| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 1, January 2023, pages 13-18
Breast to Brain: A Case Report and Literature Review of Leptomeningeal Carcinomatosis
Henrik Ghantarchyana, b, d , Suyee Winb, Essam K. Nagoria, c, Sarkis Arabiana, b, c
aDepartment of Internal Medicine, Arrowhead Regional Medical Center, Colton, CA 92324, USA
bCalifornia University of Science and Medicine, Colton, CA 92324, USA
cDepartment of Pulmonary and Critical Care Medicine, Arrowhead Regional Medical Center, Colton, CA 92324, USA
dCorresponding Author: Henrik Ghantarchyan, Department of Internal Medicine, Colton, CA 92324, USA
Manuscript submitted November 8, 2022, accepted November 25, 2022, published online December 30, 2022
Short title: Breast to Brain
doi: https://doi.org/10.14740/jmc4024
| Abstract | ▴Top |
Breast cancer is widely known as the most common cancer in women in the United States. If left untreated, it can have detrimental effects. If the breast cancer is aggressive in nature, it can metastasize to the lymph nodes, bones, liver, lungs, and brain. A rare location of metastasis is the leptomeninges, specifically the pia and arachnoid matter. This term is coined as leptomeningeal carcinomatosis. Its diagnosis can be challenging to make as patients can present with non-specific symptoms. We present the case of an elderly female with a prior history of breast cancer that was treated with 12 cycles of chemotherapy with paclitaxel, radiation to her left axilla, and daily anastrozole for 3 years who came into the emergency department for worsening confusion, urinary incontinence, and difficulty ambulating. Cerebral spinal fluid obtained from a lumbar puncture supported a diagnosis of leptomeningeal carcinomatosis.
Keywords: Breast cancer; Metastasis; Leptomeningeal carcinomatosis
| Introduction | ▴Top |
Leptomeningeal carcinomatosis (LC) is a form of cancer involving the nervous system, mainly arachnoid and pia mater. It commonly originates from primary cancers such as hematological cancers and solid tumors such as brain or breast cancer. The incidence of metastatic origin from hematological cancers is 5-15%. The incidence of metastatic origin from solid tumors ranges from breast cancer 5-8%, lung cancer 9-25%, and up to 30% in melanomas [1]. LC is primarily seen in advanced metastatic cancer; however, in rare cases, it can be the initial presentation of solid tumor (10%) or can manifest in patients in remission (20%) [2].
Literature review
This literature review was conducted to gather data on the number of case reports that have been published regarding patients with breast cancer and LC. We performed a systematic review for eligible case reports through a PubMed search using the following terms “breast cancer” AND “leptomeningeal carcinomatosis”. We included all studies published between January 2000 and November 2022 for subjects older than 65 years old.
Results
We found a total of 11 reports written in English, seven that were case reports and four that were case series. The 11 articles were evaluated for eligibility. Information about the demographic details and diagnostic modalities are included in Table 1 [3-8]. All patients below the age of 65 at the time of LC diagnosis were excluded from the literature review. Five articles were excluded as they did not include any of our inclusion criteria. There was a total of six case reports that met our inclusion criteria.
 Click to view | Table 1. Demographic Details and Diagnostic Modalities for Leptomeningeal Carcinomatosis (Review of Literature) |
In details, we looked at the gender affected, which was 100% females. The immunohistochemical staining revealed 75% were estrogen-receptor positive, 66.6% were progesterone-positive, and only 25% were human epidermal growth factor receptor 2 (HER-2)-positive. The mean duration in years from when the patient was diagnosed with breast cancer to receiving a diagnosis of LC was 4.4 years, while the median was 2 years.
