| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 1, January 2023, pages 25-30
A Case of Antisynthetase Syndrome Initially Presented With Interstitial Lung Disease Mimicking COVID-19
Mohammed Elsayeda, b, Abdelnassir Abdelgabara, Jaidev Karmania, Mohammad Majida
aDiana Princess of Wales Hospital, Grimsby, UK
bCorresponding Author: Mohammed Elsayed, Diana Princess of Wales Hospital, Grimsby DN33 2BA, UK
Manuscript submitted November 3, 2022, accepted January 5, 2023, published online December 30, 2022
Short title: ASS Presented With ILD Mimicking COVID-19
doi: https://doi.org/10.14740/jmc4031
| Abstract | ▴Top |
In this case report, we present a case of antisynthetase syndrome which is a rare disease that can be easily missed, if not specifically looked for in adults, whose initial presentation is combination of myopathic and respiratory symptoms. In clinical practice, patients presenting with coronavirus disease 2019 (COVID-19) symptoms, whose computed tomography (CT) imaging is consistent with COVID-19, were accordingly isolated and treated as COVID-19 awaiting reverse transcription polymerase chain reaction (RT-PCR) results. However, there are many COVID-19 mimics on chest CT, which can make the CT-based diagnosis of COVID-19 unsafe.
Keywords: Antisynthetase syndrome; Interstitial lung disease; Inflammatory myositis; COVID-19
| Introduction | ▴Top |
Many conditions can radiologically mimic coronavirus disease 2019 (COVID-19). These include infectious etiologies such as viral pneumonias, atypical pneumonias such as legionella and mycoplasma pneumonia. Of the most common non-infectious mimics is interstitial lung disease (ILD) associated with connective tissue disease (CTD), such as polymyositis, dermatomyositis, and scleroderma [1]. These patients not only share the same chest computed tomography (CT) changes with COVID-19 but also share the clinical presentation such as dyspnea, flue-like symptoms, fatigue and aches and pains [2].
Antisynthetase syndrome (ASS) is a rare, chronic autoimmune disease of unknown etiology. The syndrome is considered a sub-group of idiopathic inflammatory myopathies (IIMs) [3]. The hallmark of the disorder is the presence of autoantibodies against the aminoacyl-transfer ribonucleic acid (tRNA) synthetase, also known as antisynthetase antibodies, or anti-ARS [4]. Patients with this syndrome have a characteristic clinical picture consisting of fever, exanthema on the hands (also referred to as mechanic’s hands), myositis, and/or ILD, and/or articular involvement. Raynaud’s phenomenon is frequently observed. The severity and type of pulmonary involvement determines the prognosis of the disease [5].
Anti-Jo-1 was the first anti-ARS discovered. However, many other antibodies have recently been discovered. Most of the literature on ASS is based on the presence of anti-Jo-1 antibodies, which is the strongest predictor of this syndrome [6], nearly found in 70% of patients. Other less common variants of anti-ARS antibodies include: anti-PL-7, anti-PL-12, anti-OJ, anti-EJ, anti-KS, anti-ZO, and anti-tyrosyl antibodies [6].
We discuss a patient who presented with COVID-like symptoms, treated as COVID-19, later proven to have ASS.
| Case Report | ▴Top |
Investigations
A 32-year-old male presented with a 4-week history of exertional shortness of breath and dry cough. There was no chest pain, hemoptysis, edema, or weight loss. His symptoms were accompanied by intermittent fever, malaise, arthralgia, and generalized aches and pains. He is non-smoker, on no medications, has no pets at home and no occupational dust exposure. On examination, he was apyrexial, hemodynamically stable, with a respiratory rate of 18/min and a peripheral oxygen saturation of 95% on 3 L of O2. There was no clubbing or evidence of Raynaud’s phenomenon, or jaundice. Cardiac examination was normal. Chest examination revealed bibasal fine inspiratory crackles. No muscle tenderness was noted.
