| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 1, January 2023, pages 1-6
Clinically Isolated Syndrome and Frontal Lobe Arteriovenous Malformation Presenting With Behavior Issues
Chetan C. Shaha, e, Christopher J. Dudekc, Erick N. Viorrittob, John J. Sarandriad
aDepartment of Radiology, Nemours Children’s Health, Jacksonville, FL, USA
bDivision of Neurology, Nemours Children’s Health, Jacksonville, FL, USA
cDepartment of Emergency Medicine, Tampa General Hospital, Tampa, FL, USA
dDepartment of Pediatrics, Nemours Children’s Hospital, Orlando, FL, USA
eCorresponding Author: Chetan C. Shah, Department of Radiology, Nemours Children’s Health, Jacksonville, FL 32207, USA
Manuscript submitted September 6, 2022, accepted October 21, 2022, published online December 30, 2022
Short title: Clinically Isolated Syndrome and Behavior Issues
doi: https://doi.org/10.14740/jmc4005
| Abstract | ▴Top |
Prevalence of brain arteriovenous malformation ranges from 0.14% to 0.6% according to various estimates. A large number of these patients remain asymptomatic. The most common presentation is due to brain hemorrhage. A 14-year-old girl presented to the pediatrician with erratic behavior issues and hallucinations. She was diagnosed by the pediatrician and mental health facility as having schizophrenia and bipolar disorder. Once she was transferred to our children’s hospital, evaluation by a pediatric neurologist, computed tomography scan, magnetic resonance imaging, and laboratory workup including lumbar puncture confirmed a clinically isolated syndrome and frontal lobe arteriovenous malformation. Frontal lobe lesions including arteriovenous malformation in the frontal lobe can cause psychological symptoms and behavioral issues. We also discuss the differential diagnosis of acute demyelinating syndromes.
Keywords: Altered mental status; Demyelinating disorder; Arteriovenous malformation; Vascular malformation; Clinically isolated syndrome
| Introduction | ▴Top |
The frontal lobe plays an important role in personality of a human. The prefrontal cortex controls emotional regulation, social interaction, and executive functions [1]. The prefrontal cortex supports problem-solving tasks [2], strategic and sequential planning, performing mental representations of a task [3], social rule abiding [4], and processing environmental cues [5]. Lesions of the frontal lobes impair response planning, anticipation of events, goal setting, and neurobehavioral disorders. Glioma, glioblastoma, arteriovenous malformation (AVM), tumefactive multiple sclerosis, oligodendroglioma, lymphoma, and brain abscess are some of the lesions that may affect frontal lobes.
Prevalence of brain AVM ranges from 0.14% to 0.6% according to various estimates [6]. Brain AVM is more common in the supratentorial brain (85%) than in the posterior fossa (15%). A large number of these patients remain asymptomatic. The most common presentation is due to brain hemorrhage. Frontal lobe lesions can cause behavior issues. Other neurological disorders including demyelinating process can also present with behavior issues. It is important to rule out organic causes of these behavior issues before diagnosing them as a functional psychiatric illness. Demyelinating processes are relatively less common in children than in adults. Nevertheless, demyelinating processes do occur in children and should be considered while evaluating for a neurological illness in a child. Clinically isolated syndrome, typically lasting 24 h, is the first episode of neurological symptoms. The symptoms of clinically isolated syndrome include blurred vision, speech difficulty, vertigo, memory loss, depression, numbness, weakness, and bladder and bowel symptoms.
Our case presented diagnostic difficulties for the pediatrician and mental health facility as the 14-year-old girl presented with erratic behavior issues, hallucinations, and normal basic blood work results. She was diagnosed as having schizophrenia and bipolar disorder. Investigations performed at our facility diagnosed this as a clinically isolated syndrome in addition to frontal lobe AVM.
| Case Report | ▴Top |
Investigations
A 14-year-old girl with no past medical history was transferred to the emergency department from the state mental health facility for progressive difficulty swallowing. Medical records revealed she was in her normal state of health until 2 months prior, when she presented to her pediatrician with mild frontal headache, nonspecific pain in her hands, and decreased appetite. At that time, lab work revealed a normal complete blood count, comprehensive metabolic panel, thyroid function test, antinuclear antibody, and erythrocyte sedimentation rate.
