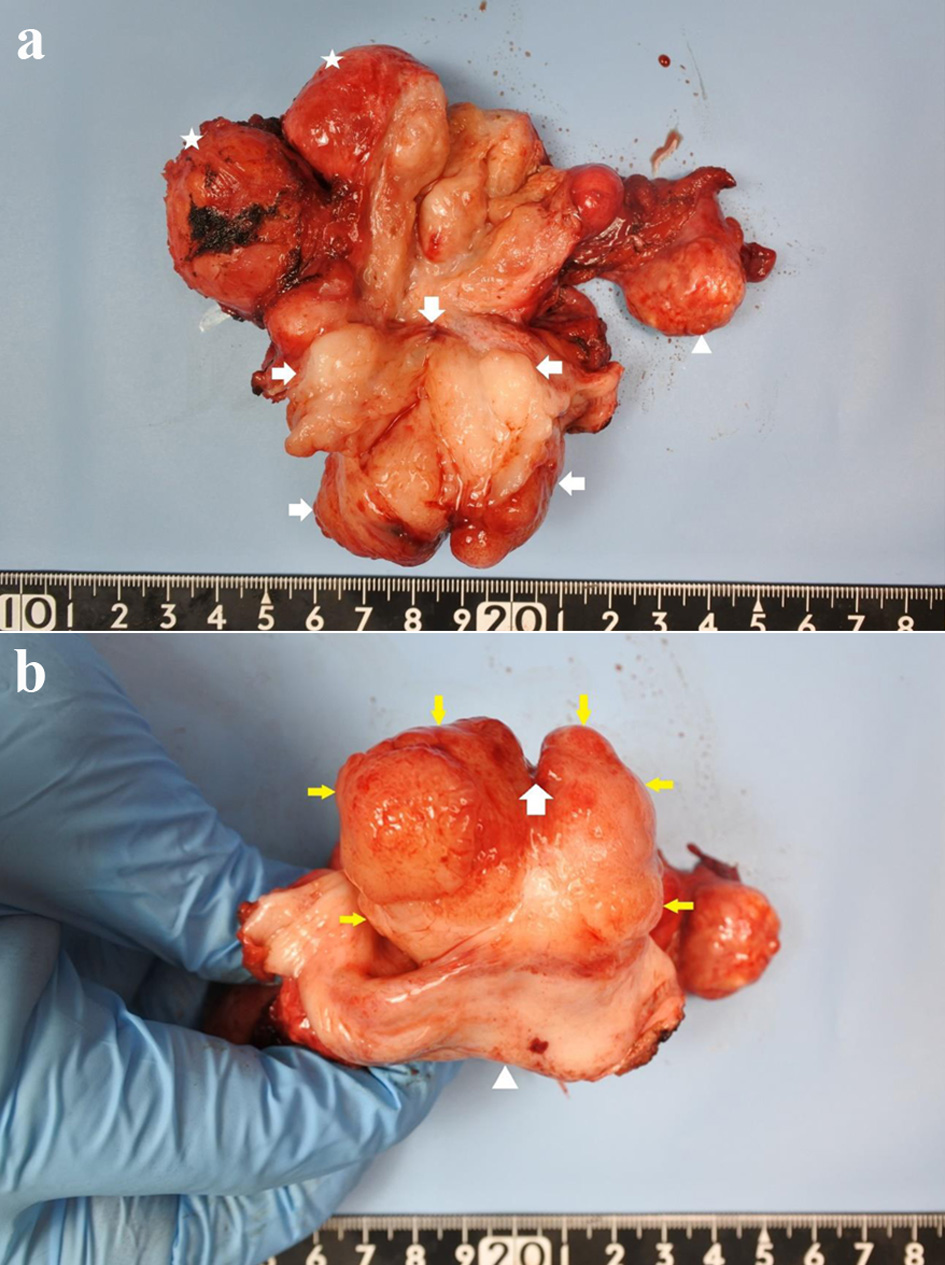 Click for large image | Figure 4. (a) Gross pathological specimen showing a mass occupying the entire cervix (arrowhead: ovary, star: leiomyoma, arrow: cervical tumor). (b) Cervix replaced by the tumor, as seen from the vaginal side (arrow: external os with an incision at 12 o’clock; yellow arrow: cervix; arrowhead: vaginal wall). |
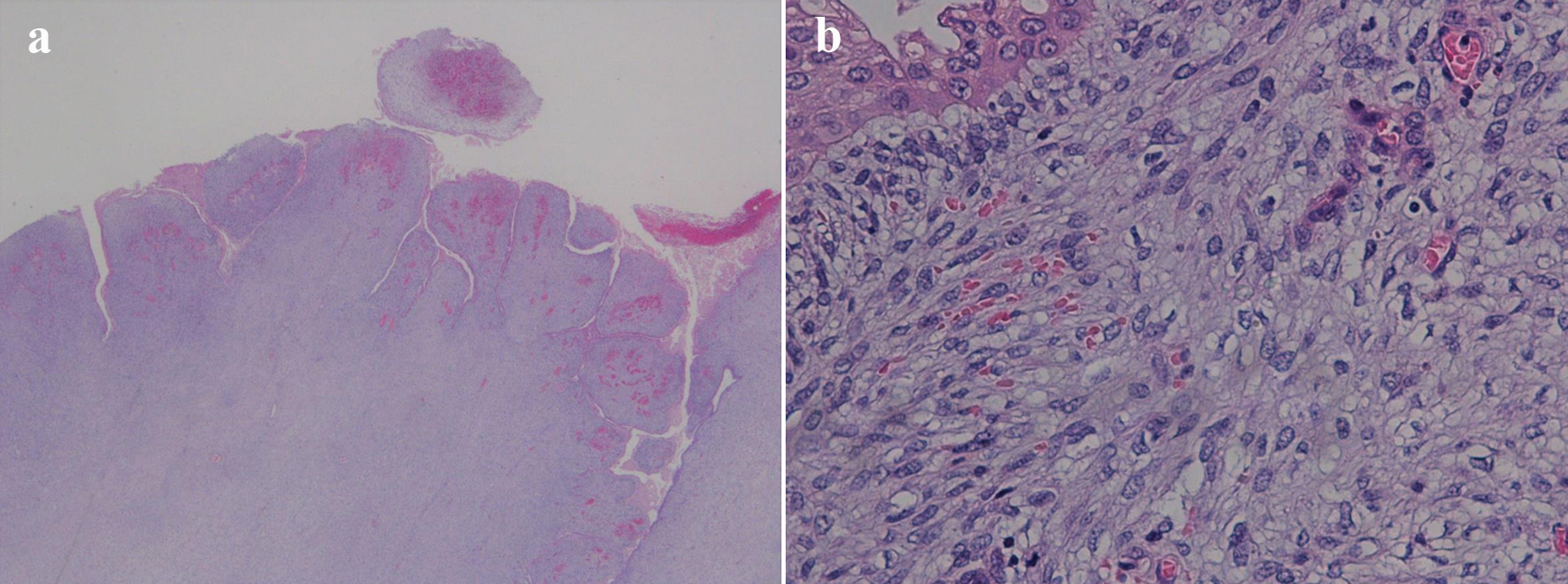 Click for large image | Figure 5. (a) Low-power field showing characteristic leaf-like architecture (hematoxylin and eosin staining, magnification × 10). (b) Papillary stromal fronds lined by benign epithelium accompanied with squamous metaplasia. The stromal cells show mild nucleal atypia and mild pleomorphism (hematoxylin and eosin staining, magnification × 200). |