There are four ways that cancer cells spread to the meninges: through arterial circulation, retrograde venous flow, consequence of a preexisting brain metastasis or migration of cancer cells through the perineural space [2]. Common presentations of LC are variable, however, exist as primarily neurological in nature. If the metastasis is chronic with resultant cell proliferation in the subarachnoid space, it can lead to cerebrospinal fluid (CSF) flow impairment, causing hydrocephalus, headaches, somnolence, nausea, and vomiting [9]. As these symptoms were observed in the presenting patient, a combination of imaging and pathological evidence helped us arrive at a diagnosis of LC.
| Case Report | ▴Top |
Investigations
A 72-year-old Hispanic female was brought into the emergency department (ED) by her family member for 3 days of worsening confusion, urinary incontinence, and difficulty ambulating after an unwitnessed fall. The patient had initially become altered at home 3 weeks prior to arrival in the ED; however, she was still able to carry out her activities of daily living (ADLs). Over the course of 3 days, the patient had a sudden decline of neurological status, inability to perform ADLs, urinary incontinence, and echolalia, which prompted the family to bring the patient to the ED. The patient was previously diagnosed with breast cancer approximately 4 years prior to presentation, during a screening mammogram. After confirmation of breast cancer, the patient underwent a bilateral mastectomy and axillary lymph node dissection. She was found to have metastatic spread to her lymph nodes. An axillary sentinel lymph node biopsy revealed three out of four axillary lymph nodes positive for metastatic disease with a pathological stage of T2N2M0. Pathology revealed estrogen-positive, progesterone-positive, HER-2 negative invasive lobular carcinoma (Fig. 1) of the left breast and ductal carcinoma in situ of the right breast. She was treated with a regimen of 12 cycles of chemotherapy with paclitaxel, external beam-radiation to her left axilla, and daily anastrozole 1 mg orally for 3 years.
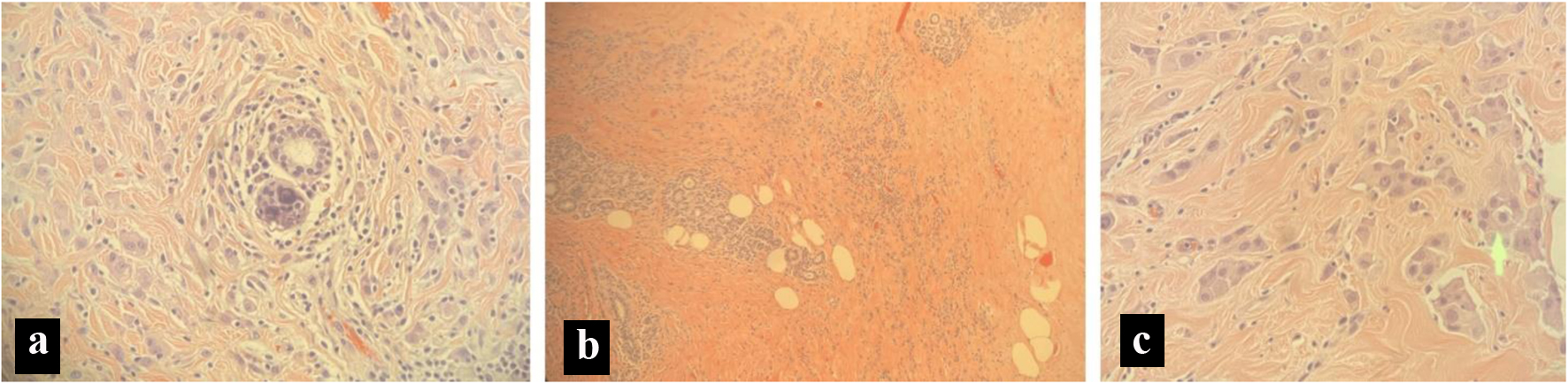 Click for large image | Figure 1. Pathology slides from a breast biopsy. (a) Micro calculi in a ductal pattern from left breast tissue. (b) Stromal sclerosis and infiltrating lobular cancer from left breast tissue. (c) Cells in a single file line, indicating lobular carcinoma from left breast tissue. |
Diagnosis
In the ED, a computed tomography (CT) of the head revealed mild hydrocephalus with transependymal CSF resorption without intracranial hemorrhage, mass, infarction, or skull fracture. Magnetic resonance imaging (MRI) of the brain revealed enlargement of the lateral and third ventricles and preserved size of cerebral aqueduct and fourth ventricle, T2 hyperintensity consistent with transependymal spread of CSF, confirming obstructive hydrocephalus.
