| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 9, September 2022, pages 432-437
SARS-CoV-2 Unmasks Type 1 Diabetes Mellitus With an Episode of Diabetic Ketoacidosis
Asimenia Haliotia, c, Maria Kitinoua, Varvara-Maria Chaliotib, Georgios Chaliotisa
aCOVID-19 Clinic, General Hospital of Chalkida, Chalkida, Evia, Greece
bMicrobiology Laboratory - Biochemistry Unit, General Hospital of Chalkida, Chalkida, Evia, Greece
cCorresponding Author: Asimenia Halioti, COVID-19 Clinic, General Hospital of Chalkida, Chalkida, 34100, Evia, Greece
Manuscript submitted June 1, 2022, accepted August 17, 2022, published online September 28, 2022
Short title: SARS-CoV-2-Induced DKA
doi: https://doi.org/10.14740/jmc3963
| Abstract | ▴Top |
During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, coronavirus disease 2019 (COVID-19) has been significantly studied for its relationship with diabetes mellitus in general. Still, the association of SARS-CoV-2 infection with diabetic ketoacidosis (DKA) is more specific and warrants a meticulous investigational approach. In this case report, we present a 23-year-old female who developed DKA as the first manifestation of SARS-CoV-2 infection. During hospitalization, the diagnosis of type 1 diabetes mellitus (T1DM) was made and the patient was treated successfully for the metabolic disorder and for SARS-CoV-2. The potential of SARS-CoV-2 to induce DKA in type 1 diabetics is highlighted. We point out that DKA and COVID-19 may have similarities in clinical presentation when gastrointestinal features predominate. In addition, we describe mechanisms that have been hypothesized to explain the negative impact of SARS-CoV-2 on the endocrine pancreatic function.
Keywords: Pandemic; Diabetic ketoacidosis; Type 1 diabetes mellitus; SARS-CoV-2; COVID-19
| Introduction | ▴Top |
In December 2019, in the region of Wuhan in China, the official announcement of a growing number of people testing positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) signaled the onset of coronavirus disease 2019 (COVID-19) pandemic [1]. COVID-19 presents with typical clinical features, which comprise fever, upper or lower respiratory tract symptoms, headache, myalgias, arthralgias and disturbances of smell and taste. The severity of the clinical picture varies from a total lack of symptoms to severe disease, hospitalization and death, and this depends on the medical and vaccination status of the individual and the pathogenic variant, among other factors. Notably, atypical and extrapulmonary manifestations develop in a substantial part of patients with COVID-19 and include cardiac adverse events, neurological complications, thromboses and abnormal hematologic findings, gastrointestinal symptoms, skin issues and secondary infections [2].
A high-risk group for exhibiting adverse outcomes due to SARS-CoV-2 infection are diabetic individuals [3]. Diabetic ketoacidosis (DKA) is a serious complication at the extreme end of the clinical spectrum of diabetes mellitus, and it may present as the first manifestation of the disease, especially in young people with type 1 diabetes mellitus (T1DM). The pathophysiology of T1DM involves principally autoimmune processes with input from the environmental and genetic background of the individual. Numerous factors have been identified as potential triggers for DKA and infections are the most common [4].
Interestingly, SARS-CoV-2 being a recently identified virus, its pathogenetic potential to induce an episode of DKA has not been fully elucidated or is widely recognized. Herein, we present the unusual case of a 23-year-old female who tested positive for SARS-CoV-2 and was in urgent need for hospitalization owing to the simultaneous development of DKA in the context of previously unknown T1DM. We highlight that SARS-CoV-2 may induce DKA in predisposed persons similarly to other viruses and this association merits further exploration. The already high mortality index of DKA rises further, up to 45%, when it is complicated with COVID-19 [5], which mandates a careful approach in the handling of these patients.
