| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 13, Number 11, November 2022, pages 541-544
An Unusual Etiology: Subarachnoid Hemorrhage Resulting in Transient Apical Ballooning Syndrome
Steven Imburgioa, c, Anmol Johala, Ndausung Udongwoa, Sherif Eltawansya, Vandan Upadhyayab, Mohammad Razab
aDepartment of Medicine, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA
bDivision of Cardiology, Department of Medicine, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA
cCorresponding Author: Steven Imburgio, Department of Medicine, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA
Manuscript submitted October 4, 2022, accepted November 2, 2022, published online November 27, 2022
Short title: An Unusual Etiology: SAH-Induced Takotusubo
doi: https://doi.org/10.14740/jmc4018
| Abstract | ▴Top |
Intracranial bleeds, such as subarachnoid hemorrhage, carry high morbidity and mortality rates. Often intracranial hemorrhages result in debilitating residual neurological symptoms but can be so extensive that cardiac complications can also be seen. We present a rare case of a patient who was found to have a subarachnoid hemorrhage that incited the development of Takotsubo cardiomyopathy, which subsequently progressed to an acute myocardial infarction. The aim of this case report is to explore the underlying pathophysiology of how cerebral hemorrhage can result in apical ballooning of the left ventricle through various mechanisms including sympathetic-induced surge in catecholamines and neurogenic damage to the myocardium. We also intend to highlight the importance for clinicians to consider brain bleeds in the differential diagnosis when a patient presents with an acute myocardial infarction as treatment with heparin is generally contraindicated.
Keywords: Takotsubo cardiomyopathy; Transient apical ballooning syndrome; Myocardial infarction; Subarachnoid hemorrhage; Cerebral aneurysm; Syncope; Catecholamine surge
| Introduction | ▴Top |
A subarachnoid hemorrhage (SAH) is a life-threatening type of head bleed where blood accumulates in the space between the arachnoid and pia layers of the meninges [1]. While initial presentations vary, patients typically report the acute onset of an excruciating “thunderclap” headache [2]. Additional symptoms including nausea, vomiting, diplopia, and signs of meningeal irritation are often seen [3]. Most commonly, SAH results from the rupture of a cerebral aneurysm with smoking, hypertension, and alcohol use being the strongest risk factors [4, 5].
SAHs are associated with a host of adverse events including seizures, vasospasm, hydrocephalus, brain herniation, and cerebral infarction [6]. Occasionally, SAH can be so debilitating that it can affect organ systems outside of the nervous system. To date, there has been a scarcity of data regarding cardiac complications resulting from SAHs reported in the literature. This paper aims to explore the possible underlying pathophysiology for how brain bleeds such as SAH can result in structural and ischemic cardiac changes. Our goal is to improve patient outcomes by bringing awareness to how intracranial hemorrhage should be on the differential diagnosis when patients present with acute myocardial infarction (AMI) and associated neurological symptoms. We report a rare case of a patient who presented with SAH and subsequently developed a myocardial infarction with non-obstructive coronary arteries (MINOCA) as a result of stress-induced Takotsubo cardiomyopathy.
| Case Report | ▴Top |
Investigations
A 64-year-old female with a past medical history of smoking presented to an outside medical facility following a home unwitnessed syncopal event. The patient was found on the floor unresponsive by her daughter. Emergency medical services were called, and cardiopulmonary resuscitation (CPR) was initiated with return of spontaneous circulation achieved after 5 min of compressions without shocks. However, the patient required intubation in the field for airway protection and etomidate was given for sedation induction.
She had no history of alcohol or illicit drug use in the past. Her family history was remarkable for hypertension and coronary artery disease in her mother and father, respectively.
On initial presentation, the patient had a blood pressure of 106/72 mm Hg, pulse rate of 88 beats per minute, respiratory rate of 16 breaths per minute, temperature of 36.6 °C, and oxygen saturation of 95% while on the ventilator. Neurological assessment was limited by sedation with the rest of the physical exam unremarkable.
Diagnosis
Labs revealed a leukocytosis of 14.9 × 103/µL (4.5 - 11 × 103/µL), glucose of 222 mg/dL (70 - 99 mg/dL), phosphorus of 5.3 mg/dL (2.5 - 4.6 mg/dL), lactic of 2.7 mmol/L (0 - 2.0 mmol/L), and troponin of 0.03 ng/mL (< 0.04 ng/mL). An electrocardiogram (EKG) in the emergency department (ED) demonstrated ST elevations in the anterolateral leads V3 - V6 (Fig. 1), for which a code ST segment elevation myocardial infarction (STEMI) was called. In the ED, the patient was given aspirin 300 mg suppository and 4,000 units intravenous (IV) heparin and transferred to the cardiac catheterization lab. A left heart catheterization revealed patent coronary arteries with elevated left ventricular end-diastolic pressure at 28 mm Hg, along with a reduced ejection fraction (EF) of 35%. Anteroapical hypokinesia was also noted with a pattern that was characteristic of Takotsubo cardiomyopathy. Coronary artery vasospasm was ruled out with the coronary angiogram. Inflammatory markers were not initially drawn but based on the EKG with specific V3 - V6 ST elevations and following catheterization, myocarditis, pericarditis as well as pheochromocytoma were less likely to be the underlying pathology.