An electroencephalogram (EEG) was negative for seizure-like activity. Initial lumbar puncture (LP) revealed CSF with an elevated white blood cell (WBC) count with lymphocytic pleocytosis, normal opening pressure, elevated glucose, and very elevated protein as shown in Table 2. She became febrile to 101 °F and encephalopathic, and was started on empiric antibiotics, antivirals, and antifungals. CT imaging was negative for metastatic disease, but revealed bilateral pleural effusions, moderate right basilar consolidations, and intraluminal filling defects in the right pulmonary arteries suggestive of right pulmonary embolism. During her hospital course, she had a rapid deterioration in her neurological status. Eventually she was upgraded to the intensive care unit (ICU) for poor mentation and acute hypercapnic respiratory failure requiring intubation and mechanical ventilation. After ICU upgrade, a CT scan with intravenous contrast revealed a pulmonary embolism (PE). She was ultimately started on a heparin drip to prevent worsening of the PE.
 Click to view | Table 2. Significant Laboratory Results From the Cerebrospinal Fluid |
On initial presentation, given her immunocompromised state, there was concern for tuberculosis. A quantiferon gold test was positive for tuberculosis; however, acid fast bacilli smears were negative on three separate occasions. CSF studies did not reveal any infectious etiology. Given her breast cancer history, LC remained high on our differential list. However, considerations were made for acute encephalitis secondary to an infectious etiology due to CSF findings of elevated glucose, protein, and WBCs. The patient was started on acyclovir due to suspicion of viral encephalitis. The patient was also on vancomycin and ceftriaxone for a suspected hospital acquired pneumonia. CSF studies were found to be negative for herpes simplex virus (HSV), varicella zoster virus (VZV), and coccidioidomycosis (antibodies were < 1:2).
