| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 2, February 2023, pages 45-49
Pericardial Tamponade in a Patient With a History of Pneumonectomy
Nicholas Georgea, Brian China, Jamshid Mistrya, Rodney Borgera, Fanglong Donga, Michael M. Neekia, b, c
aDepartment of Emergency Medicine, Arrowhead Regional Medical Center, Colton, CA 92324, USA
bCalifornia University of Science and Medicine, Colton, CA 92324, USA
cCorresponding Author: Michael M. Neeki, Department of Emergency Medicine, Arrowhead Regional Medical Center, Colton, CA 92324, USA
Manuscript submitted November 8, 2022, accepted January 16, 2023, published online February 25, 2023
Short title: Pericardial Tamponade in a Post-Pneumonectomy Patient
doi: https://doi.org/10.14740/jmc4033
| Abstract | ▴Top |
Shock is the clinical presentation of circulatory failure with impaired perfusion that results in inadequate cellular oxygen utilization. Treatment requires properly identifying the type of shock that is impacting the patient (obstructive, distributive, cardiogenic, and/or hypovolemic). Complex cases may involve numerous contributors to each type of shock and/or multiple types of shock which can present interesting diagnostic and management challenges to the clinician. In this case report, we present a 54-year-old male with a remote history of a right lung pneumonectomy presenting with multifactorial shock including cardiac tamponade, with initial compression of the expanding pericardial effusion by the postoperative fluid accumulation within the right hemithorax. While in the emergency department, the patient gradually became hypotensive with worsening tachycardia and dyspnea. A bedside echocardiogram revealed an increase in size of the pericardial effusion. An emergent ultrasound-guided pericardial drain was inserted with gradual improvement of his hemodynamics followed by placement of thoracostomy tube. This unique case highlights the importance of utilizing point-of-care ultrasound along with emergent intervention in critical resuscitation.
Keywords: Pneumonectomy; Pericardial effusion; Cardiac tamponade; Shock
| Introduction | ▴Top |
Pericardial tamponade is caused by the accumulation of pericardial fluid that limits the diastolic filling of the heart, resulting in obstructive shock with hypotension and tachycardia. The pericardial space normally contains approximately 50 mL of serous fluid. However, additional fluid may collect in the pericardial space due to various etiologies including infectious/inflammatory, traumatic, neoplastic, and/or cardiovascular causes among others [1]. The most common causes are reported to be viral pericarditis in the developed world such as the United States, and infection with Mycobacterium tuberculosis in developing areas [2]. The development of tamponade depends on the amount of fluid, the rate of the filling in the pericardial space, and the compliance of the pericardium. As little as 100 mL of rapidly accumulating fluid can cause cardiac tamponade [1]. The management of cardiac tamponade is to evacuate the fluid in the pericardial space, as well as treating the underlying condition.
Pericardial tamponade is a rare complication in patients receiving a pneumonectomy, the surgical removal of a lung most commonly for an advanced malignancy or inflammatory lung disease [3]. Pneumonectomy is also indicated for unstable trauma patients with significant parenchymal destruction, central hilar vascular destruction, and/or extensive bronchial injuries [4]. The incidence of post-traumatic pneumonectomy is 0.01% with a mortality rate exceeding 50% [4]. Complications of pneumonectomy are generally divided into early and late complications [5, 6]. Examples of early complications include cardiac arrhythmias, heart failure, pneumonia, acute respiratory distress syndrome, bronchopleural fistulas, and cardiac herniation [5, 6]. Late complications include the post-pneumonectomy syndrome, pulmonary artery stump thrombosis, bronchopleural fistula, and empyema [5, 6]. Patients also experience physiologic changes, as the space previously occupied by the lung becomes filled with air, which over time gets absorbed and replaced with fluid [3].