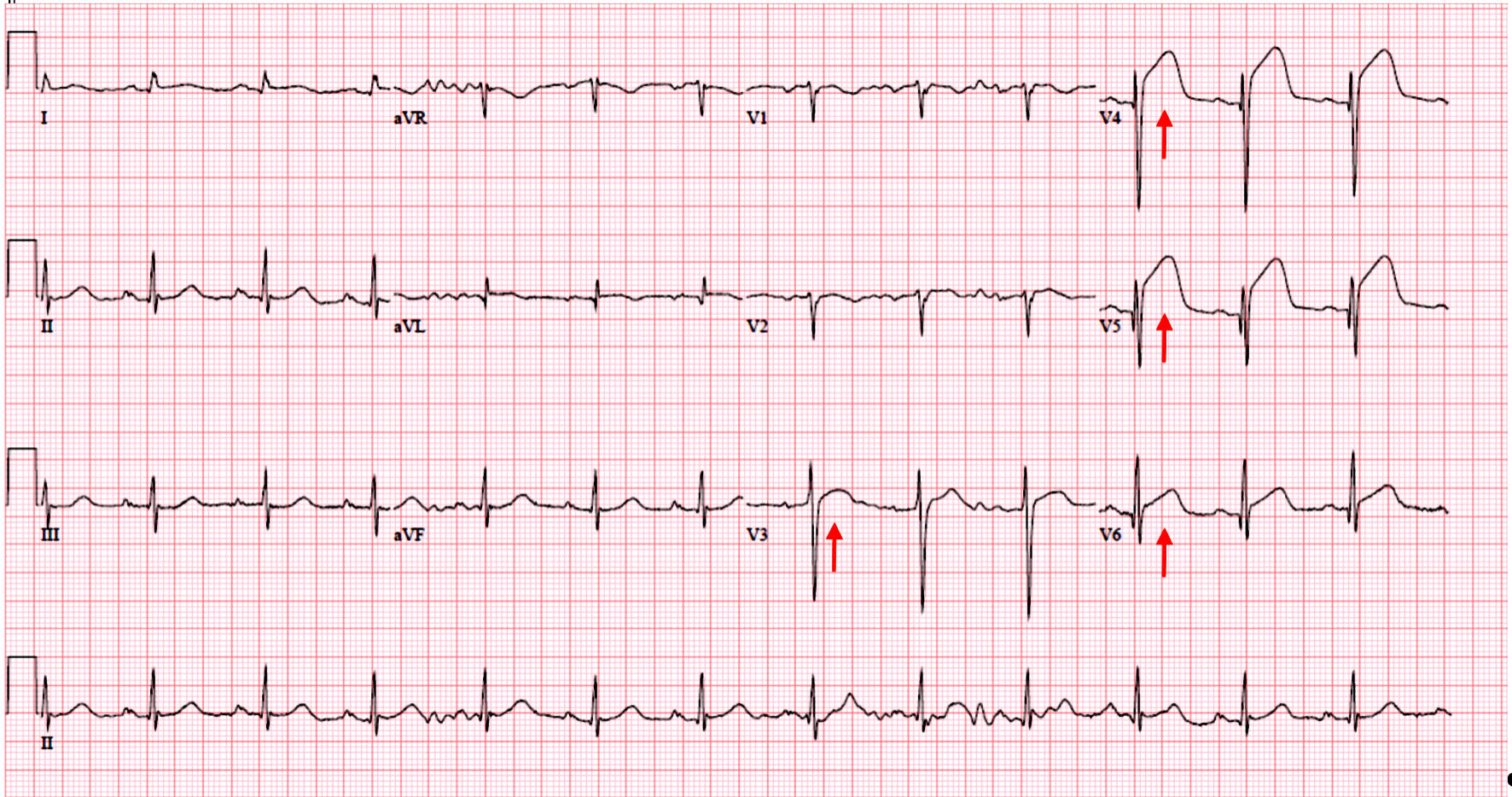 Click for large image | Figure 1. Initial electrocardiogram showing sinus rhythm, rate of 78 beats per minute, normal axis, with ST segment elevations (arrows) in leads V3 - V6. |
Treatment
The decision was made to start metoprolol due to previous studies demonstrating that beta-blockers reduce the incidence of major adverse cardiac events in patients with MINOCA. Following the procedure, the patient had a stat computed tomography of the head which revealed an acute SAH with Hunt and Hess grade 5. She was given protamine for reversal of the heparin given during the catheterization and subsequently transferred to our facility for higher level of care including possible neurosurgical intervention. She underwent an angiogram which revealed a large middle cerebral artery aneurysm which was treated with coil embolization and placement of a ventricular drain.