Diagnosis
Over the next 4 weeks, her behavior became increasingly erratic. Her demeanor was described as defiant and inappropriate in social situations, and she began to experience agitation along with auditory and visual hallucinations. She developed an abnormal gait that became concerning to school officials, so she was involuntarily institutionalized at the local state mental health facility for psychosis. There, the psychiatrist made diagnoses of schizophrenia and bipolar disorder and she was placed on quetiapine.
Seven days later, she developed verbal and motor tics leading to an additional diagnosis of Tourette’s syndrome and her medication was switched from quetiapine to olanzapine. Because her symptoms failed to abate with the new medication, she was referred to child neurology and a clinic appointment was scheduled. The morning of her transfer, 2 days before her scheduled outpatient neurology appointment, she awoke with tachycardia, sialorrhea, and dysphagia. These symptoms were suspected to be adverse effects of olanzapine resulting in discontinuation of olanzapine. No weight loss, fever, or seizure activity was reported.
On arrival to our facility, she was alert and oriented, scoring 15 out of 15 on the Glasgow Coma Scale, and in no acute distress. She was tachycardic at 144 beats per minute. She had difficulty executing complex commands. She was both overly complimentary and apologetic when interacting with the staff. She exhibited frontal lobe and limbic/amygdaloid dysfunction with disinhibition and sexual suggestion. Her speech exhibited dysprosody and was at times scanning and halting. Neurological examination revealed psychomotor agitation as well as left-sided ptosis, dysphagia, absent gag reflex, gait ataxia, and occasional myoclonic jerks of the proximal upper extremities. Binocular eye movements were disconjugate and she intermittently covered her left eye to compensate for diplopia. Intermittent bursts of rotary, horizontal, and vertical nystagmus were present. Mild dysmetria was observed on finger-to-nose testing.
A head computed tomography scan without contrast was performed, which although markedly limited by movement, revealed a vascular right frontal lobe abnormality suggestive of AVM without bleeding, surrounding edema, or mass effect. While supine for imaging, her mental status quickly deteriorated, and she required endotracheal intubation to maintain her airway. She was transferred to the pediatric intensive care unit. Based on the size and location of the AVM, it was considered an incidental finding.
Magnetic resonance imaging (MRI) (Fig. 1) of the brain and spine was then performed to explore other causes of her neurological deterioration. Results revealed bilateral diffuse white matter lesions involving the bilateral temporal lobes, subinsular cortex, inferior bilateral frontal cortex, basal ganglia, and both the cervical and thoracic spinal cords.