The current case report describes an adult patient with a history of right lung pneumonectomy 25 years prior secondary to a gunshot wound who presented with multifactorial obstructive shock. After his right hemithorax was drained, the patient clinically deteriorated with serial ultrasound examinations demonstrating the development of cardiac tamponade. Based on his clinical course, the authors suspect that the postoperative physiologic fluid accumulation within the right hemithorax caused initial compression of the expanding pericardial effusion, thereby effectively “tamponading the tamponade.”
| Case Report | ▴Top |
Investigations
A 54-year-old male with a history of hypertension and post-traumatic right lung pneumonectomy 25 years prior due to a gunshot wound presented to the emergency department (ED) via Emergency Medical Services complaining of worsening shortness of breath and chest pain. The patient was found to be hypoxic in the field and was placed on continuous positive airway pressure (CPAP). The patient was unable to carry a conversation without significant dyspnea. His daughter reported the patient had been experiencing dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, and bilateral lower extremity swelling with gradual exacerbation for about 3 to 4 weeks prior to presentation. His primary doctor had recently initiated an outpatient evaluation for his symptoms with a presumptive diagnosis of congestive heart failure and started him on a diuretic. He denied any other medical or surgical history and was not taking any other medications. He denied any recent fever, illness, travel, or trauma. He also denied the use of illicit drugs, tobacco, or alcohol.
His vital signs upon arrival to the ED were a blood pressure of 125/83 mm Hg, heart rate (HR) of 111 beats per minute, respiratory rate of 27 breaths per minute, oxygen saturation of 100% while on CPAP, and temporal temperature of 36.1 °C. His physical examination was notable for an increased work of breathing, jugular venous distention bilaterally, absent breath sounds on the right side, distant heart sounds, and 3+ pitting edema of bilateral lower extremities extending to the knees.
Diagnosis
His initial workup included an electrocardiogram (ECG) which revealed sinus tachycardia with a normal axis, and low voltages throughout all leads (Fig. 1a). Pertinent laboratory studies included hyponatremia of 119 mEq/L (reference range 135 - 145 mEq/L), hypochloremia of 79 mEq/L (reference range 95 - 105 mEq/L). An arterial blood gas (ABG) revealed acidemia with a pH of 7.21 (reference range 7.35 - 7.45), pCO2 of 73 mm Hg (reference range 35 - 45 mm Hg), pO2 of 191 mm Hg (reference range 75 - 100 mm Hg), and bicarbonate of 29 mmol/L (reference range 22 - 26 mmol/L) after 1 h on high flow nasal cannula with the initial settings at 60 L/min on 60% fraction of inspired oxygen. His N-terminal brain natriuretic peptide (BNP) level was only mildly elevated to 266 pg/mL (reference range < 125 pg/mL). His troponin, creatinine, lactate, complete blood count, thyroid-stimulating hormone, and liver function tests were within the normal ranges. He had an unremarkable urinalysis, a negative urine drug screen, and a negative BioMerieux BioFire polymerase chain reaction viral swab which included coronavirus disease 2019 (COVID-19) testing.