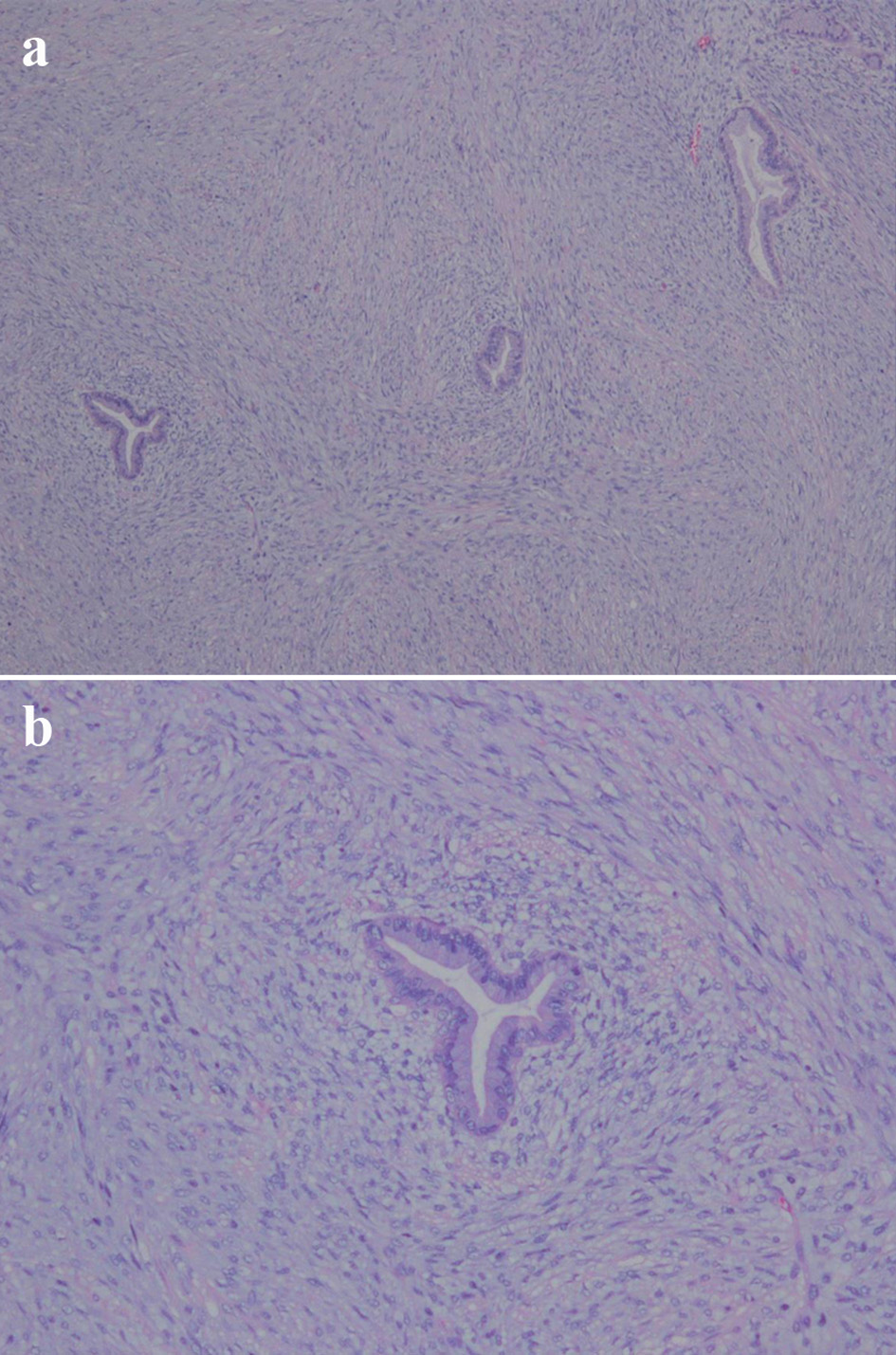 Click for large image | Figure 6. (a) Subepithelial condensation of the stroma; periglandular cuffing (hematoxylin and eosin staining, magnification × 40). (b) Hypercellular stroma and high cellularity beneath the epithelium (hematoxylin and eosin staining, magnification × 100). |
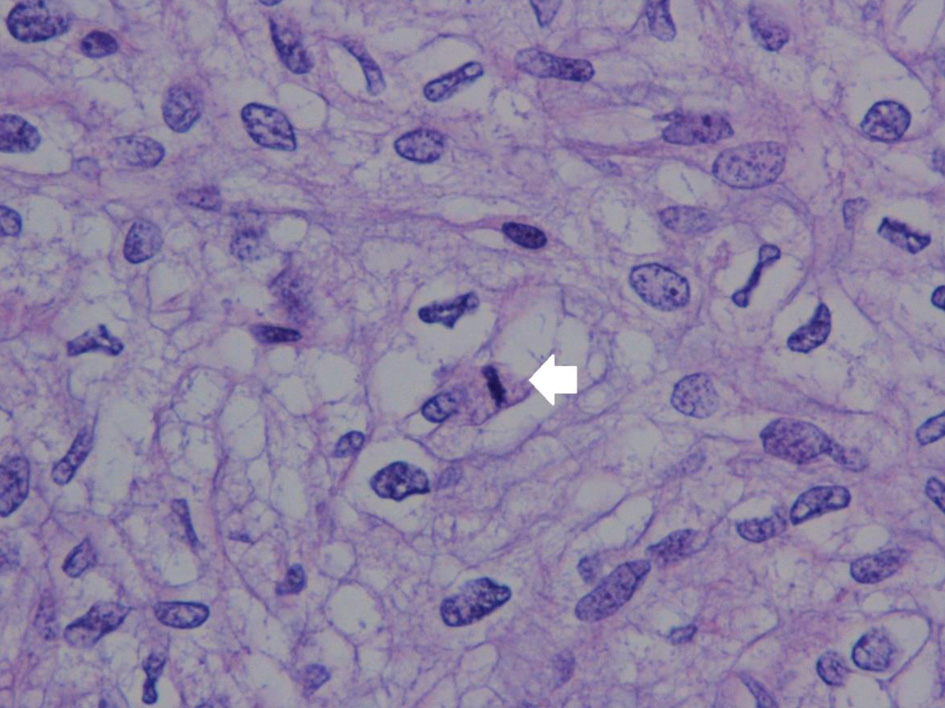 Click for large image | Figure 7. Stromal atypia with mitosis in the stromal element (arrow) (hematoxylin and eosin staining, magnification × 400). |
No immunostaining is specifically positive for adenosarcoma. However, the cytoplasm in adenosarcoma stromal cells is often positive for CD10, and the nucleus is often positive for estrogen