| Case Report | ▴Top |
Investigations
A 23-year-old woman presented to the emergency department (ED) in a serious clinical state. She reported - with difficulty, owing to gasping - that she had approximately six episodes of emesis accompanied by intense, diffuse abdominal pain in the last 24 h, and also, she had been febrile with a body temperature up to 38.5 °C (101.3 °F) the previous day. She did not report headache, sore throat or cough. She had been feeling relatively well until 2 days ago. However, the previous 3 months she had noticed an intense feeling of thirst, which prompted her to increase her daily intake of water and home-prepared beverages, and her daily urine volume was also increased, especially at sleeping hours. Also, the patient had unintentionally lost 5 kg of body weight the previous 3 months, weighting 43 kg at presentation. She had no significant personal medical history apart from an abortion 3 years ago and did not take any medications. She worked as a waitress, smoked lightly and consumed alcohol on rare social events. She had not recently travelled abroad or had contact with animals. She was in a stable monogamous relationship. She was not vaccinated against SARS-CoV-2 and, to her knowledge, she had not recently come in contact with people who tested positive for COVID-19 or had suspicious symptoms. Her maternal grandmother had diabetes mellitus, though the patient could not recall on its specific type or the treatment being followed, and the rest of her family was in good health.
On initial physical examination, the patient appeared very thin (the body mass index (BMI) for her height of 1.64 m was calculated at 16 kg/m2) and she was breathing heavily and rapidly (a respiratory rate of 32 breaths per minute), but without evident hypoxemia (SpO2 99% on room air). The heart rate was 119 beats per minute with a sinus mode on electrocardiogram, the blood pressure was 115/57 mm Hg and her body temperature was 37.1 °C (98.7 °F). There were no rales or wheezing on lung auscultation, the heart beating was rapid and rhythmic without any heart murmurs and the abdomen was diffusely tender but without rebound pain and with normal bowel sounds. Despite her agitated clinical condition, she exhibited mental clarity, she was responsive and cooperative during the physical examination and did not have any focal neurological signs. A rapid antigenic test for SARS-CoV-2 was performed that was positive.