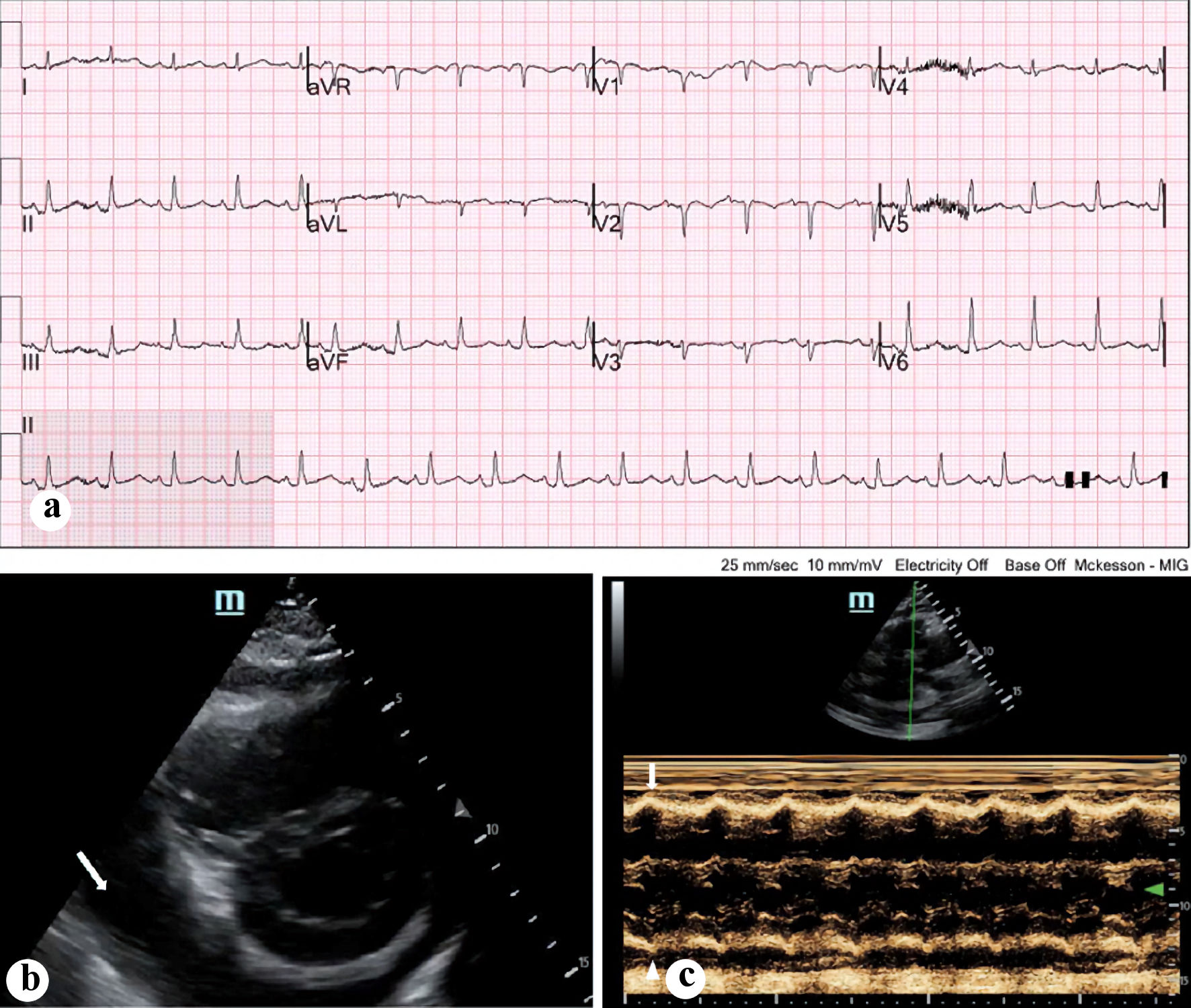 Click for large image | Figure 1. (a) Electrocardiogram done on presentation, demonstrating sinus tachycardia with relatively low voltages. (b) Bedside echocardiogram done on presentation, with a parasternal view demonstrating circumferential pericardial effusion (arrow). (c) M-mode ultrasonography demonstrating early right ventricular collapse during diastole (arrow). |
An initial portable chest X-ray revealed right-sided white-out with leftward mediastinal shift and cardiomegaly (Fig. 2a). A point-of-care ultrasound (POCUS) revealed a large pericardial effusion on the parasternal short axis view (Fig. 1b). In addition, early evidence of right ventricular (RV) diastolic collapse was noted on the parasternal long axis view on M-mode (Fig. 1c) as well as a dilated inferior vena cava (IVC). In addition, there was some mass effect from an opacity most likely a large fluid collection in the right hemithorax without significant respiratory variation, which raised concern for obstructive shock from both mass effect of the right lung fluid collection as well as early tamponade. Cardiology was consulted and performed an emergent bedside echocardiogram, which again demonstrated the findings of the initial POCUS, including a large pericardial effusion and RV dilatation with a dilated IVC. However, they did not note evidence of RV diastolic collapse or any other signs of tamponade. Cardiology recommended monitoring the patient and scheduling a non-emergent pericardiocentesis in the cardiac lab unless the patient’s status changes.