Full blood count (FBC) was normal. Urea and electrolytes were normal. C-reactive protein (CRP) was high at 128 mg/L (0 - 5 mg/L). Liver function test (LFT) was deranged with high alanine transaminase (ALT) at 315 U/L (0 - 41 U/L). Gamma-glutamyl transferase (GGT) was 165 U/L (10 - 71 U/L) with normal values of bilirubin and alkaline phosphatase (ALP). Troponin was high at 64 ng/L (0 - 15 ng/L). D-dimer was positive. Reverse transcription polymerase chain reaction (RT-PCR) was negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (COVID-19) on three consecutive occasions. Throat swab was negative for influenza A, influenza B and respiratory syncytial virus (RSV) using PCR. Urinary legionella and pneumococcal antigens were negative. Electrocardiogram (ECG) was normal as well as cardiac echo. Chest X-ray (Fig. 1) showed patchy air space shadowing in both lungs, prominently in mid and lower zones. Appearances are likely due to infection/COVID-19. CT pulmonary angiography (CTPA) (Fig. 2) showed no pulmonary embolism but multiple ground glass lesions covering more than 50% of the alveolar surface, concerning for pulmonary infection including moderate form of COVID pneumonitis.
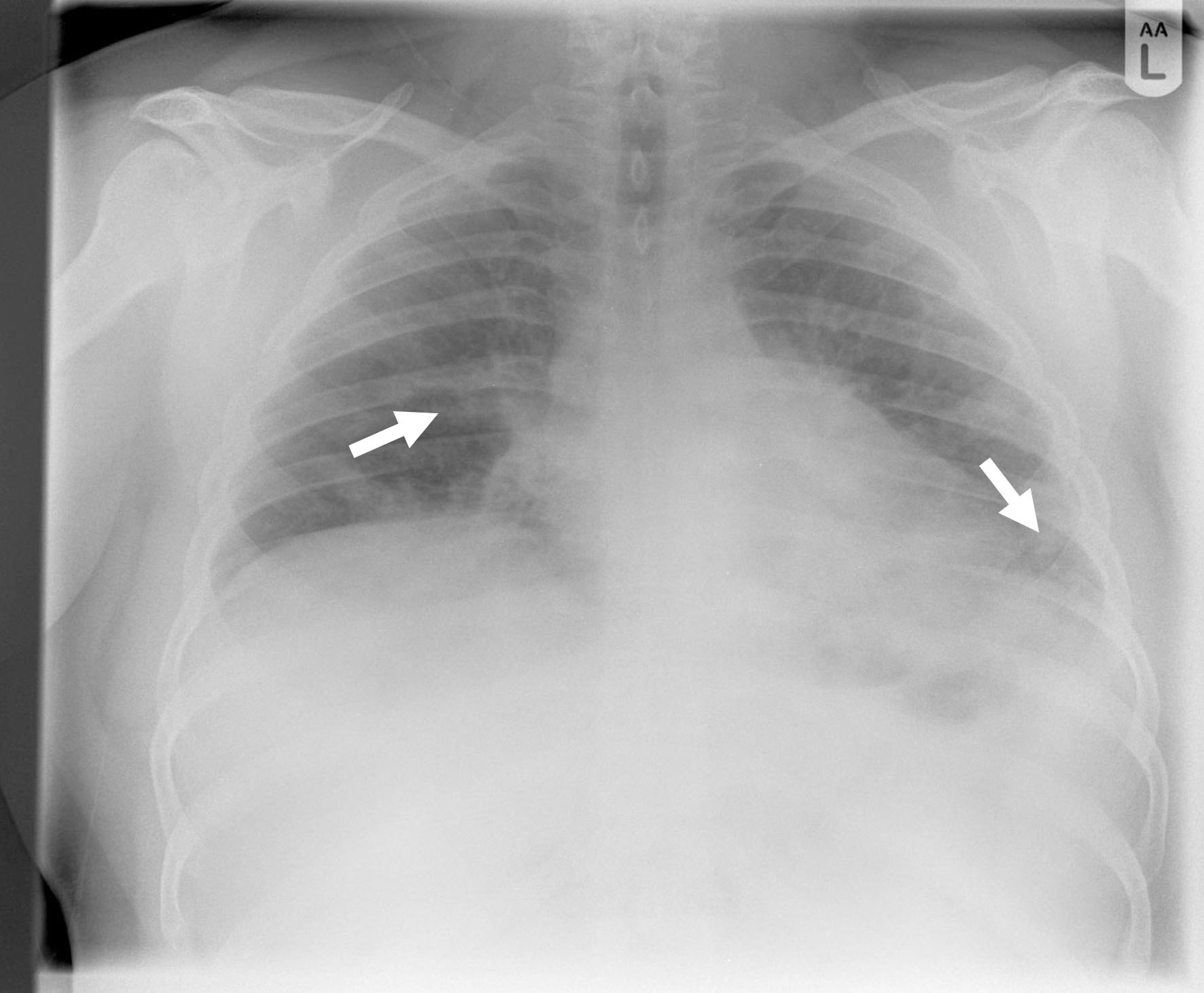 Click for large image | Figure 1. Chest X-ray showing patchy air space shadowing in both lungs, prominently in mid and lower zones (arrows). |
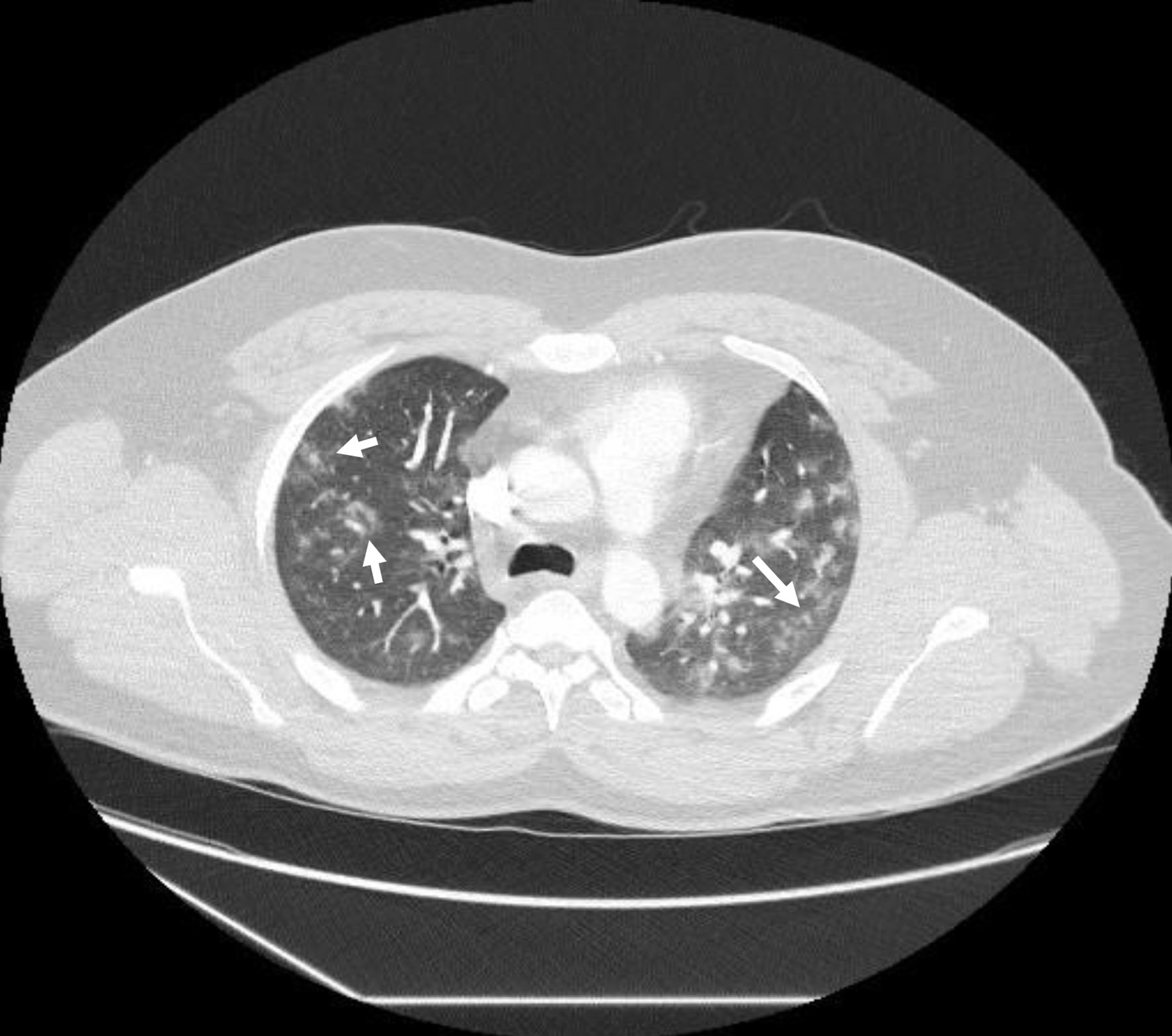 Click for large image | Figure 2. Computed tomography pulmonary angiography showing no pulmonary embolism but multiple ground glass lesions covering more than 50% of the alveolar surface, concerning for pulmonary infection including moderate form of covid pneumonitis (arrows). |
Diagnosis
Despite the negative coronavirus PCR tests, he was presumed to have COVID-19, based on his symptoms and chest CT findings. Eventually, after more investigations including autoimmune screen, he was diagnosed with ASS.