Diagnosis
The diagnosis of COVID-19 was hypothesized. Notably, however, her finger stick blood glucose was found at 580 mg/dL. The full blood count was normal and the remainder of the routine laboratory workup was remarkable for moderately elevated markers of inflammation (C-reactive protein, ferritin) and a strikingly high glycosylated hemoglobin (HbA1c) at 14% (Table 1). Arterial blood gases revealed metabolic acidosis with an arterial pH of 7.06 and bicarbonates at the level of 3.1 mmol/L with a high anion gap at 32.5 mmol/L (Table 2). Glycosuria, proteinuria and ketonuria were found in the urinalysis (Table 3). Blood and urine cultures that were performed for the investigation of fever were sterile. A plain chest X-ray did not show abnormalities suggestive of pneumonia (Fig. 1). A nasopharyngeal swab was examined with RT-PCR for SARS-CoV-2 and was positive, with a Cycle threshold (Ct) of 29.7.
 Click to view | Table 1. Venous Blood Laboratory Findings on Admission |
 Click to view | Table 2. Arterial Blood Gases on Admission (FiO2 21%) |
 Click to view | Table 3. Urine Analysis on Admission |
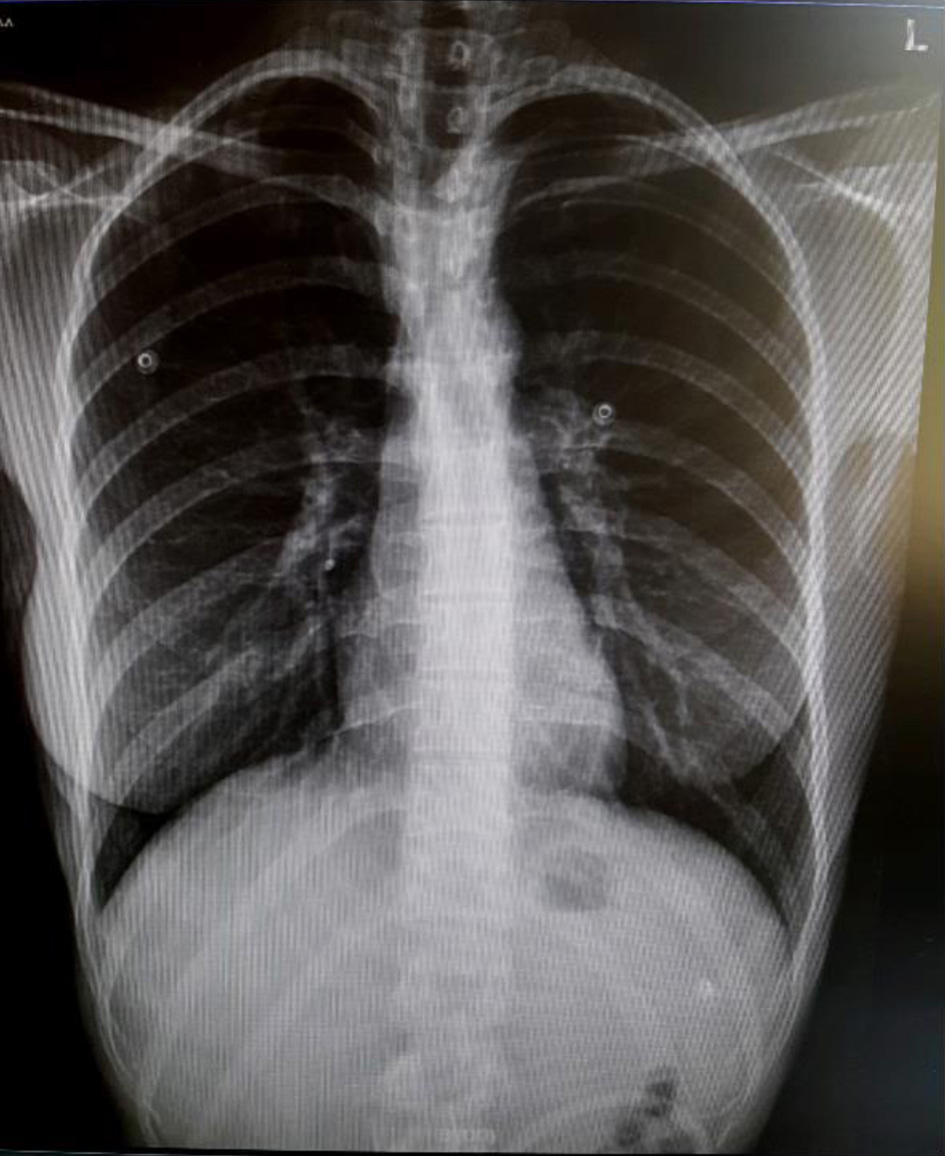 Click for large image | Figure 1. A normal chest X-ray of the patient, taken on the day of admission. |
Treatment
Taking into account the above clinical and laboratory results, the patient was treated for DKA and a co-occurrent SARS-CoV-2 infection. She initially received an intravenous (IV) bolus dose of 10 units of human insulin and 2 L of normal saline in the first couple of hours. She was then started on a continuous IV infusion of regular short-acting insulin at a rate of 0.1 units/kg/h with continuation of IV rehydration. In addition, the patient was put on nil per mouth, she received remdesivir (in a single dose of 200 mg IV on day 1 followed by single doses of 100 mg the next 4 days for 5 days in total) and empirical administration of ceftriaxone and she was given omeprazole and enoxaparin for prophylaxis against stress-induced peptic ulcer and venous thrombosis, respectively. The use of corticosteroids was withheld in order to avoid worsening of hyperglycemia. No need for supplemental oxygen therapy was identified. The clinical status of the patient (vital signs, urine output, mental state) was frequently assessed and her metabolic and biochemical parameters (blood glucose, electrolytes, arterial blood gases) were monitored and corrected accordingly.