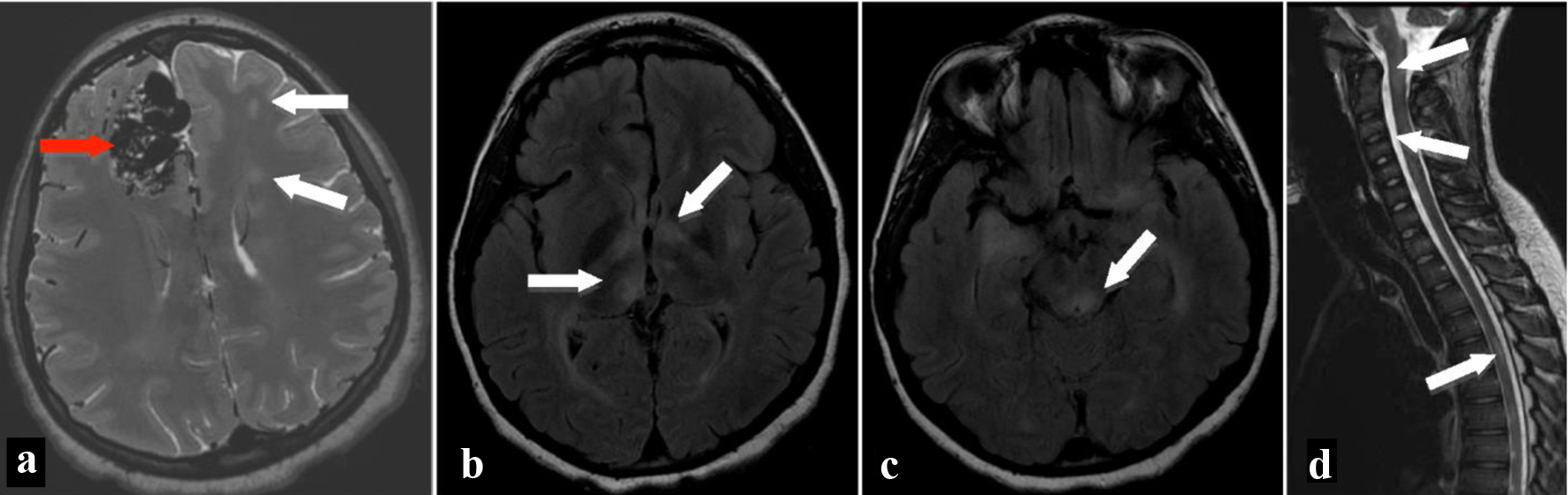 Click for large image | Figure 1. MRI of brain on axial T2-weighted image (a) shows multiple flow voids in the right frontal lobe (red arrow) consistent with arteriovenous malformation as well as multiple hyperintense T2 signal areas (white arrows) in the white matter bilaterally. Fluid-attenuated inversion recovery axial images show hyperintensities in the bilateral thalami (b), left globus pallidus, left putamen, bilateral cerebral white matter, and brainstem (c). MRI of the spine shows patchy hyperintense signal areas (arrows) in the brainstem, cervical spinal cord, and thoracic spinal cord on T2-weighted sagittal image (d). MRI: magnetic resonance imaging. |
Laboratory workup to distinguish inciting causes for the demyelinating disorder including urine organic acids, thyroid peroxidase antibodies, and a toxicology screen was negative. Levels of ammonia, lactate, pyruvate, plasma amino acids, folate, ceruloplasmin, and vitamin B12 were normal. Aside from evidence of past infection with Epstein-Barr virus, infectious meningitis workup including rapid plasma reagin, human immunodeficiency virus, herpes simplex virus, mycoplasma, and influenza was negative.
Lumbar puncture (Table 1) revealed increased protein in the cerebrospinal fluid (CSF). Electrophoresis revealed oligoclonal banding and increases in CSF immunoglobulin G (IgG), CSF IgG index, CSF IgG/albumin ratio, and CSF IgG synthesis rate. Her CSF was negative for N-methyl D-aspartate receptor antibodies and angiotensin-converting enzyme. Electroencephalogram monitoring showed no evidence of seizures or focal epileptiform discharges.
 Click to view | Table 1. Results of Lumbar Puncture |
Treatment
The patient received high-dose steroid therapy (1 g of methylprednisolone per day) over 3 days, and her bulbar dysfunction improved considerably. However, the patient’s speech and disinhibition remained unchanged. Plasma exchange therapy was administered, but no further improvement in her new baseline was appreciated. A preliminary diagnosis of a clinically isolated syndrome was made, and a follow-up MRI was scheduled for 6 months after discharge.
Follow-up and outcomes
Transfer to a rehabilitation facility was arranged. She later underwent successful operative repair of her AVM. On follow-up visit 23 months after her initial presentation, her behavioral symptoms were unchanged. Repeat MRI at that time (Fig. 2) showed a decrease in the number and size of the thalamic and brainstem lesions without new lesions, further supporting the diagnosis of clinically isolated syndrome.