Repeat CT of head showed interval improvement of the mild hydrocephalus, and MRI (Fig. 2) of the cervical and thoracic spine did not demonstrate any metastatic lesions. A CA 27-29 level was checked, which was found to be negative.
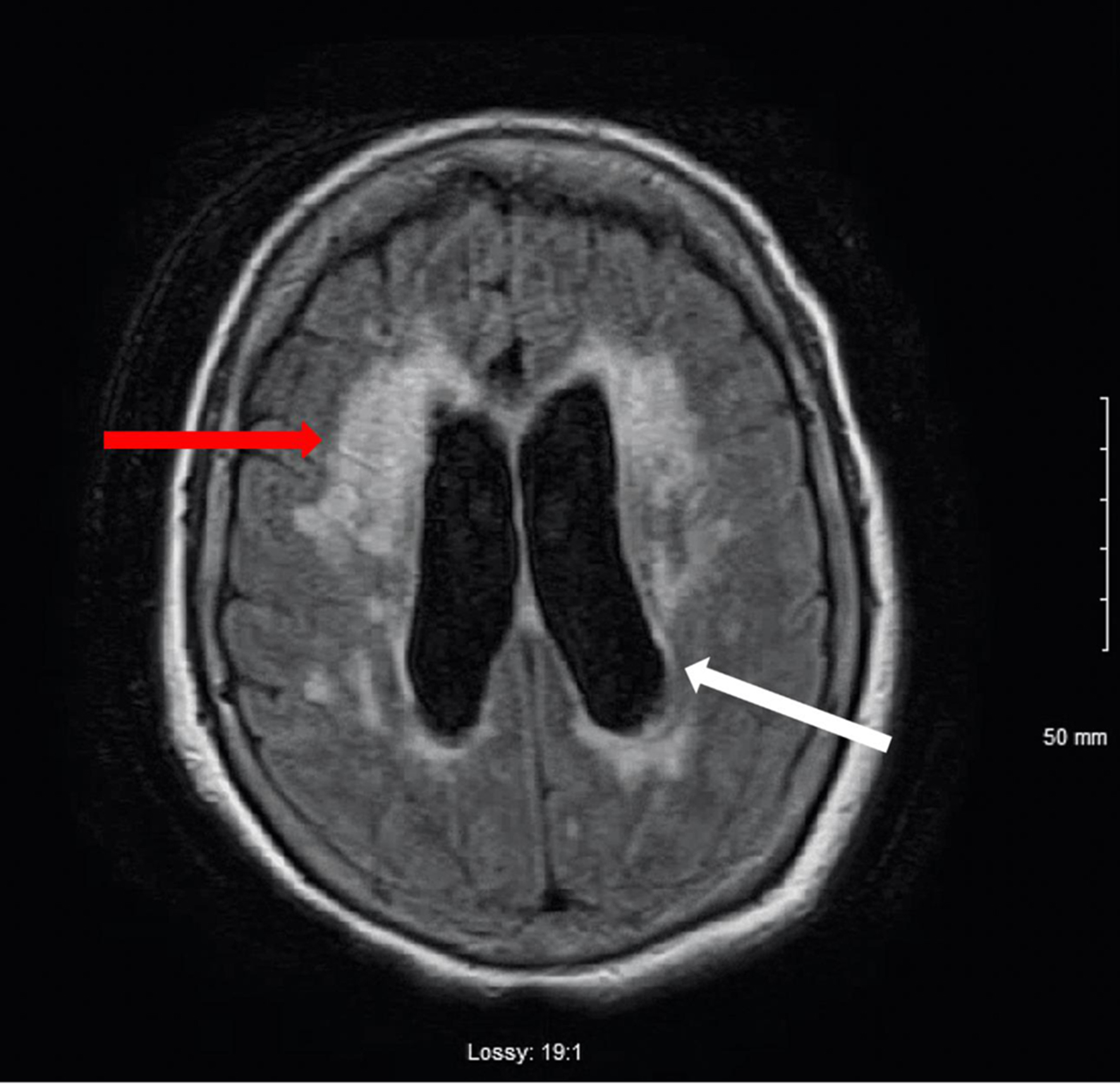 Click for large image | Figure 2. T1-weighted MRI image of the brain. Mild to moderate hydrocephalus (white arrow). T2 hyperintensity consistent with transependymal spread of CSF (red arrow). There is no evidence of mass. There is no abnormal gadolinium enhancement. CSF: cerebrospinal fluid; MRI: magnetic resonance imaging. |
Throughout the patient’s hospital course, there was noted improvement in mentation, which was noticeable especially after 26 mL of CSF was obtained after a lumbar drain was placed. This was seen as the patient had spontaneous opening of her eyes and started to follow minimal commands while in the ICU. Even though she was intubated, she was able to communicate with the medical staff and with her family members by answering to yes or no questions with her eyes. During her ICU stay, there were multiple spontaneous breathing trials attempted with the hopes of possible extubation; however, the patient unfortunately failed on these attempts.
CSF studies from lumbar drain revealed small groups of large vacuolated epithelial cells having circular nuclei with distinct nucleoli consistent with metastatic mammary adenocarcinoma (Fig. 3). The oncology department was heavily involved in the patient’s hospital course as the decision was made to stop the anastrozole therapy given the progression of disease.