receptors, progesterone receptors, and P53 [8]. In the present case, the stromal cell cytoplasm was positive for CD10, and the glandular epithelium nuclei were positive for estrogen receptors but negative for progesterone receptors. Nonspecific nuclear staining for P53 was noted, which was interpreted as wild-type (Fig. 8).
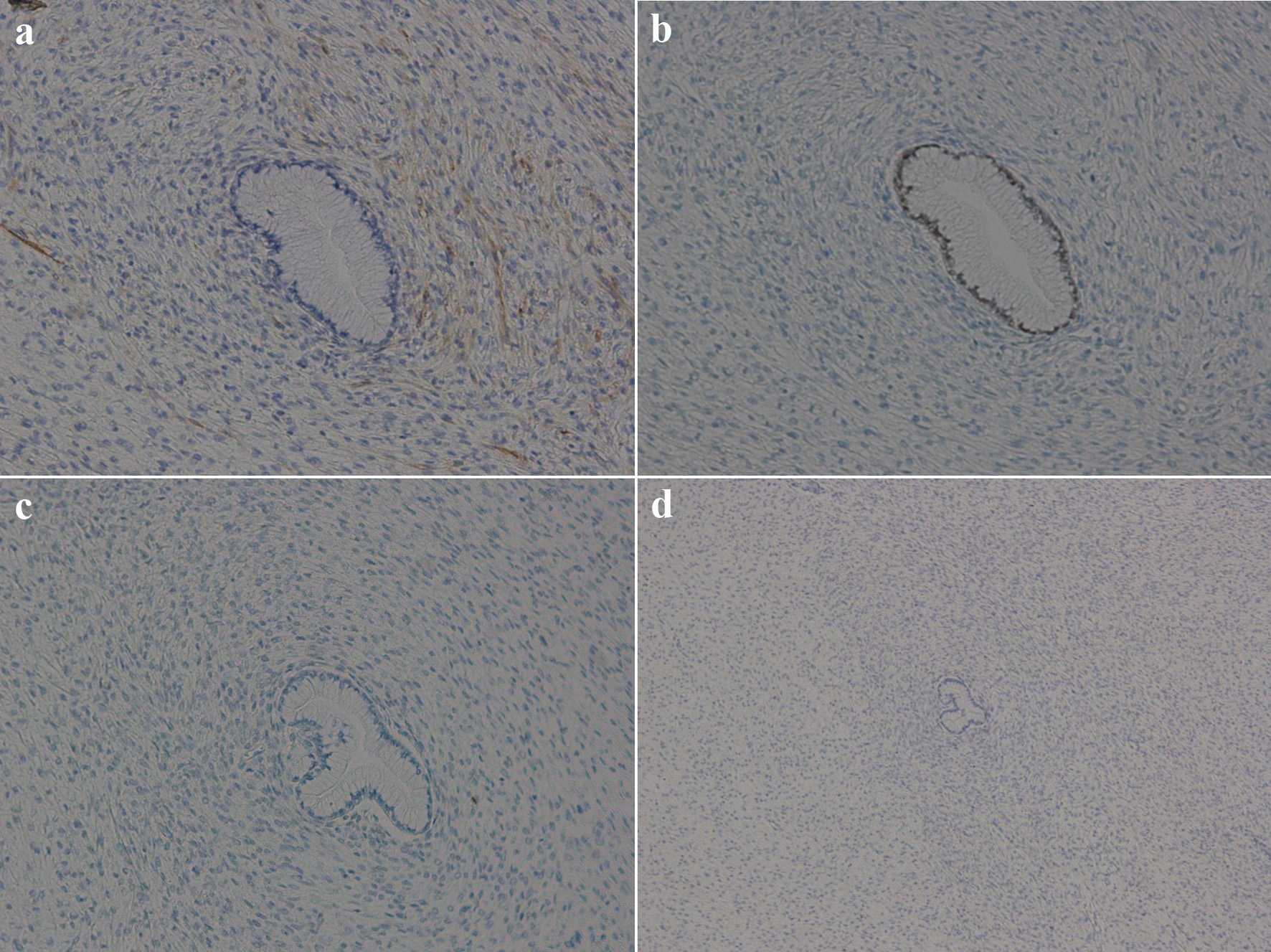 Click for large image | Figure 8. Immunohistochemical findings of the tumor. (a) Stromal cell cytoplasm positive for CD10 (magnification, ×100); (b) glandular epithelia nuclei positive but stromal cell nuclei negative for estrogen receptors (magnification, ×100); (c) both glandular epithelia nuclei and stromal cell nuclei negative for progesterone receptors (magnification, ×100); (d) stromal cell showing wild-type pattern for P53 (magnification, ×40). |
Follow-up and outcomes
She has been followed up for the last 10 months without any adjuvant therapy after the surgery.
| Discussion | ▴Top |
An accurate diagnosis of adenosarcoma solely on the basis of preoperative imaging, clinical symptoms, and pathological biopsy findings without surgery is difficult. This may be because of the following three reasons.
First, in diagnostic imaging for adenosarcoma, several studies have reported cysts in polypoid lesions that appear hypo- and hyperintense on T1-weighted images and T2WI, respectively [9, 10]. However, this finding is not unique to adenosarcomas; it is also observed in adenomyomas or atypical polypoid adenomyomas with intimal glands within the myometrium [11]. In our case, the tumor had high attenuation with hyperintense cysts on T2WI and moderately low ADC. In sarcomas, ADC restriction is known to demonstrate high attenuation on DWI; however, our case demonstrated high attenuation on DWI and mild diffusion restriction on ADC. These findings are similar to those of cystic/myxoid degeneration, which is difficult to distinguish from sarcomas [12]. In addition, the cervix in our case was found to have moderately increased uptake by PET, which prompted further examination. Although a negative FDG image may exclude the possibility of uterine sarcoma, even benign uterine tumors can be FDG-positive, which is said to be related to age and inflammation [13].