Follow-up and outcomes
The patient recovered gradually, exhibiting a slower pattern of breathing and remission of fever and vomiting. Blood glucose measurements reached normal range, arterial pH increased slowly to over 7.3 and ketones disappeared from the urine. On day 2 of hospitalization, the patient was started on a diabetic diet and she was transitioned to a subcutaneous basal-bolus insulin regimen with short-acting insulin doses given three times a day based on pre-prandial glucose values and a standard dose of 10 units of insulin glargine. No recurrence of the metabolic derangements was noted. The patient received detailed information on the type of diet to be followed onwards and on the daily self-monitoring of glucose and self-administration of insulin and on day 5 she was discharged from the hospital in a significantly improved and stable condition. On a follow-up assessment 3 weeks later, the patient reported having gained 4 kg of body weight while efficiently controlling her diabetes at home with the prescribed insulin treatment. On that meeting, a C-peptide measurement was brought in, which was 0.14 ng/mL (reference range 0.8 - 3.8 ng/mL).
| Discussion | ▴Top |
The patient initially presented to the ED with fever, breathing distress, vomiting and abdominal pain. She tested positive for SARS-CoV-2 with an RT-PCR Ct value of 29.7 (Ct values in a RT-PCR test for SARS-CoV-2 point to the number of cycles that the viral nucleic acid needs to be amplified in a sample for the detection of the virus; the lesser the value, the higher the viral load, and usually Ct values of less than 30 indicate recent infection [6]) and she was not vaccinated against the virus. The principal suggestive diagnosis was COVID-19. However, she surprisingly fulfilled the diagnostic criteria for DKA, which consist of hyperglycemia, ketonemia or ketonuria and a high anion gap metabolic acidosis [7]. The co-occurrence of SARS-CoV-2 infection with DKA in patients without a prior diagnosis of diabetes mellitus is uncommon in clinical practice.
This 23-year-old woman developed DKA in the context of a recent history of polydipsia, polyuria and weight loss, having a low BMI indicative of being underweight and she showed a favorable clinical response only with the exogenous administration of insulin after hospital discharge. This presentation coincides with the classic phenotype described for T1DM patients who initially present in the symptomatic stage of the disease [8] and so the diagnosis of T1DM was made with certainty. We did not measure serum autoantibodies against the endocrine unit of the pancreas (anti-glutamic acid decarboxylase 65, anti-islet antigen 2, anti-insulin, anti-zinc transporter 8) owing to the corroborative value of the previously described clinical information in favor of T1DM, the unavailability of these tests in our community-based hospital and their high cost. These biomarkers are evidence of the ongoing autoimmune-mediated destruction of the endocrine pancreas in T1DM and have high diagnostic value for the distinction between T1DM and type 2 diabetes mellitus (T2DM), especially in cases with overlapping clinical features [8]. Nevertheless, C-peptide was measured close to zero in this patient, which indicates the underlying endocrine pancreatic insufficiency.
DKA may present with fever, nausea, vomiting and acute abdominal pain. This is also true for 10-20% of COVID-19 patients, who exhibit predominantly gastrointestinal symptoms [9]. Owing to this possible overlap in the clinical picture of these potentially life-threatening disorders, DKA and COVID-19, a high index of suspicion and careful approach are necessary in the investigation of any patient who presents with fever and gastrointestinal features in the COVID-19 pandemic. This case report serves as an example of an episode of DKA mimicking severe COVID-19. More broadly speaking, the differential diagnosis of fever in association with gastrointestinal features in a young person comprises a wide spectrum of potential causes, for example food-borne illnesses, inflammatory bowel disease and acute appendicitis [10], so that even with the finding of a positive SARS-CoV-2 test in such a patient, the remaining possible causes should also be examined. Notably, however, Nassar et al conclude in their systematic review of the literature that fever, nausea, vomiting, hyperglycemia, as well as cough, may indeed be the most common presenting clinical features of COVID-19 in patients with T1DM [11].