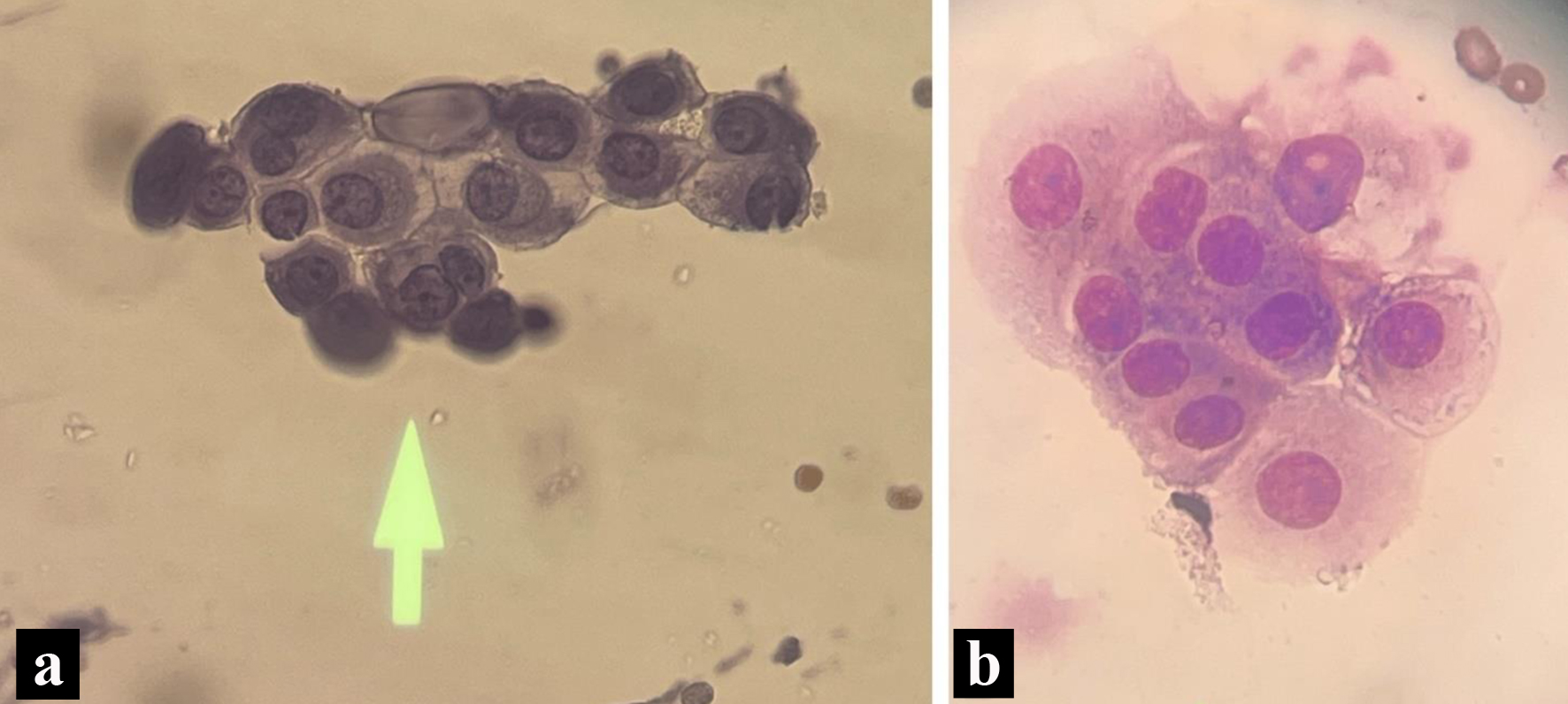 Click for large image | Figure 3. Pathology slides form the cerebrospinal fluid. (a) Enlarged nucleoli with cytoplasmic vacuoles on cerebrospinal fluid cytology (May Grunwald-Giemsa stain). (b) Wright stain indicating a nest of tumors with increased cytoplasm, non-spindled from cerebrospinal fluid cytology. |
Treatment
The diagnosis of LC was made. After extensive discussions with the family members, the patient’s family decided to pursue comfort care given the extent of disease complicated by a PE. The patient was weaned off sedation then transitioned to dexmedetomidine, and then was successfully extubated. Per the family request, the patient was discharged home with family and was followed by hospice care.
Follow-up and outcomes
All therapies were discontinued, and she was successfully extubated with discharge to home hospice. There was no follow-up regarding length of survival after discharge from the hospital.
| Discussion | ▴Top |
LC is meningeal infiltration from a metastatic cancer, which can originate from varying etiologies such as hematological cancer and solid tumors [1]. The rarity in making the diagnosis of LC exists primarily due to the resistance of the blood-brain barrier (BBB) and the blood-CSF barrier to metastasis [10, 11]. Proposed theories of the pathogenesis of LC include hematogenous and lymphatic spread, direct spread from the brain parenchyma or transendothelial migration via vascular endothelial growth factors (VEGFs) [11, 12]. According to Altundag et al when observing 420 patients diagnosed with breast cancer with resultant CNS metastasis, it was found that CNS metastasis develops more commonly in younger patients with larger tumors [13]. The retrospective study revealed only 3.8% had lobular histologic type and isolated LC [13]. This was identified in our presented case as estrogen-positive, progesterone-positive, HER-2-negative invasive lobular carcinoma of the left breast and ductal carcinoma in situ of the right breast.