Follow-up and outcomes
Unfortunately, the patient developed residual quadriplegia and became nonverbal. She was unable to be weaned off the ventilator and underwent tracheostomy and percutaneous endoscopic gastrostomy tube placement. Repeat echocardiogram 32 days later showed recovery in EF from 35% to 55% with no signs of regional wall motion abnormalities. However, despite a lengthy hospital stay, the patient’s neurological status failed to improve, and the decision was made to transfer to hospice care.
| Discussion | ▴Top |
The purpose of our case is to demonstrate how a cerebral hemorrhage can induce Takotsubo cardiomyopathy and eventually result in AMI. Takotsubo syndrome was first characterized in 1990 by Sato et al to describe an acute form of non-ischemic heart failure that results from systolic dysfunction in the absence of coronary artery disease [7]. The name Takotsubo comes from the Japanese term for a unique octopus trap, which describes the apical ballooning appearance of the left ventricle typically in response to a stressor that induces significant regional cardiomyopathy [8]. Despite multiple possible etiologies of Takotsubo cardiomyopathy, very few cases report intracranial hemorrhage as an underlying cause [9-12]. One prospective study by Molnar et al estimates the incidence of severe Takotsubo cardiomyopathy in SAH cases at approximately 8% [13].
The term, neurogenic stunned myocardium (NSM), has been slowly growing in the literature to describe the pathologic process that results in rapidly reversible cardiac dysfunction following an acute brain injury [14]. While the mechanism for how this neurocardiogenic injury develops is not exactly known; the leading theory proposes that the intracranial bleed leads to significant sympathetic activation and a corresponding surge in catecholamines [15, 16]. In a retrospective study of 142 patients with SAH, Sugimoto et al found that patients with wall motion abnormalities on transthoracic echocardiogram (TTE) had significantly higher levels of plasma norepinephrine compared to those without wall motion abnormalities [17]. Another study by Salem et al found that SAH patients had sustained increased levels of catecholamines on both admission and follow-up, with echocardiogram often showing left ventricular dysfunction [18]. It has also been proposed that intracranial hemorrhages can lead to direct neurogenic toxicity to cardiac myocytes [19]. To highlight this point, one study by Naidech et al enrolled 253 patients with SAH and found that elevated levels of cardiac troponin I were seen in 68% (172) of cases [9]. Additionally, increased aortic wall stiffness has been reported in the early phase of SAH, which likely worsens the pre-existing ventricular dysfunction through increased filling pressures in the left ventricle [20]. With increased mortality associated with wall-motion abnormalities and increased cardiac markers post-SAH, recognizing the early signs and possibility of brain bleed is essential to mounting a proper treatment plan [21]. After reviewing the literature, it is clear that the underlying pathophysiology for the myocardial damage and associated left ventricular failure induced by an acute intracranial hemorrhage needs further exploration but is likely multifactorial in nature.
The clinical importance of recognizing a concurrent brain bleed when a patient presents with AMI stems from the fact that clinicians are incumbent to act and heparinize this patient demographic [22]. Since anticoagulating patients with an intracranial hemorrhage is contraindicated, it becomes challenging for clinicians to make this decision when neurological symptoms are present or there is limited history available as with our patient. In our case, the patient’s critical condition combined with the presence of ST elevations on EKG was concerning and potential cardiac causes were worked up in the catheterization lab before obtaining imaging of the head to rule-out bleeding. Ultimately, this case report demonstrates the importance of considering intracranial hemorrhage in patients with suspected Takotsubo cardiomyopathy or AMI who present with neurological symptoms.
Learning points
Our case aims to explore the proposed pathophysiology for how brain bleeds can induce structural and ischemic cardiac dysfunction, particularly via the catecholamine surge pathway. While Takotsubo cardiomyopathy is generally a reversible process, it is paramount to recognize the underlying etiologies that may have initiated the disease. We also highlight the challenging dilemma that clinicians experience in deciding whether to heparinize patients with suspected acute coronary syndrome (ACS) when other potential etiologies, such as cerebral hemorrhage, have not been ruled out. Ultimately, we aim to potentially improve patient outcomes by bringing awareness to this underrecognized pathogenesis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Verbal informed consent was obtained from the patient for their anonymized information to be published in this article.