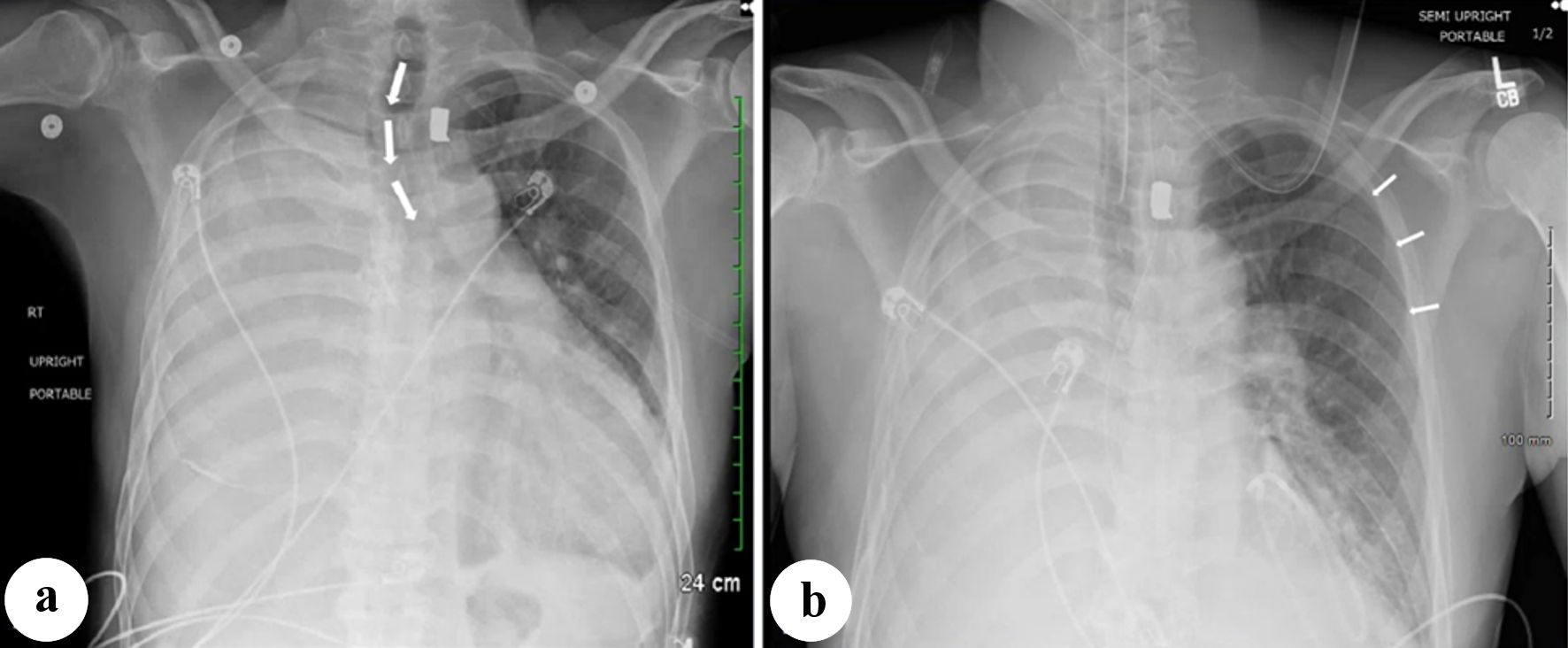 Click for large image | Figure 2. (a) Portable chest X-ray done on presentation, demonstrating right-sided white-out and cardiomegaly along with shift in the mediastinal airway structure (arrows). (b) Significant improvement in left lung volume following pericardiocentesis (arrows). |
Treatment
He was later transitioned to high-flow nasal cannula at 30 L/min and 100% oxygen, which was gradually titrated down. However, while in the ED, the patient gradually became hypotensive with worsening tachycardia and dyspnea. A repeat bedside echocardiogram revealed an increase in size of the pericardial effusion. He was given 1 L of normal saline intravenously and started on a norepinephrine drip peripherally with a starting rate of 8 µg/min to support his blood pressure. The patient developed worsening respiratory distress despite noninvasive airway management. Consequently, a delayed sequence intubation with ketamine 200 mg (intravenous (IV)) and rocuronium 100 mg (IV) was performed for airway protection. A left subclavian central IV line and a left radial arterial were placed for administration of vasoactive medications and close hemodynamic monitoring. The patient was given on fentanyl and midazolam drips for pain control and sedation.