Malignant spread to the CNS may have numerous presentations varying from cranial nerve deficits, headaches, diplopia, seizures, or even respiratory compromise [13]. Certain presentations can often be dismissed, as it can be related to the patient’s primary malignancy or side effects of therapies. The mechanism of symptom development can be related to direct parenchymal compression, parenchymal invasion, and vessel invasion resulting in ischemic effects [14].
The challenge lies in diagnosing LC, often initially seen with a high-quality MRI with gadolinium of the brain. This has a sensitivity of 70% and a specificity ranging from 77% and 100%. On an MRI, one can notice leptomeningeal enhancement, hydrocephalus, and even spinal cord involvement presented as patchy enhancements of nerve roots and extramedullary nodules [1]. This was similar to our case in which a mild to moderate hydrocephalus was noticed on brain MRI. The confirmatory test relies on a lumbar puncture with identification of malignant cells in the CSF. Analysis of these studies included CSF findings significant for elevated leukocytes ≥ 5 per mm3, elevated protein ≥ 50 mg/dL, glucose ≤ 40 mg/dL, and an opening pressure ≥ 20 cm H2O [15]. An algorithm for the correct approach to arrive at a diagnosis of LC in these subsets of patients has been outlined in Figure 4.
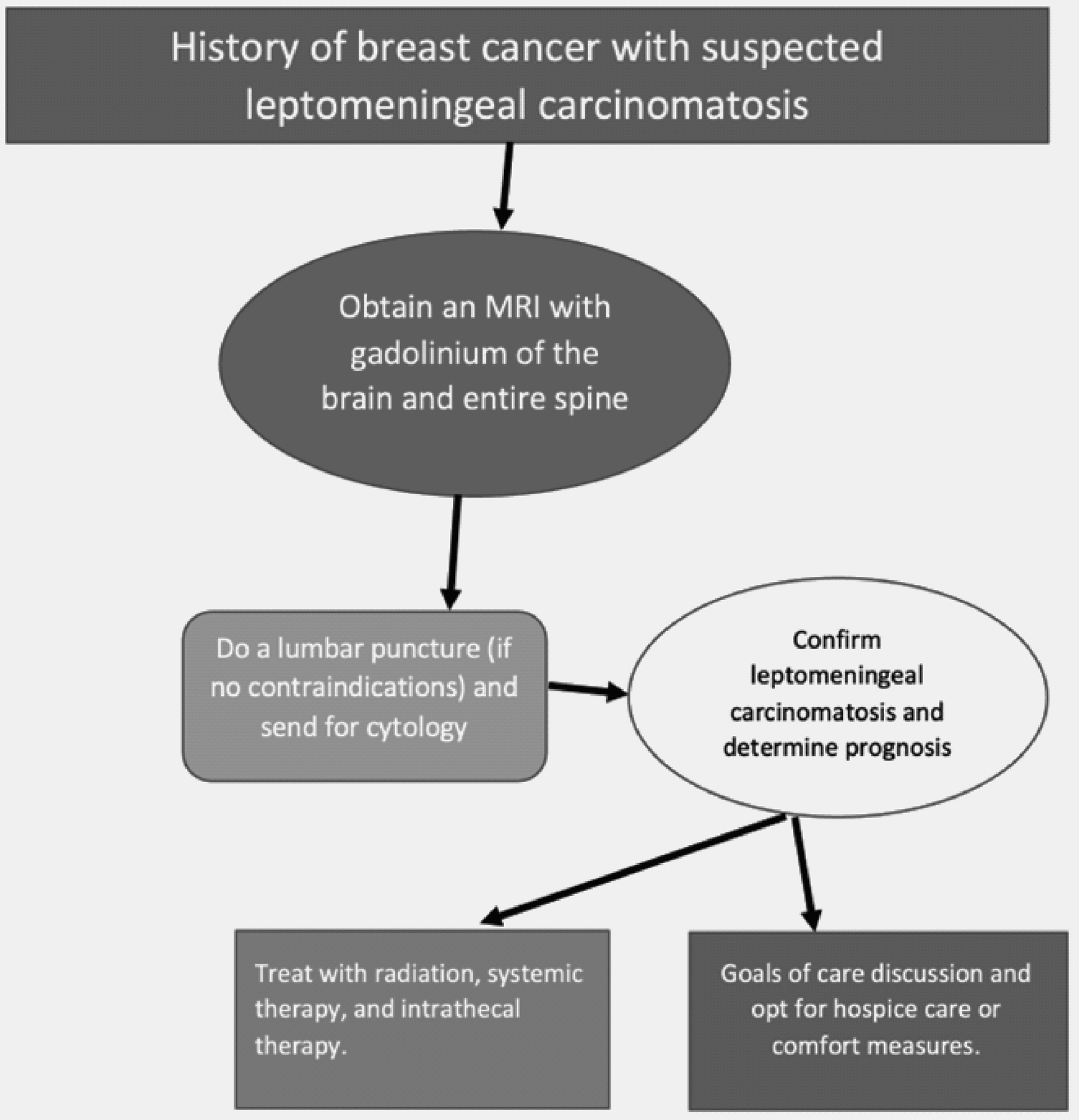 Click for large image | Figure 4. Algorithm for the correct diagnostic approach to leptomeningeal carcinomatosis. |
After confirming a diagnosis of LC, it is important to consider prognosis prior to treatment initiation. A retrospective study of 187 patients with LC, which included 150 solid tumors and 37 hematopoietic malignancies by Clark et al looked at the median overall survival of patients with LC and noticed a median overall survival of 2.4 months. When separated into patients with solid vs. hematopoietic malignancies, the overall survival was 2.3 months vs. 4.7 months, respectively. Additionally, given the correlation of a poor prognosis with solid malignancies, the authors separated the solid malignancies into breast and lung cancer, and found that the median overall survival was 2.8 and 2.2 months, respectively [15].
Treatment of LC includes radiation therapy, systemic therapy, and intrathecal therapy. Radiation therapy is important as it targets the entire CSF space, allowing for palliation of symptoms, and improving quality of life [16]. A better response has been noted to radiation therapy in patients with triple negative breast cancer and intracranial metastases [16]. Systemic therapies exist, which are limited by the BBB and the