Treatment
He received dexamethasone, intravenous antibiotics and O2 therapy. He improved, weaned off O2 and discharged home with a plan for a repeat chest CT and a follow-up with the respiratory team.
Follow-up
Two months following his discharge, he was seen in the chest clinic. He was completely asymptomatic with unlimited exercise tolerance and normal O2 saturation. A high-resolution chest CT showed significant improvement in the ground glass appearance compared to the previous study (Fig. 3).
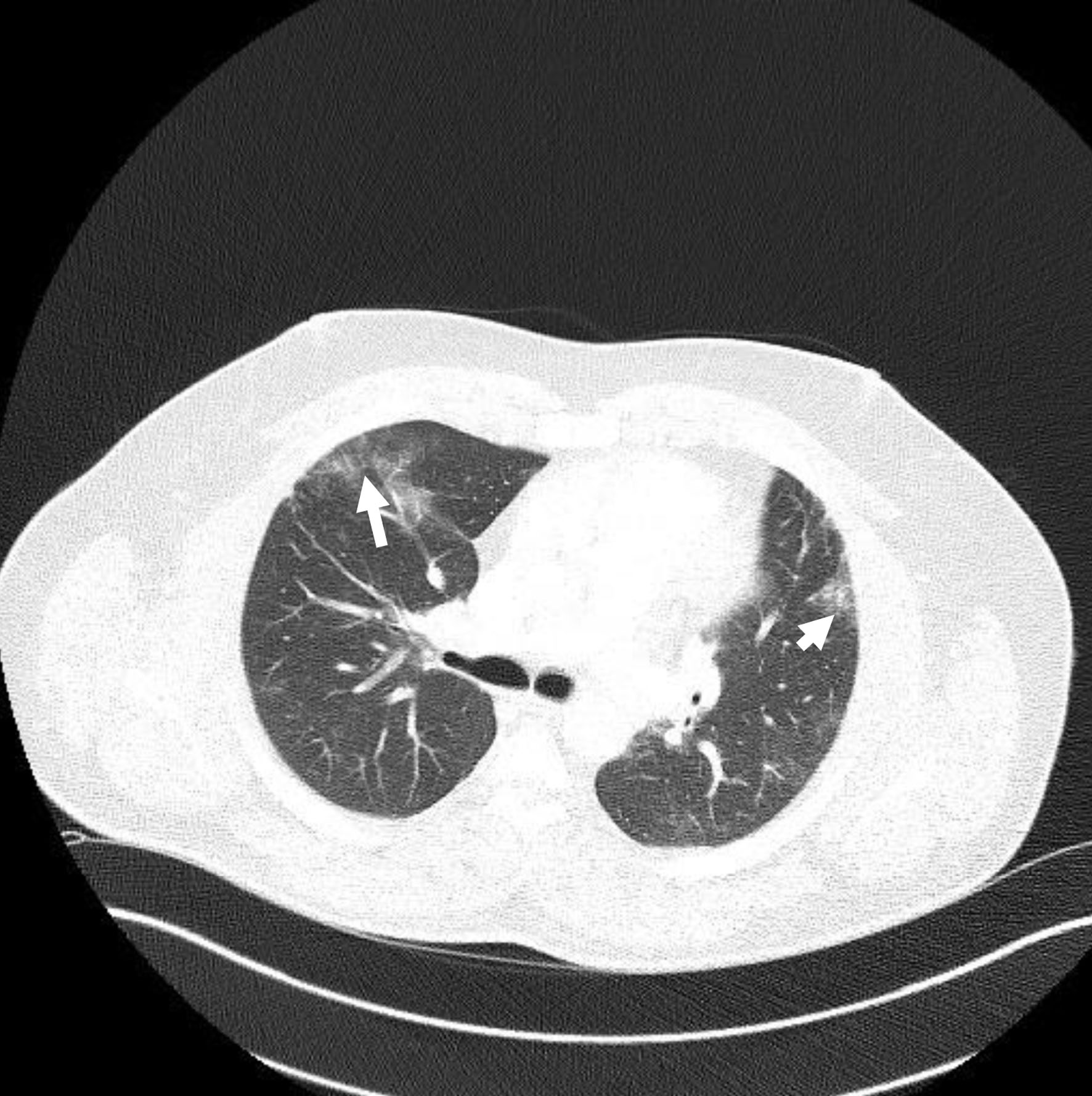 Click for large image | Figure 3. First follow-up computed tomography chest showing improvement of ground glass appearance (arrows). |
Two months following his visit to the respiratory clinic, he presented to same day emergency care (SDEC) with 2 weeks history of on and off palpitation, chest pain and fatigue. There was no shortness of breath or hemoptysis. Clinical examination was unremarkable in particular no pleural or pericardial rub. D-dimer was positive. Troponin was high at 850 ng/L (0 - 15 ng/L) and ECG was normal. CTPA showed no PE with diffuse patchy lung