Second, cervical adenosarcomas are often extroverted and appear as polypoid tumors. Therefore, cervical adenosarcomas protrude into the vagina and are often misdiagnosed as cervical polyps [14, 15]. However, in our case, the tumor developed such that it replaced the cervix and exhibited an endophytic growth pattern. Tumors showing an exophytic growth pattern can often be biopsied as sufficient tissue samples are available for pathological diagnosis. In contrast, tumors showing endophytic growth pattern may not be diagnosed even with adenosarcoma as the amount of tumor tissue available for a biopsy is small. In our case, the biopsy did not indicate adenosarcoma as mitosis, phyllodes-like architecture, and periglandular cuffing were not observed in the tumor cells. However, pathological analysis of the resected uterus revealed mitosis of stromal cells, phyllodes-like architecture, and periglandular cuffing, leading to the diagnosis of cervical adenosarcoma.
Despite the tumor measuring 4 cm, vaginal bleeding was noted only once at 6 months before diagnosis as faint brown discharge. This may be attributed to the tumor’s growth pattern; tumors showing an exophytic growth pattern usually appear polypoid or papillary. Such tumors then grow into the vaginal cavity and cause vaginal bleeding. In contrast, tumors showing an endophytic growth pattern infiltrate and eventually replace the cervix without any visible surface growth [16]. Therefore, the squamous epithelium of the cervix may remain intact even if the diameter of the tumor exceeds 4 cm. As the squamous epithelium is intact, bleeding may not occur. The tumor in the present case exhibited an endophytic growth pattern; therefore, no bleeding occurred despite the entire cervix being replaced by the tumor.
Third, although we performed a biopsy on the tumor, no definitive pathological diagnosis was made. In this case, cervical adenosarcoma was pathologically diagnosed after hysterectomy. If biopsy results indicate a mesenchyal tumor, the presence of adenosarcoma should still be considered even in the absence of mitosis or other malignancy characteristics on imaging. Hence, it is necessary to completely excise the tumor and evaluate it pathologically.
A well-established poor prognostic factor of uterine adenosarcoma is the surgical stage, which primarily indicates deep muscle infiltration and extrauterine extension [17]. Although the examination of pathological factors is limited to single-center data, an examination of previous cases of uterine adenosarcoma indicates that sarcomatous overgrowth or heterologous elements, such as pathological factors, indicate a poor prognosis [18, 19]. Systemic anticancer drug treatment is the best option for patients with relapse or distant metastasis. However, the optimal first-line treatment remains unclear because of the lack of large-scale, multicenter, prospective studies. Our case did not present with sarcomatous overgrowth or heterologous elements; however, the prognosis was poor as the tumor had replaced the cervix entirely. Moreover, the efficacy of adjuvant therapy for patients with a poor prognosis has not been established; therefore, we decided to follow-up the patient without adjuvant therapy.
Conclusions
Adenosarcomas are low-grade stromal tumors with benign epithelium. Therefore, nuclear mitosis or atypia of tumor cells may be overlooked depending on the amount of tissue collected. As a result, several reports have indicated that recurring cervical polyps are finally diagnosed as adenosarcoma [14, 15].
If cervical biopsy reveals a mesenchymal tumor, the presence of an adenosarcoma should be considered. Tumor resection, including hysterectomy, should be performed, and the whole tumor should be pathologically evaluated even if the biopsied specimen does not show mitosis and there is no evidence of malignancy on imaging.
Learning points
Adenosarcomas can originate in the cervix. Although adenosarcomas usually cause genital bleeding, cervical adenosarcoma showing an endophytic growth pattern may not lead to vaginal bleeding. Even if the cervical biopsy results reveal a mesenchymal tumor and there is no evidence of malignancy on imaging, the presence of adenosarcoma should be considered.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient prior to completing the case report.
Author Contributions
ELS and SiI contributed to the writing of the manuscript. TK, RK, MM, and YS reviewed and adjusted the manuscript prior to submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
MRI: magnetic resonance imaging; DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient; T2WI: T2-weighted imaging
| References | ▴Top |
- D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131-139.
doi pubmed - Harlow BL, Weiss NS, Lofton S. The epidemiology of sarcomas of the uterus. J Natl Cancer Inst. 1986;76(3):399-402.