blood-CSF barrier. Current treatments like trastuzumab-based therapy in HER-2-positive breast cancer do not effectively cross the BBB. Another medication with better BBB penetration that can be used in patients with HER-2-positive breast cancer is lapatinib. This was seen with 7-9-fold higher concentrations of the drug in brain metastases when compared to surrounding tissue. Finally, intrathecal therapy exists as a means to bypass the BBB and blood-CSF barrier in patients with LC. Current agents that are approved by the United States FDA include methotrexate, cytosine arabinoside, liposomal cytarabine arabinoside, and thiotepa, with a known overall survival of 2 - 4.5 months [16, 17]. With such a poor prognosis, it is important for a goals of care discussion to occur prior to initiating therapeutic options.
With the current advancements in medicine, it is important to shine light on the novel therapies for LC. One unique and novel treatment modality that is currently undergoing research is the depletion of one specific nutrient in the CSF, iron. Given that LC is anatomically devoid of a blood-tumor capillary network, it is found that free iron in the CSF is one of the main nutrients that LC survives off [18]. An iron-binding transporter that is possessed by cancer cells is lipocalin-2, along with its receptor, SLC22A17 [18]. Current pre-clinical studies in mice show that by restricting the cancer cells from iron by intrathecal administration of an iron chelator, deferoxamine, there is an observed reduction in tumor burden correlated with prolonged survival. Other novel therapies that exist are targeted radioimmunotherapy, commonly used in the pediatric population, and brachytherapy using liposomally encapsulated radionucleotides, used in solid tumor LC [18].
Learning points
This case highlights the importance of doing a thorough investigation into encephalopathy especially with individuals with a history of malignancy. Symptoms of LC that usually exist include head or neck pain, cranial nerve deficits, diplopia, seizures, or even respiratory compromise, as seen in this patient. For these patients, workup includes imaging, such as obtaining an MRI, and obtaining CSF via a lumbar puncture. The CSF results should be sent for evaluation with cytology. With a very poor prognosis, it is important to consider having goals of care discussions with the patient, the family, and the multidisciplinary team as was highlighted through this case. There is potential to advance the definitive treatments for this disease, with prospective studies in conjunction with ongoing advancement in genetics and targeted therapies.