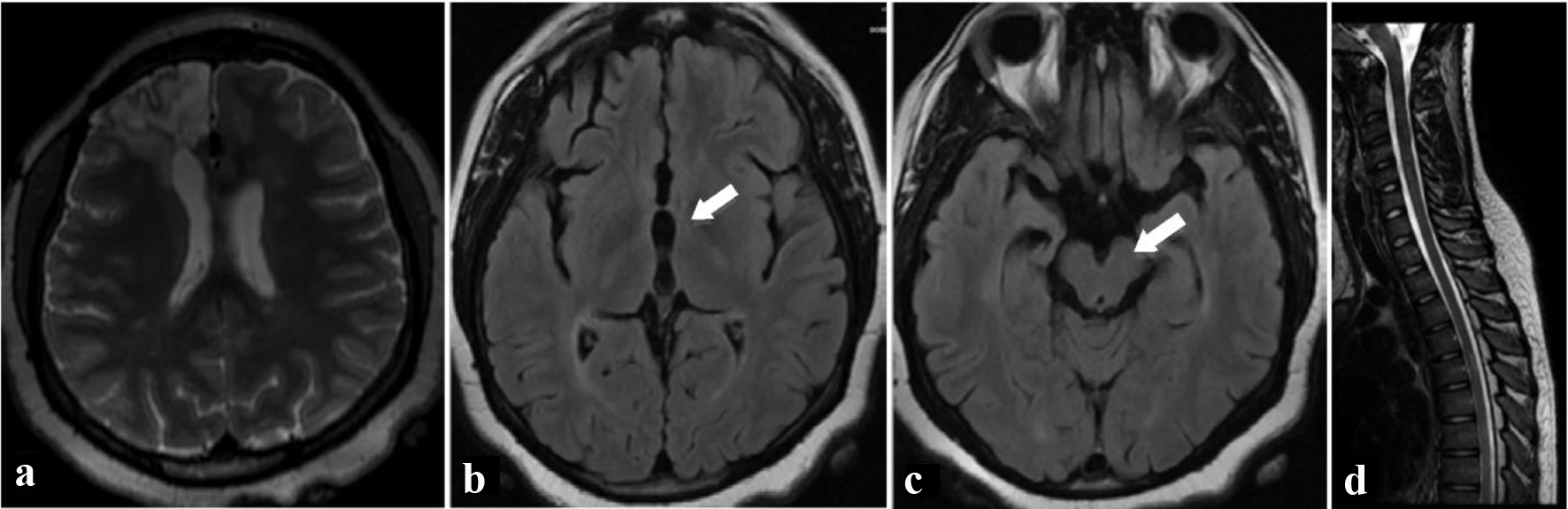 Click for large image | Figure 2. Two years after initial presentation, T2-weighted axial (a) MRI image shows resection bed of removed arteriovenous malformation from right frontal lobe. Fluid-attenuated inversion recovery axial (b, c) MRI images of the brain show resolution of white matter lesions in the thalamus (a) and brainstem (b). There was a decrease in the number and size of other lesions during the interval, and no new lesions were found. T2-weighted sagittal (d) MRI image of spine shows interval improvement in hyperintense T2 signal areas in the spinal cord. MRI: magnetic resonance imaging. |
| Discussion | ▴Top |
Encephalopathy in children is rare, with an incidence of 10.5/100,000/year [7]. This number includes encephalopathies such as acute disseminated encephalomyelitis (ADEM), where the incidence may be 0.4/100,000/year [8] and pediatric onset multiple sclerosis (POMS), where the incidence varies by country but is estimated at 0.19-2.85/100,000/year [9]. Childhood onset schizophrenia by comparison has a lower estimated incidence of 0.11/1,000 to 0.70/1,000 children per year [10].
The differential diagnosis for white matter disease of the central nervous system (CNS) in children is broad, including infectious, autoimmune, rheumatological, metabolic, and vascular conditions. Evaluation of blood and CSF can aid in lowering the likelihood of some etiologies (Table 2). However, acute demyelinating syndromes such as ADEM, neuromyelitis optica spectrum disorder, transverse myelitis (TM), optic neuritis (ON), and POMS require neuroimaging to meet the major criteria for diagnosis.
 Click to view | Table 2. Laboratory Evaluation of Acute Central Nervous System Demyelination in Children: CSF and Serum Studies and Their Corresponding Disease Processes |
Since ADEM and POMS remain diagnoses of exclusion, it is important to use a combination of laboratory and imaging evidence to identify potentially treatable intracranial infections or processes. Differentiating POMS from other acute demyelinating syndromes such as ADEM, ON, neuromyelitis optica spectrum disorder, and TM is imperative not only to predict long-term prognosis, but also for prompt initiation of immunotherapy to prevent recurrent demyelination [11].
Nonetheless, distinguishing between these diagnoses can be challenging as all are autoimmune-mediated demyelinating diseases of the CNS white matter, each with varying presentations. In fact, POMS may initially present identically to ADEM [12], ON, and TM [13]. Still, encephalopathy as a presenting symptom has been described in only 10% to 16% of pediatric multiple sclerosis (MS) cases [14, 15]. More commonly, initial MS attacks are monosymptomatic and range from sensory impairment to motor dysfunction such as tremor, dysmetria, or internuclear ophthalmoplegia [13, 16, 17]. Oligoclonal bands in CSF, as seen in this case, are present in 89% of individuals with MS but only about 10% with ADEM [18]. Therefore, familiarizing oneself with the most up-to-date diagnostic criteria is paramount.