DKA signifies hyperglycemia on the basis of low or absent circulating insulin and increased counterregulatory hormones (glucagon, catecholamines, cortisol and growth hormone), that also drive unopposed lipolysis and ketone production in the liver and lead to metabolic acidosis [12]. DKA is a life-threatening condition that may present as the first manifestation of the slow but unrelenting autoimmune destruction of the beta islet cells of the pancreas in children and young adults with T1DM, although an increasing proportion of the overall incidence of DKA is associated with T2DM [12]. The widely recognized events that can precipitate an episode of DKA include acute illnesses such as infections, acute pancreatitis and myocardial ischemia, stress-inducing medical conditions such as surgery and pregnancy, inadequate or missed doses of insulin and the intake of certain drugs, such as glucocorticoids and sodium-glucose co-transporter 2 (SGLT2) inhibitors. All types of infectious agents (bacterial, viral, mycobacterial, fungal, parasitic) have been associated with DKA [13]. In this context, the role of SARS-CoV-2 virus as a potential trigger for DKA has only recently started being evaluated in the course of the COVID-19 pandemic. As an infectious agent, SARS-CoV-2 can theoretically precipitate hyperglycemic metabolic derangements in many different clinical scenarios: for example, either in type 1 or in type 2 diabetic patients, in those with an established diagnosis of diabetes mellitus or in those who are unaware of their underlying chronic hyperglycemic state, and also irrespectively of whether SARS-CoV-2 infection is symptomatic or not. Considering the significantly higher prevalence of T2DM in comparison with T1DM in the general population (approximately 95% versus 5%, respectively) [14] and the fact that diabetes mellitus in general is a negative prognostic factor in COVID-19 associated with more severe disease and worse outcomes in diabetic patients in opposition to euglycemic patients [3], it is to be expected that most recent case reports and series for the association of SARS-CoV-2 with DKA are described for type 2 diabetic patients [15, 16]. However, the association of SARS-CoV-2 with DKA in T1DM is interesting and deserves further exploration, in part because it is highly unexpected.
SARS-CoV-2 leads to the development of an acute systemic inflammatory response, characterized as a type of “cytokine storm”, that can impact adversely on the function of any specific organ-system, the pancreas included, and which underlies the diverse clinical manifestations - pulmonary and extrapulmonary - of COVID-19 [17]. Interestingly, this acute oxidative stress-related immune response against SARS-CoV-2 can even be so intense as to enter a chronic phase of clinical decline termed the “long COVID” syndrome. In the case of T1DM, the smoldering, progressive injury of beta islet pancreatic cells on an autoimmune basis is central for the pathogenesis of the disease and becomes clinically evident with an absolute need for the exogenous administration of insulin for the preservation of metabolic homeostasis. In one study, this acute-on-chronic dysregulation of the immune system and its potential effects on the endocrine pancreas have been deemed plausible to construe the development of DKA in SARS-CoV-2 patients with T1DM [18]. Finally, endothelial dysfunction and hypercoagulable mechanisms may participate synergistically with these skew immune processes to increase the strain on the endocrine pancreas and eliminate its potential for insulin synthesis and secretion.
The role of angiotensin-converting enzyme type 2 (ACE-2) has been studied significantly in the pathogenesis of COVID-19. ACE-2 on the surface of the host cells binds the Spike protein on the outer membrane of the virus and allows the entry of the virus into the human cells. It is presumed that SARS-CoV-2 infects the pancreatic cells on the same molecular basis, thus causing acute pancreatitis as a complication of COVID-19 [19] when the exocrine part or supporting tissues of the organ are affected in excess, as well as acquiring a diabetogenic potential, when the endocrine unit is injured in excess [18, 20]. Thus, the relationship of SARS-CoV-2 with diabetes mellitus can be examined as a bidirectional one, for in addition to the fact that diabetes mellitus is an important negative prognostic factor in COVID-19 [3], SARS-CoV-2 seems to disrupt glycemic control in patients with known diabetes mellitus and can possibly precipitate acute hyperglycemic complications in persons with undiagnosed or new-onset diabetes mellitus [21, 22]. In this patient, hyperglycemic symptoms had a duration of several months as is inferred from the medical history and the two-digital value of HbA1c. Her clinical presentation is consistent with the acute-onset subtype of T1DM (either of autoimmune or idiopathic etiology, which corresponds to the presence or absence of serum autoantibodies against the pancreas, respectively [23]). However, the exact time point that the molecular pathogenetic mechanisms come into play for the ultimate destruction of the pancreas is difficult if not impossible to be specified in patients with T1DM. In this patient, DKA was classified as moderate to severe based on the significantly low level of arterial pH albeit with a normal mental status [12], and she did not develop any of the fearful complications of COVID-19, such as respiratory failure, circulatory compromise or any thromboses. Her in-hospital course was uneventful with a positive clinical outcome, and this can probably be attributed to her young age, the lack of other comorbidities and the prompt recognition and correction of hyperglycemia.