The patient underwent computed tomography (CT) scans of the chest, abdomen, and pelvis with IV contrast which revealed mild cardiomegaly with leftward mediastinal shift secondary to a large amount of fluid occupying the right hemithorax (Fig. 3a, b). A right-sided thoracentesis was performed with initial drainage of 1 L of dark fluid, resulting in a transient improvement in his hemodynamics. However, shortly afterwards, the patient exhibited severe hypotension requiring an increase in the norepinephrine rate and the addition of a vasopressin drip. An additional 1 L of lactated Ringer’s solution was administered. A repeat POCUS by the ED team revealed an increase in size of the pericardial effusion. An emergent ultrasound-guided pericardial drain was inserted with an initial return of approximately 700 mL of dark blood, and gradual improvement of the patient’s hemodynamics. The pericardial drain was left in place to drain to gravity.
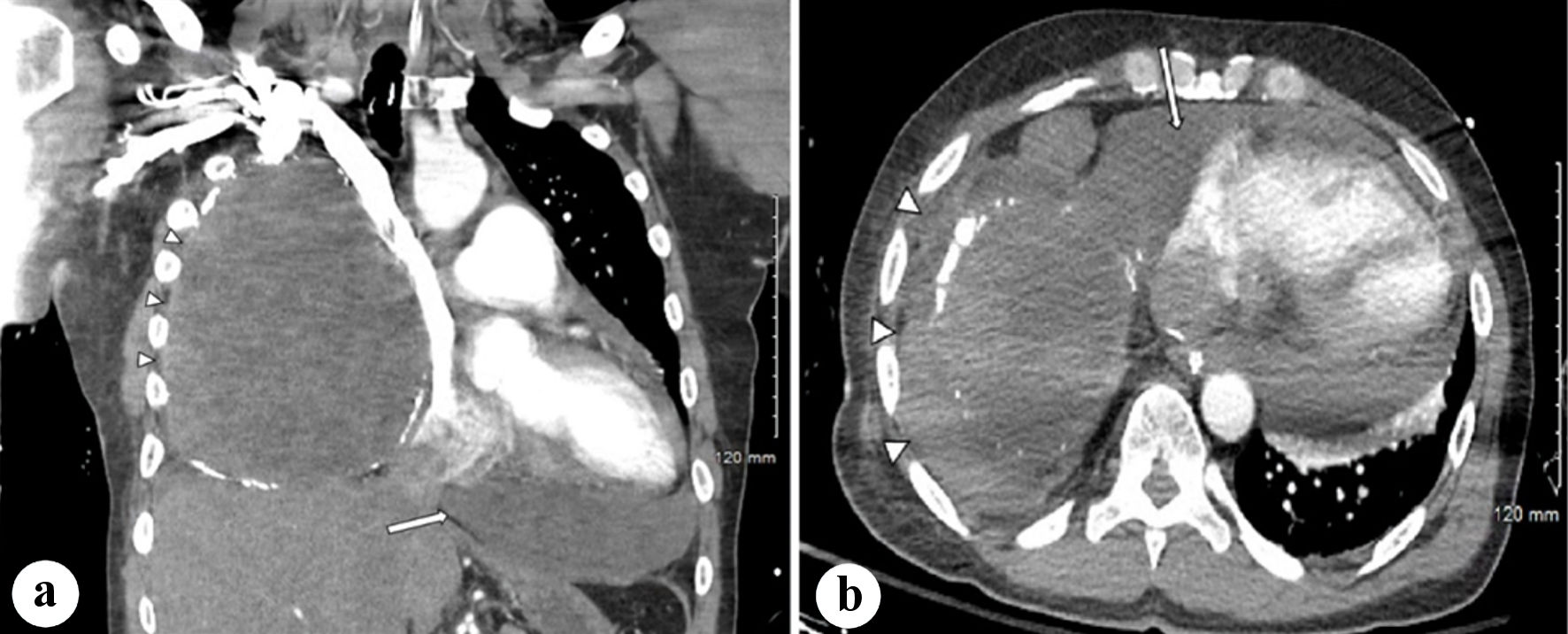 Click for large image | Figure 3. (a) Coronal CT scan of the thorax with IV contrast demonstrating moderate-large pericardial effusion and fluid collection within the right thorax with surrounding calcifications, with mediastinal shift (arrows). (b) Axial CT scan of the thorax with IV contrast demonstrating right-sided fluid collection with calcifications as well as the pericardial effusion (arrows). CT: computed tomography; IV: intravenous. |
Follow-up and outcomes
The patient was transferred to the intensive care unit. A repeat chest radiograph demonstrated improvement in his left lung volume. The pericardial drain had minimal output the following day and was removed after 2 days (Fig. 2b). A bronchoscopy was ordered by the inpatient team revealing normal left upper and lower lobes, a terminal right main-stem bronchus, and was negative for any bronchopleural fistula on the right side. Repeat echocardiogram by the cardiology team revealed an absence of pericardial effusion and was otherwise unremarkable. The patient was extubated after 7 days. He remained hemodynamically stable and was transferred to the telemetry unit. The right chest tube drained a total of 5 L of fluid over the course of 12 days and was then discontinued 5 days after extubation. He was discharged home after 18 total days of hospitalization with outpatient follow-up.
| Discussion | ▴Top |