infiltrates, predominantly in both lung bases, with some variation from previous imaging (Fig. 4). Echo was completely normal. In view of the high troponin and normal echo and CTPA, serum creatine kinase (CK) was checked and found to be significantly high at 6,744 U/L (40 - 320 U/L). Lactate dehydrogenase (LDH) was also high at 1,482 U/L (135 - 225 U/L). Rheumatology opinion was sought, and muscle weakness was clinically confirmed, therefore further investigations were made. Electromyography (EMG) was found to be abnormal with occasional denervation and myopathic features. Magnetic resonance imaging (MRI) of the femur (Fig. 5) was highly suspicious of acute polymyositis. JO-1, La (SS-B), RO (SS-A) and ENA autoantibodies were positive and a diagnosis of polymyositis JO-1 positive was made. His lung function test showed a restrictive pattern with mildly reduced carbon monoxide uptake (TLCO).
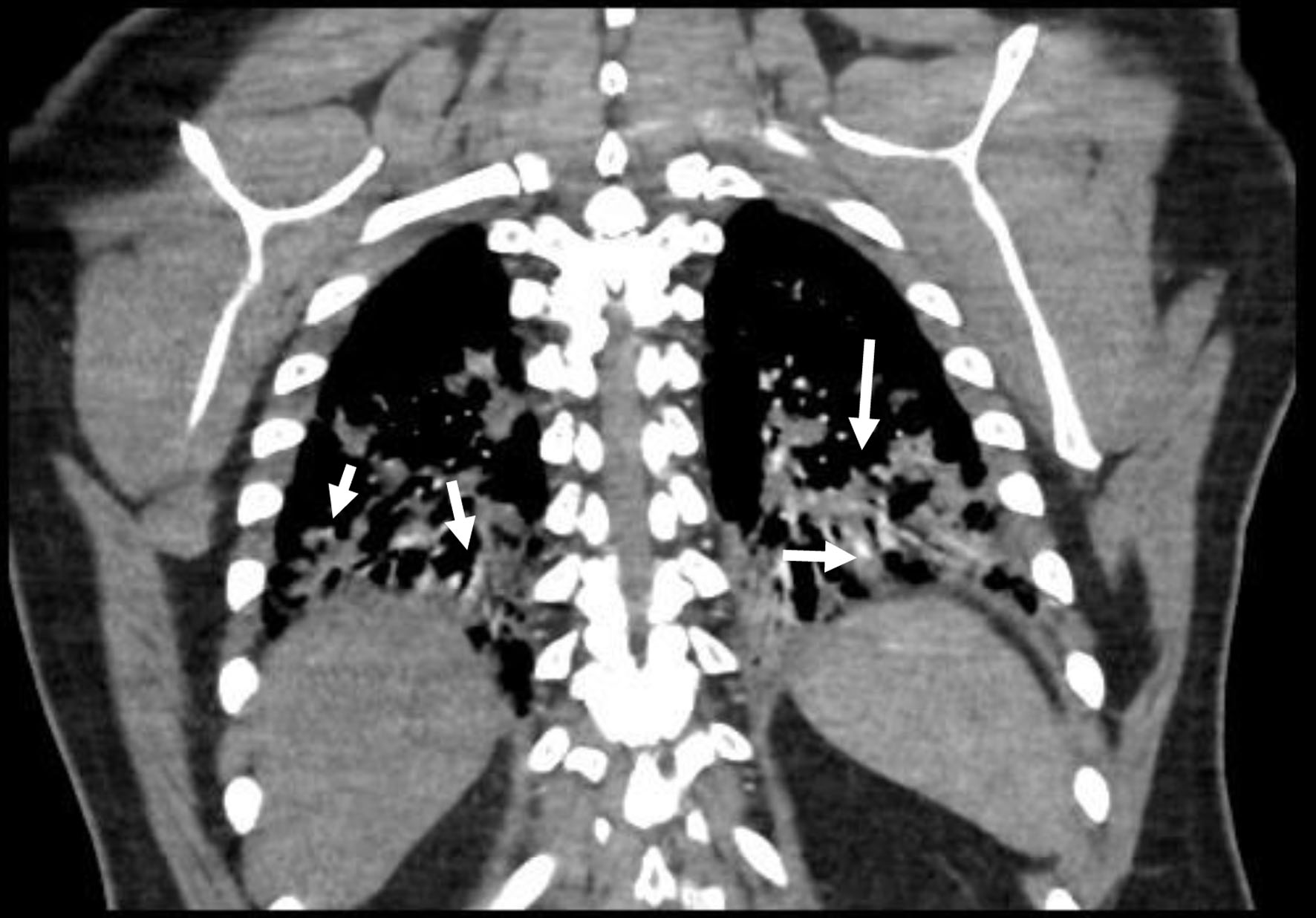 Click for large image | Figure 4. Diffuse patchy lung infiltrates, predominantly in both lung bases, with some variation from previous imaging (arrows). |