Some interesting aspects of the clinical management of this patient that we consider useful to point out are the following. First, she was a young woman without known comorbidities and without COVID-19 pneumonia, which permitted the administration of a rigorous type of IV fluid therapy, giving her more than 4 L of normal saline IV on day 1 of hospitalization. This would not have been advisable had the patient been significantly older or with underlying cardiac or renal failure, in which case caution is necessary for the avoidance of volume overload and exacerbation of dyspnea [24]. Second, the patient was initially treated with IV continuous insulin therapy, but some authors present the use of standard doses of subcutaneous insulin as an alternative when DKA is mild or moderate [12]. This method would be particularly appreciated during a pandemic for it would minimize the need for frequent exposure of health care personnel to infected patients for the close adjustment of insulin infusion rate to glucose measurements, as well as contribute to the economic use of personal protective equipment. Third, although corticosteroids were omitted from the treatment plan against SARS-CoV-2 in this patient, we administered the antiviral agent remdesivir for 5 days, bearing in mind some recently published guidelines that advocate the early use of remdesivir for the prevention of progression to severe COVID-19 after infection in unvaccinated persons [25].
This case report describes the occurrence of DKA which was triggered possibly by a simultaneous SARS-CoV-2 infection in a woman and the final diagnosis of T1DM was made. We acknowledge that case reports have limited power for their conclusions to be generalized in clinical practice, yet they have value for their potential to spur research efforts in order to interpret pathogenetic mechanisms, disease associations and therapeutic challenges encountered in medical cases.
Learning points
In the evolution of COVID-19 pandemic, we have come across a wide spectrum of typical (respiratory) and atypical (non-respiratory) clinical manifestations of this viral infection. While the former are easily recognized and systematically treated according to formal guidelines, a high index of suspicion is essential for the optimal management of patients who present with the latter. Moreover, regarding the widely studied association of COVID-19 with diabetes mellitus, SARS-CoV-2 can lead to clinically significant glycemic metabolic derangements, more so in persons with previously undiagnosed and, therefore, uncontrolled diabetes mellitus.
Acknowledgments
The authors acknowledge the important contribution of the nursing staff of the COVID-19 Clinic of the General Hospital of Chalkida, Greece, in the successful management of the patient.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
We obtained a written informed consent from the patient in order to submit this case report for publication.
Author Contributions
AH, MK and GC participated in the medical management of the patient. AH wrote the original draft. AH, VMC and GC further edited the manuscript. All authors reviewed the final manuscript prior to its publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACE-2: angiotensin-converting enzyme type 2; BMI: body mass index; COVID-19: coronavirus disease 2019; Ct: cycle threshold; DKA: diabetic ketoacidosis; ED: emergency department; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SGLT2: sodium-glucose co-transporter 2; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus
| References | ▴Top |
- Kumar A, Singh R, Kaur J, Pandey S, Sharma V, Thakur L, Sati S, et al. Wuhan to world: the COVID-19 pandemic. Front Cell Infect Microbiol. 2021;11:596201.
doi pubmed - Long B, Carius BM, Chavez S, Liang SY, Brady WJ, Koyfman A, Gottlieb M. Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation. Am J Emerg Med. 2022;54:46-57.
doi pubmed - Varikasuvu SR, Dutt N, Thangappazham B, Varshney S. Diabetes and COVID-19: A pooled analysis related to disease severity and mortality. Prim Care Diabetes. 2021;15(1):24-27.
doi pubmed - Shahid W, Khan F, Makda A, Kumar V, Memon S, Rizwan A. Diabetic ketoacidosis: clinical characteristics and precipitating factors. Cureus. 2020;12(10):e10792.
doi - Pal R, Banerjee M, Yadav U, Bhattacharjee S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: A systematic review of literature. Diabetes Metab Syndr. 2020;14(6):1563-1569.