Acknowledgments
The authors acknowledge the Department of Pathology at Arrowhead Regional Medical Center for providing the pathology slides.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
Informe consent has been obtained.
Author Contributions
HG participated in patient care, writing of the case report, revisions, and submission process. SW participated in patient care and writing of the case report. EN participated in patient care, writing of the case report, and revisions. SA participated in patient care and revisions of the case report.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Batool A, Kasi A. Leptomeningeal carcinomatosis. In: StatPearls. Treasure Island (FL); 2022.
- Lanfranconi S, Basilico P, Trezzi I, Borellini L, Franco G, Civelli V, Pallotti F, et al. Optic neuritis as isolated manifestation of leptomeningeal carcinomatosis: a case report and systematic review of ocular manifestations of neoplastic meningitis. Neurol Res Int. 2013;2013:892523.
doi pubmed - Almajed MM, Esfahani K, Pelmus M, Panasci L. Complete response and long-term survival of leptomeningeal carcinomatosis from breast cancer with maintenance endocrine therapy. BMJ Case Rep. 2016;2016:bcr2016215525.
doi pubmed - Kapke JT, Schneidewend RJ, Jawa ZA, Huang CC, Connelly JM, Chitambar CR. High-dose intravenous methotrexate in the management of breast cancer with leptomeningeal disease: Case series and review of the literature. Hematol Oncol Stem Cell Ther. 2019;12(4):189-193.
doi pubmed - Sekhar A, Corbo B, Das K, Biswas S. Leptomeningeal carcinomatosis: easy to miss. J R Coll Physicians Edinb. 2017;47(4):351-352.
doi pubmed - Lavaud P, Rousseau B, Ajgal Z, Arrondeau J, Huillard O, Alexandre J, Hulin A, et al. Bi-weekly very-high-dose lapatinib: an easy-to-use active option in HER-2-positive breast cancer patients with meningeal carcinomatosis. Breast Cancer Res Treat. 2016;157(1):191-192.
doi pubmed - Wostrack M, Pape H, Kreutzer J, Ringel F, Meyer B, Stoffel M. Surgical treatment of spinal intradural carcinoma metastases. Acta Neurochir (Wien). 2012;154(2):349-357.
doi pubmed - Madgula IM, Hemmerdinger CM, Clark P. Metastatic breast cancer presenting as sequential cranial nerve palsy: a case report. J Med Case Rep. 2014;8:430.
doi pubmed - Parker N, Forge J, Lalich D. Leptomeningeal carcinomatosis: a case report of metastatic triple-negative breast adenocarcinoma. Cureus. 2019;11(3):e4278.
doi - Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: Review and update on management. Cancer. 2018;124(1):21-35.
doi pubmed - Damaske M, Panarese V, Casey S, Feeney M, Liuzzi FJ. Leptomeningeal carcinomatosis secondary to adenocarcinoma of the breast: a cadaveric case report. Cureus. 2021;13(1):e12693.
doi pubmed - Nayar G, Ejikeme T, Chongsathidkiet P, Elsamadicy AA, Blackwell KL, Clarke JM, Lad SP, et al. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget. 2017;8(42):73312-73328.
doi pubmed - Altundag K, Bondy ML, Mirza NQ, Kau SW, Broglio K, Hortobagyi GN, Rivera E. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer. 2007;110(12):2640-2647.
doi pubmed - Chowdhary S, Chamberlain M. Leptomeningeal metastases: current concepts and management guidelines. J Natl Compr Canc Netw. 2005;3(5):693-703.
doi pubmed - Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449-1454.
doi pubmed - Kak M, Nanda R, Ramsdale EE, Lukas RV. Treatment of leptomeningeal carcinomatosis: current challenges and future opportunities. J Clin Neurosci. 2015;22(4):632-637.
doi pubmed - Franzoi MA, Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol. 2019;135:85-94.
doi pubmed - Wilcox JA, Li MJ, Boire AA. Leptomeningeal metastases: new opportunities in the modern era. Neurotherapeutics. 2022;19(6):1782-1798.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