The 2001 McDonald criteria, formed by the International Panel on the Diagnosis of MS, are classically used to bolster a diagnosis of MS [11, 19]. According to McDonald criteria, dissemination of contrast-enhanced CNS lesions in time (persistent lesions or symptoms) and/or space (persistence of previous or appearance of new brain lesions) represents a second clinical event and, only then, can the diagnosis be made. Revisions in 2017, however, add that a second clinical event can be demonstrated by positive oligoclonal band in lieu of the characteristic evolution of lesions on CNS imaging [20]. Therefore, as with this case, when MS is highly suspected based on laboratory and imaging findings, the diagnosis cannot be made until repeat imaging and/or CSF oligoclonal band testing has been obtained.
During the period between the first attack and subsequent follow-up, the patient should be diagnosed with a clinically isolated syndrome [20]. Of note, the time between the initial attack and follow-up testing, previously recommended as 30 days, is now described as any amount of time [20]. Additionally, the second MS attack must be without encephalopathy, which helps distinguish it from conditions such as multiphasic ADEM [20, 21]. It is difficult to predict the prognosis of patients suffering from their first demyelinating event. In patients with POMS, the course is more commonly relapsing-remitting [22], and patients tend to reach permanent disability earlier in life. As relapse rates are high in POMS [23], patients suffer higher rates of fatigue, depression, and cognitive impairment [9, 24]. The key to improving long-term outcomes seems to be a combination of early recognition and treatment [25] as well as encouragement of both physical activity and weight control [26].
Our case was complicated by a large AVM, which in the absence of bleeding, is usually an incidental finding. Frontal lobe lesions including AVM in the frontal lobe can cause psychological symptoms and behavioral issues. Also, considering the future risk of bleeding, the lesion was removed.
Learning points
This case highlights the importance of a proper physical exam and consideration of differential diagnosis when encountering a child with erratic behavior issues. While psychiatric diagnoses are part of the differential, due diligence to rule out neurological disorders when an acute change in behavioral baseline is encountered is imperative to avoid misdiagnosis and delayed treatment. Frontal lobe lesions including AVM in the frontal lobe can cause psychological symptoms and behavioral issues. Finally, it shows that the often ill-defined and difficult to diagnose acute demyelinating syndrome must be considered with a presentation of altered mental status.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was not required as the images have been anonymized and there are no patient identifiers.
Author Contributions
Each author has been individually involved in and has made substantial contributions to conceptions and designs, acquisition of data, analysis, interpretation of data, drafting, and editing the manuscript. Chetan C. Shah contributed to the conceptualization, organization of the project, and editing of the manuscript. Christopher J. Dudek contributed to writing the first draft of the manuscript and editing of the manuscript. Erick N. Vioritto contributed to the compilation of data, provided neurology input to the case and follow-up of recovery, and editing of the manuscript. John J. Sarandria contributed to collecting clinical data and editing of the manuscript.
Data Availability
The data supporting the findings of this case report are available from the authors.
Abbreviations
ADEM: acute disseminated encephalomyelitis; AVM: arteriovenous malformation; CSF: cerebrospinal fluid; CNS: central nervous system; IgG: immunoglobulin G; MRI: magnetic resonance imaging; MS: multiple sclerosis; ON: optic neuritis; POMS: pediatric onset multiple sclerosis; TM: transverse myelitis
| References | ▴Top |
- Takayama M, Kashiwagi M, Hara K, Matsusue A, Waters B, Kubo SI. Giant intracranial arteriovenous malformation as a possibility of epileptic seizures in a case of drowning. Leg Med (Tokyo). 2022;59:102144.
doi pubmed - Friedman NP, Robbins TW. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology. 2022;47(1):72-89.
doi pubmed - Monk AM, Dalton MA, Barnes GR, Maguire EA. The role of hippocampal-ventromedial prefrontal cortex neural dynamics in building mental representations. J Cogn Neurosci. 2021;33(1):89-103.
doi pubmed - Rozzi S, Fogassi L. Neural coding for action execution and action observation in the prefrontal cortex and its role in the organization of socially driven behavior. Front Neurosci. 2017;11:492.