doi pubmed - Al Bayat S, Mundodan J, Hasnain S, Sallam M, Khogali H, Ali D, Alateeg S, et al. Can the cycle threshold (Ct) value of RT-PCR test for SARS CoV2 predict infectivity among close contacts? J Infect Public Health. 2021;14(9):1201-1205.
doi pubmed - Dhatariya KK. Defining and characterising diabetic ketoacidosis in adults. Diabetes Res Clin Pract. 2019;155:107797.
doi pubmed - DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449-2462.
doi - McDermott CV, Cox EJ, Scanlan JM, Alicic RZ. COVID-19 and gastrointestinal tract symptoms: recognition, containment, and learning from the past. Mayo Clin Proc. 2020;95(11):2320-2324.
doi pubmed - Govender I, Rangiah S, Bongongo T, Mahuma P. A primary care approach to abdominal pain in adults. S Afr Fam Pract (2004). 2021;63(1):e1-e5.
doi - Nassar M, Nso N, Baraka B, Alfishawy M, Mohamed M, Nyabera A, Sachmechi I. The association between COVID-19 and type 1 diabetes mellitus: A systematic review. Diabetes Metab Syndr. 2021;15(1):447-454.
doi pubmed - Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: An update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507-521.
doi pubmed - Murunga AN, Owira PM. Diabetic ketoacidosis: an overlooked child killer in sub-Saharan Africa? Trop Med Int Health. 2013;18(11):1357-1364.
doi pubmed - World Health Organization. November 10, 2021). Diabetes. Retrieved from: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- Emara MH, Mazid U, Atta MA, Elshahat S, Mahros AM. Ketonuria with or without ketoacidosis as the presenting manifestation of SARS-CoV-2 (COVID-19) among uncontrolled type 2 diabetic patients. Med Hypotheses. 2020;144:110226.
doi pubmed - Croft A, Bucca A, Jansen JH, Motzkus C, Herbert A, Wang A, Hunter BR. First-time diabetic ketoacidosis in type 2 diabetics with COVID-19 infection: a novel case series. J Emerg Med. 2020;59(5):e193-e197.
doi pubmed - Chernyak BV, Popova EN, Prikhodko AS, Grebenchikov OA, Zinovkina LA, Zinovkin RA. COVID-19 and oxidative stress. Biochemistry (Mosc). 2020;85(12):1543-1553.
doi pubmed - Kountouri A, Korakas E, Ikonomidis I, Raptis A, Tentolouris N, Dimitriadis G, Lambadiari V. Type 1 diabetes mellitus in the SARS-CoV-2 pandemic: oxidative stress as a major pathophysiological mechanism linked to adverse clinical outcomes. Antioxidants (Basel). 2021;10(5):752.
doi pubmed - Jablonska B, Olakowski M, Mrowiec S. Association between acute pancreatitis and COVID-19 infection: what do we know? World J Gastrointest Surg. 2021;13(6):548-562.
doi pubmed - Ibrahim S, Monaco GSF, Sims EK. Not so sweet and simple: impacts of SARS-CoV-2 on the beta cell. Islets. 2021;13(3-4):66-79.
doi pubmed - Meza JL, Triana A, De Avila I, Del Rio-Pertuz G, Viasus D. Diabetic ketoacidosis precipitated by COVID-19 in patients without respiratory symptoms: case reports. Cureus. 2020;12(8):e10031.
doi pubmed - Palermo NE, Sadhu AR, McDonnell ME. Diabetic Ketoacidosis in COVID-19: Unique Concerns and Considerations. J Clin Endocrinol Metab. 2020;105(8):dgaa360.
doi pubmed - Kawasaki E, Maruyama T, Imagawa A, Awata T, Ikegami H, Uchigata Y, Osawa H, et al. Diagnostic criteria for acute-onset type 1 diabetes mellitus (2012): Report of the Committee of Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus. J Diabetes Investig. 2014;5(1):115-118.
doi pubmed - Luckey AE, Parsa CJ. Fluid and electrolytes in the aged. Arch Surg. 2003;138(10):1055-1060.
doi pubmed - Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, Oguchi G, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386(4):305-315.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