- Abeler VM, Royne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54(3):355-364.
doi pubmed - Verschraegen CF, Vasuratna A, Edwards C, Freedman R, Kudelka AP, Tornos C, Kavanagh JJ. Clinicopathologic analysis of mullerian adenosarcoma: the M.D. Anderson Cancer Center experience. Oncol Rep. 1998;5(4):939-944.
doi pubmed - Togami S, Kawamura T, Fukuda M, Yanazume S, Kamio M, Kobayashi H. Clinical management of uterine cervical mullerian adenosarcoma: A clinicopathological study of six cases and review of the literature. Taiwan J Obstet Gynecol. 2018;57(4):479-482.
doi pubmed - Liu L, Davidson S, Singh M. Mullerian adenosarcoma of vagina arising in persistent endometriosis: report of a case and review of the literature. Gynecol Oncol. 2003;90(2):486-490.
doi - Pontrelli G, Cozzolino M, Stepniewska A, Bruni F, Pesci A, Ceccaroni M. Primary vaginal adenosarcoma with sarcomatous overgrowth arising in recurrent endometriosis: feasibility of laparoscopic treatment and review of the literature. J Minim Invasive Gynecol. 2016;23(5):833-838.
doi pubmed - Gallardo A, Prat J. Mullerian adenosarcoma: a clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am J Surg Pathol. 2009;33(2):278-288.
doi pubmed - Yoshizako T, Wada A, Kitagaki H, Ishikawa N, Miyazaki K. MR imaging of uterine adenosarcoma: case report and literature review. Magn Reson Med Sci. 2011;10(4):251-254.
doi pubmed - Takeuchi M, Matsuzaki K, Yoshida S, Kudo E, Bando Y, Hasebe H, Kamada M, et al. Adenosarcoma of the uterus: magnetic resonance imaging characteristics. Clin Imaging. 2009;33(3):244-247.
doi pubmed - Connors AM, deSouza NM, McIndoe GA. Adenomyoma mimicking an aggressive uterine neoplasm on MRI. Br J Radiol. 2003;76(901):66-68.
doi pubmed - Li HM, Liu J, Qiang JW, Zhang H, Zhang GF, Ma F. Diffusion-weighted imaging for differentiating uterine leiomyosarcoma from degenerated leiomyoma. J Comput Assist Tomogr. 2017;41(4):599-606.
doi pubmed - Sun S, Bonaffini PA, Nougaret S, Fournier L, Dohan A, Chong J, Smith J, et al. How to differentiate uterine leiomyosarcoma from leiomyoma with imaging. Diagn Interv Imaging. 2019;100(10):619-634.
doi pubmed - Kerner H, Lichtig C. Mullerian adenosarcoma presenting as cervical polyps: a report of seven cases and review of the literature. Obstet Gynecol. 1993;81(5 Pt 1):655-659.
- Hino A, Hirose T, Seki K, Uehara H, Sano N. Adenosarcoma of the uterine cervix presenting as a cervical polyp. Pathol Int. 1998;48(8):649-652.
doi pubmed - https://screening.iarc.fr/doc/colpochapter03.pdf.
- Arend R, Bagaria M, Lewin SN, Sun X, Deutsch I, Burke WM, Herzog TJ, et al. Long-term outcome and natural history of uterine adenosarcomas. Gynecol Oncol. 2010;119(2):305-308.
doi pubmed - Kaku T, Silverberg SG, Major FJ, Miller A, Fetter B, Brady MF. Adenosarcoma of the uterus: a Gynecologic Oncology Group clinicopathologic study of 31 cases. Int J Gynecol Pathol. 1992;11(2):75-88.
doi pubmed - Tate K, Watanabe R, Yoshida H, Shimizu H, Uehara T, Ishikawa M, Ikeda SI, et al. Uterine adenosarcoma in Japan: Clinicopathologic features, diagnosis and management. Asia Pac J Clin Oncol. 2018;14(4):318-325.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