doi pubmed - Hall-McMaster S, Millar J, Ruan M, Ward RD. Medial orbitofrontal cortex modulates associative learning between environmental cues and reward probability. Behav Neurosci. 2017;131(1):1-10.
doi pubmed - Berman MF, Sciacca RR, Pile-Spellman J, Stapf C, Connolly ES, Jr., Mohr JP, Young WL. The epidemiology of brain arteriovenous malformations. Neurosurgery. 2000;47(2):389-396; discussion 397.
doi pubmed - Koskiniemi M, Korppi M, Mustonen K, Rantala H, Muttilainen M, Herrgard E, Ukkonen P, et al. Epidemiology of encephalitis in children. A prospective multicentre study. Eur J Pediatr. 1997;156(7):541-545.
doi pubmed - Leake JA, Albani S, Kao AS, Senac MO, Billman GF, Nespeca MP, Paulino AD, et al. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004;23(8):756-764.
doi pubmed - Alroughani R, Boyko A. Pediatric multiple sclerosis: a review. BMC Neurol. 2018;18(1):27.
doi pubmed - Messias EL, Chen CY, Eaton WW. Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am. 2007;30(3):323-338.
doi pubmed - Pohl D, Tenembaum S. Treatment of acute disseminated encephalomyelitis. Curr Treat Options Neurol. 2012;14(3):264-275.
doi pubmed - Smyk DS, Alexander AK, Walker M, Walker M. Acute disseminated encephalomyelitis progressing to multiple sclerosis: are infectious triggers involved? Immunol Res. 2014;60(1):16-22.
doi pubmed - Venkateswaran S, Banwell B. Pediatric multiple sclerosis. Neurologist. 2010;16(2):92-105.
doi pubmed - Yeh EA, Chitnis T, Krupp L, Ness J, Chabas D, Kuntz N, Waubant E, et al. Pediatric multiple sclerosis. Nat Rev Neurol. 2009;5(11):621-631.
doi pubmed - Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224-1231.
doi pubmed - Atzori M, Battistella PA, Perini P, Calabrese M, Fontanin M, Laverda AM, Suppiej A, et al. Clinical and diagnostic aspects of multiple sclerosis and acute monophasic encephalomyelitis in pediatric patients: a single centre prospective study. Mult Scler. 2009;15(3):363-370.
doi pubmed - Chou IJ, Whitehouse WP, Wang HS, Tanasescu R, Constantinescu CS. Diagnostic modalities in multiple sclerosis: perspectives in children. Biomed J. 2014;37(2):50-59.
doi pubmed - Franciotta D, Columba-Cabezas S, Andreoni L, Ravaglia S, Jarius S, Romagnolo S, Tavazzi E, et al. Oligoclonal IgG band patterns in inflammatory demyelinating human and mouse diseases. J Neuroimmunol. 2008;200(1-2):125-128.
doi pubmed - Sadaka Y, Verhey LH, Shroff MM, Branson HM, Arnold DL, Narayanan S, Sled JG, et al. 2010 McDonald criteria for diagnosing pediatric multiple sclerosis. Ann Neurol. 2012;72(2):211-223.
doi pubmed - Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
doi - Boesen MS, Blinkenberg M, Koch-Henriksen N, Thygesen LC, Uldall PV, Magyari M, Born AP. Implications of the International Paediatric Multiple Sclerosis Study Group consensus criteria for paediatric acute disseminated encephalomyelitis: a nationwide validation study. Dev Med Child Neurol. 2018;60(11):1123-1131.
doi pubmed - Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D, UBC MS Clinic Neurologists. Early onset multiple sclerosis: a longitudinal study. Neurology. 2002;59(7):1006-1010.
doi pubmed - Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66(1):54-59.
doi pubmed - Parrish JB, Weinstock-Guttman B, Smerbeck A, Benedict RH, Yeh EA. Fatigue and depression in children with demyelinating disorders. J Child Neurol. 2013;28(6):713-718.
doi pubmed - Kavaliunas A, Manouchehrinia A, Stawiarz L, Ramanujam R, Agholme J, Hedstrom AK, Beiki O, et al. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult Scler. 2017;23(9):1233-1240.
doi pubmed - Sikes EM, Motl RW, Ness JM. Pediatric multiple sclerosis: current perspectives on health behaviors. Pediatric Health Med Ther. 2018;9:17-25.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