Pericardial effusion is a rare complication of post-pneumonectomy, and the literature is scant. Tomimaru et al reported an incidence of 0.0034% of pericardial effusion following pulmonary resection [7]. They also noted that patients who underwent left pneumonectomy returned within 30 days postoperatively with pericardial effusions requiring drainage, while patients who had undergone right pneumonectomy thoracotomies generally did well with diuretic medications alone and required no further surgical intervention [7]. Case reports describe development of pericardial effusions secondary to development of chylous fistulas in patients who underwent right upper lung lobectomy [8]. Development of a pericardial effusion has been more frequently described as a complication of cardiac surgery such as heart transplant, though the reported incidence varies widely between 1% and 77%. Risk factors including hypertension, larger body surface area, immunosuppression, pulmonary thromboembolism, and renal failure [9]. While pericardial effusions may be visualized on CT scans or magnetic resonance imaging, ultrasound via either transthoracic or transesophageal echocardiography is the diagnostic modality of choice [2].
Evidence in the literature suggests that in a post-pneumonectomy patient, fluid will accumulate in the hemithorax at a variable rate following pneumonectomy and the hemithorax will be completely obliterated within weeks to months postoperatively [10]. The patient in this case appeared to have presented in multifactorial obstructive shock. Given the result of the POCUS findings and other radiographic evidence, it appeared that the mediastinal shift secondary to the large amount of fluid within the right hemithorax was the initial culprit behind the obstructive shock, as it appeared to have been compressing the inferior vena cava resulting in decreased preload. This was further complicated by a pericardial effusion which had not yet progressed to cardiac tamponade upon presentation to the ED.
This case presents a unique challenge since the patient underwent pneumonectomy 25 years prior to the ED visit. The continued accumulation of intra-thoracic fluid over such a long period of time has not been reported in the literature. Given that the patient did not present in cardiac tamponade on initial presentation, it was reasonable to evacuate the right hemithorax to alleviate the leftward mediastinal shift and relieve the obstruction of the inferior vena cava. Immediately after the thoracentesis, the patient’s clinical condition and his overall hemodynamic parameters improved for a brief period, however his status deteriorated shortly thereafter.