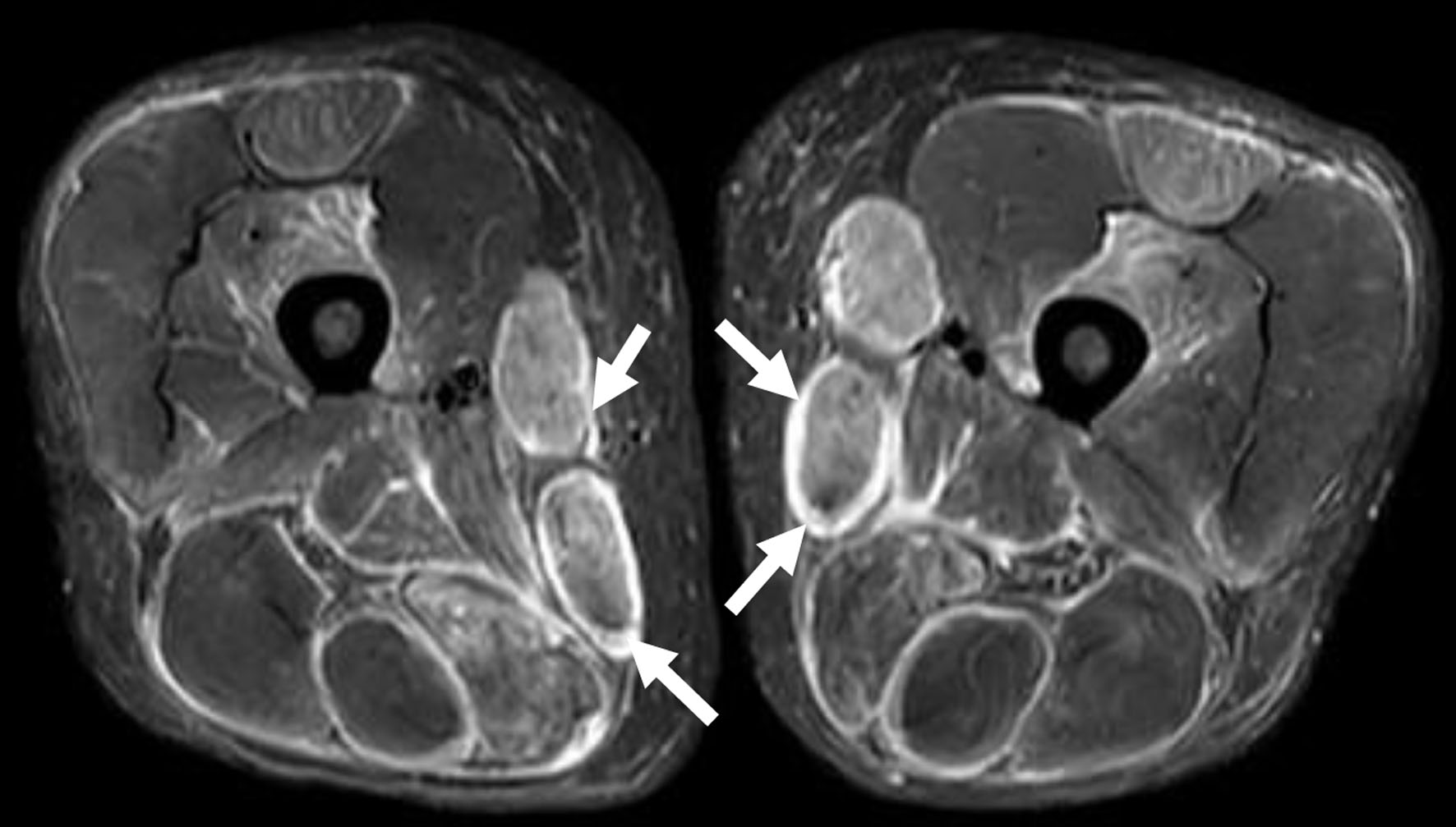 Click for large image | Figure 5. Magnetic resonance imaging of femur with evidence of myositis (arrows). |
The patient was given a 3-day course of intravenous methylprednisolone and discharged on a reducing dose of oral prednisolone and a gradual increase dose of methotrexate, with regular follow-up in both respiratory and rheumatology teams.
| Discussion | ▴Top |
Our case highlights the importance of awareness of other conditions that imitate COVID-19 both clinically and radiologically. A degree of scepticism for the diagnosis of COVID-19 should be maintained in RT-PCR negative testing.
The cardinal features of ASS are a triad of ILD, arthritis and myositis. Beside the typical triad, other features may be present, including Raynaud’s phenomenon, unexplained fever and hyperkeratotic fissured plaques on hands and feet, called mechanic’s hands and hiker’s feet respectively. These other symptoms occur in only 40% of patients [7]. The main difficulty in diagnosing this syndrome is not only its rarity but also the fact that only 20% of the patients present with the full triad. In this case, our patient presented mainly with the ILD form, which shares the same radiological changes with COVID-19, a reason why COVID-19 was strongly suspected even though the patient had three negative RT-PCR results. He complained of fatigue, which is a muscle symptom, that can be caused by myositis and hence lead to checking the muscle enzymes, such as CK. It seems that fatigue, in this case, was also attributed to COVID-19. This is not unusual as fatigue, myalgia and muscle weakness are common in viral pathologies, not only with coronavirus [8]. Muscles enzymes are also reported to be high in 33-46% of COVID-19 patients, particularly in severe cases [9]. The degree of muscle inflammation is dependent on the duration of illness and that the skeletal muscles are more frequently affected than the cardiac muscles. The viral load was also found to be low in the affected muscles, which makes the muscle inflammation is most likely due to circulating RNA, rather than direct infection of the muscle cells. This suggests that myopathy in COVID-19 is most likely post infectious, immune-mediated myopathy [10].