Author Contributions
Steven Imburgio and Anmol Johal wrote the original manuscript draft and reviewed the literature. Ndausung Udongwo reviewed the literature and edited the manuscript. Sherif Eltawansy edited the manuscript. Vandan Upadhyaya provided the concept idea and edited the manuscript. Mohammad Raza supervised and edited the manuscript. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Ziu E, Khan Suheb MZ, Mesfin FB. Subarachnoid hemorrhage. In: StatPearls. Treasure Island (FL), 2022.
- van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124(Pt 2):249-278.
doi pubmed - Kairys N, J MD, Garg M. Acute subarachnoid hemorrhage. In: StatPearls. Treasure Island (FL), 2022.
- Rabinstein AA. Subarachnoid hemorrhage. Neurology. 2013;80(5):e56-59.
doi pubmed - Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, Anderson CS. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36(12):2773-2780.
doi pubmed - Welty TE, Horner TG. Pathophysiology and treatment of subarachnoid hemorrhage. Clin Pharm. 1990;9(1):35-39.
- Chhabra L, Butt N, Ahmad SA, Kayani WT, Sangong A, Patel V, Bharaj G, et al. Electrocardiographic changes in Takotsubo cardiomyopathy. J Electrocardiol. 2021;65:28-33.
doi pubmed - Ahmad SA, Brito D, Khalid N, Ibrahim MA. Takotsubo cardiomyopathy. In: StatPearls. Treasure Island (FL), 2022.
- Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, Fitzsimmons BF, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112(18):2851-2856.
doi pubmed - Barsoum E, Elhosseiny S, Patel B, Pathak S, Patel A, Vaidya P. Successful use of the impella ventricular assist device for management of reverse Takotsubo Cardiomyopathy in the setting of acute intracranial hemorrhage. Heart Lung. 2021;50(2):313-315.
doi pubmed - Russell L, Stockwell P. A case of intracranial hemorrhage causing stress-induced cardiomyopathy. R I Med J (2013). 2013;96(6):33-35.
- Wang F, Darby J. Case Report: Takotsubo Cardiomyopathy After Traumatic Brain Injury. Front Neurol. 2021;12:727754.
doi pubmed - Molnar C, Gal J, Szanto D, Fulop L, Szegedi A, Siro P, Nagy EV, et al. Takotsubo cardiomyopathy in patients suffering from acute non-traumatic subarachnoid hemorrhage-A single center follow-up study. PLoS One. 2022;17(5):e0268525.
doi pubmed - Mierzewska-Schmidt M, Gawecka A. Neurogenic stunned myocardium - do we consider this diagnosis in patients with acute central nervous system injury and acute heart failure? Anaesthesiol Intensive Ther. 2015;47(2):175-180.
doi pubmed - Shimada M, Rose JD. Takotsubo cardiomyopathy secondary to intracranial hemorrhage. Int J Emerg Med. 2014;7:33.
doi pubmed - Inamasu J, Nakamura Y, Saito R, Kuroshima Y, Mayanagi K, Ohba S, Ichikizaki K. Normokalemia and hyperglycemia in subarachnoid hemorrhage patients resuscitated from prehospital cardiopulmonary arrest. Resuscitation. 2002;54(3):255-258.
doi - Sugimoto K, Inamasu J, Kato Y, Yamada Y, Ganaha T, Oheda M, Hattori N, et al. Association between elevated plasma norepinephrine levels and cardiac wall motion abnormality in poor-grade subarachnoid hemorrhage patients. Neurosurg Rev. 2013;36(2):259-266; discussion 266.
doi pubmed - Salem R, Vallee F, Depret F, Callebert J, Maurice JP, Marty P, Mateo J, et al. Subarachnoid hemorrhage induces an early and reversible cardiac injury associated with catecholamine release: one-week follow-up study. Crit Care. 2014;18(5):558.
doi pubmed - Chen S, Li Q, Wu H, Krafft PR, Wang Z, Zhang JH. The harmful effects of subarachnoid hemorrhage on extracerebral organs. Biomed Res Int. 2014;2014:858496.
doi pubmed - Papanikolaou J, Makris D, Karakitsos D, Saranteas T, Karabinis A, Kostopanagiotou G, Zakynthinos E. Cardiac and central vascular functional alterations in the acute phase of aneurysmal subarachnoid hemorrhage. Crit Care Med. 2012;40(1):223-232.
doi pubmed - van der Bilt IA, Hasan D, Vandertop WP, Wilde AA, Algra A, Visser FC, Rinkel GJ. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: a meta-analysis. Neurology. 2009;72(7):635-642.
doi pubmed - Granger CB. Heparin management in acute myocardial infarction (AMI). Aust N Z J Med. 1998;28(4):541-547.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