Serial POCUS studies revealed expansion of the pericardial effusion which led into pericardial tamponade. The etiology of this patient’s pericardial effusion remains uncertain. The leading differentials include a transudative effusion secondary to congestive heart failure, injury to pericardial vessels secondary to mass effect from the large fluid collection of the right hemithorax, post-myocardial infarction, and idiopathic. There was no clinical evidence of a primary cardiac tumor, lung cancer, aortic vascular injury, chronic hepatic or renal disease, and the patient denied other medical history, recent illness, drug use, or recent trauma, making these other etiologies less likely.
It is generally accepted that the development of cardiac tamponade depends on both the rate of accumulation of pericardial fluid as well as the compliance of the pericardium [1]. In this case, the rapid accumulation of further fluid within the pericardial space following the removal of fluid from the right hemithorax may have been the cause for the patient’s rapid development of cardiac tamponade [1]. Effectively, the large amount of fluid in the right hemithorax was most likely “tamponading the tamponade”.
Learning points
This case report highlights the importance of a detailed evaluation of patients with a history of pneumonectomy and maintaining a broad differential diagnosis for the presentation of shock, as well as the utility of POCUS in the emergent management of critical patients.
Acknowledgments
We appreciate Dr. Louis Tran, MD, for proofreading the revision of this case report.
Financial Disclosure
This study did not receive funding.
Conflict of Interest
All authors declare no conflict of interest.
Informed Consent
The Institutional Review Board at Arrowhead Regional Medical Center (ARMC) approved this study as exempt study with approval number 21-20. Informed consent was waived based on the retrospective nature of the study. No patient identifier was included in this manuscript. Data were deidentified before conducting data analysis. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Author Contributions
NG, BC, JM, RB, and FD: design of the study, introduction, case presentation, discussion, and overall manuscript editing. MMN: expert emergency medicine (EM) physician consult, worked with team to create, draft, edit, proofread, and give direction to manuscript. All authors read and approved the final manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Jensen JK, Poulsen SH, Molgaard H. Cardiac tamponade: a clinical challenge. EJ Cardiol Pract. 2017;15(17):107-113.
- Willner DA, Goyal A, Grigorova Y, Kiel J. Pericardial effusion. In: StatPearls. Treasure Island (FL). 2022.
- Beshara M, Bora V. Pneumonectomy. In: StatPearls. Treasure Island (FL). 2022.
- Phillips B, Turco L, Mirzaie M, Fernandez C. Trauma pneumonectomy: A narrative review. Int J Surg. 2017;46:71-74.
doi pubmed - Alpert JB, Godoy MC, Degroot PM, Truong MT, Ko JP. Imaging the post-thoracotomy patient: anatomic changes and postoperative complications. Radiol Clin North Am. 2014;52(1):85-103.
doi pubmed - Tsukada G, Stark P. Postpneumonectomy complications. AJR Am J Roentgenol. 1997;169(5):1363-1370.
doi pubmed - Tomimaru Y, Kodama K, Okami J, Oda K, Takami K, Higashiyama M. Pericardial effusion following pulmonary resection. Jpn J Thorac Cardiovasc Surg. 2006;54(5):193-198.
doi pubmed - Yang W, Luo C, Liu Z, Cheng C. Chylous pericardial effusion after pulmonary lobectomy. Interact Cardiovasc Thorac Surg. 2017;25(1):145-146.
doi pubmed - Ashikhmina EA, Schaff HV, Sinak LJ, Li Z, Dearani JA, Suri RM, Park SJ, et al. Pericardial effusion after cardiac surgery: risk factors, patient profiles, and contemporary management. Ann Thorac Surg. 2010;89(1):112-118.
doi pubmed - Chae EJ, Seo JB, Kim SY, Do KH, Heo JN, Lee JS, Song KS, et al. Radiographic and CT findings of thoracic complications after pneumonectomy. Radiographics. 2006;26(5):1449-1468.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