In our patient, the possibility of inflammatory myositis was not entertained, due to lack of any specific features and failure of checking serum muscles enzymes. It is only during the patient’s second visit to hospital, presenting with chest pain and sob of breath, a possibility of a different diagnosis has started to emerge. The high troponin in the second visit, in the absence of cardiac sounding chest pain, negative CTPA for pulmonary embolism and normal echo, ruling out myocarditis, have led to the exploration of the skeletal muscles as a source for the troponin. This was confirmed by typical history of proximal myopathy and high serum CK. Following this, the patient had all the necessary workup to make the diagnosis of ASS.
Many factors have interacted together resulting in the delay of the diagnosis of ASS. The first factor was the similarity of presentation with COVID-19 (fever, aches and pains, dry cough, fatigue, and shortness of breath). The other factor is the similarity of the chest CT findings in both conditions. The absence of the myopathic and other ASS symptoms at the initial presentation did not alert the treating team to consider other diagnoses. ASS itself is rare and uncommon, and it is not surprising that such a diagnosis be missed even at normal times. During times of COVID-19 pandemic, the mindset and cognitive biases are more towards COVID. The dangers and implications of the false-negative RT-PCR have also led to multiple testing, which could also have resulted in the delayed diagnosis and management of other conditions that mimic COVID-19 [11].
Conclusion
ASS is a rare medical syndrome and one of the COVID-19 mimics that can be easily missed if not specifically looked for.
Learning points
Several potentially fatal diseases remain masked among the wave of COVID-19 mimics. It is imperative that a thorough differential diagnostic panel be considered prior to consideration of a COVID-19 diagnosis. The implications of delayed or missed diagnosis might result in serious complications and poor quality of life, that could have been avoided.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent has been obtained.
Author Contributions
Dr. Elsayed performed literature search related to the case, and he also wrote the case report. Dr. Abdelgabar and Dr. Karmani contributed to writing the case report, with chasing the investigation results. Dr. Majid contributed to images upload and obtaining the consent form from the patient.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kligerman SJ, Groshong S, Brown KK, Lynch DA. Nonspecific interstitial pneumonia: radiologic, clinical, and pathologic considerations. Radiographics. 2009;29(1):73-87.
doi pubmed - Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier AA. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics. 2007;27(3):595-615.
doi pubmed - Trallero-Araguas E, Grau-Junyent JM, Labirua-Iturburu A, Garcia-Hernandez FJ, Monteagudo-Jimenez M, Fraile-Rodriguez G, Les-Bujanda I, et al. Clinical manifestations and long-term outcome of anti-Jo1 antisynthetase patients in a large cohort of Spanish patients from the GEAS-IIM group. Semin Arthritis Rheum. 2016;46(2):225-231.
doi pubmed - Mirrakhimov AE. Antisynthetase syndrome: a review of etiopathogenesis, diagnosis and management. Curr Med Chem. 2015;22(16):1963-1975.
doi - Mahler M, Miller FW, Fritzler MJ. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun Rev. 2014;13(4-5):367-371.
doi pubmed - Nishikai M, Reichlin M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum. 1980;23(8):881-888.
doi pubmed - Cavagna L, Nuno L, Scire CA, Govoni M, Longo FJL, Franceschini F, Neri R, et al. Clinical spectrum time course in anti Jo-1 positive antisynthetase syndrome: results from an international retrospective multicenter study. Medicine (Baltimore). 2015;94(32):e1144.
doi pubmed - Mall S, Buchholz U, Tibussek D, Jurke A, An der Heiden M, Diedrich S, Schweiger B, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30(8):e142-146.
doi pubmed - Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
doi pubmed - Aschman T, Schneider J, Greuel S, Meinhardt J, Streit S, Goebel HH, Buttnerova I, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol. 2021;78(8):948-960.
doi pubmed - Kelly S, Waters L, Cevik M, Collins S, Lewis J, Wu MS, Blanchard TJ, et al. Pneumocystis pneumonia, a COVID-19 mimic, reminds us of the importance of HIV testing in COVID-19. Clin Med (Lond). 2020;20(6):590-592.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


