| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 2, February 2023, pages 59-63
Chronic Oral Amiodarone as a Cause of Acute Liver Failure
Evelyn Calderon-Martineza, c , Samanta Landazuri-Navasb, Gizem Kayaa, John Cinicolaa
aDepartment of Internal Medicine Residency, UPMCH Harrisburg Hospital, Harrisburg, PA, USA
bDepartment of Internal Medicine, University of the Americas, Quito, Ecuador
cCorresponding Author: Evelyn Calderon-Martinez, Department of Internal Medicine, UPMC Harrisburg (Brady Building), PA 17104, USA
Manuscript submitted December 29, 2022, accepted January 28, 2023, published online February 25, 2023
Short title: Oral Amiodarone and Acute Liver Failure
doi: https://doi.org/10.14740/jmc4044
| Abstract | ▴Top |
Acute liver failure (ALF) is a rare condition that can have a variable clinical course and potentially fatal outcomes. Medication toxicity is a known etiology, however liver failure induced by amiodarone is rare and has been reported mostly in the setting of intravenous (IV) infusion. We present an 84-year-old patient who developed ALF after chronic use of oral amiodarone. The patient received supportive care and her symptoms improved.
Keywords: Acute liver failure; Amiodarone; Adverse reaction; Hepatotoxicity
| Introduction | ▴Top |
Acute liver failure (ALF) is defined as a loss of liver function characterized by encephalopathy, and elevation of liver enzymes and prothrombin time [1]. It is mainly caused by acetaminophen overdose, viral hepatitis, idiosyncratic drug reactions, or hypoperfusion of the liver [1, 2]. Up to 30% of the patients die without liver transplantation [3]. Drug-induced ALF can be challenging to diagnose; it should be suspected in patients with unexplained acute liver injury who are using a medication that can potentially cause hepatocellular damage. We present a case of ALF induced by chronic oral amiodarone that improved after discontinuation of the drug and supportive care.
| Case Report | ▴Top |
Investigations
An 84-year-old woman came to the emergency room for nausea, vomiting, diarrhea (up to 10/day), and anorexia. Her past medical history included paroxysmal atrial fibrillation, hypertrophic cardiomyopathy, coronary artery disease (CAD), hyperlipidemia, osteoporosis, hypertension, essential tremor, chronic kidney disease (CKD), chronic diastolic heart failure, and multinodular goiter. Her mother had a history of dementia and died at the age of 96. Her father died at 58 years old of a stroke. Both paternal grandparents died young with apparent heart disease.
She had undergone left ventricular septal myectomy 10 years earlier. The postoperative course was complicated by recurrent episodes of atrial fibrillation. She was given intravenous (IV) amiodarone and metoprolol at that time to reestablish sinus rhythm. She was discharged on verapamil CR 180 mg daily and warfarin, which was later switched to rivaroxaban. Three years ago, after persistent atrial fibrillation with rapid ventricular response (RVR), she was placed on oral amiodarone at a loading dose of 400 mg twice a day (BID) for 15 days, followed by 200 mg daily. The patient did not tolerate metoprolol due to excessive fatigue.
Two years prior to the admission, the patient noticed a worsening of her essential tremor, which was attributed to amiodarone. Her tremor did not improve after adding propranolol, and amiodarone was discontinued. It was resumed at 200 mg daily after an episode of atrial fibrillation with RVR 7 months later. She also underwent a transesophageal echocardiogram with cardioversion the same month. Three months before this presentation, laboratory results showed mildly elevated liver enzymes (Table 1) and the dose of amiodarone was reduced to 100 mg daily. The treating physician decided that due to her history of chronic diastolic heart failure, CKD, and CAD, other anti-arrhythmic options would not be appropriate. Additionally, she was on amlodipine, propranolol, rivaroxaban, furosemide, and calcium supplement.
 Click to view | Table 1. Liver Function Test Trend Before Acute Presentation |
One day before presenting to the emergency room, she attended a family gathering. On the day of admission, she experienced acute onset non-bloody, non-bilious emesis and numerous loose watery stools with no blood. She was unable to tolerate oral intake but denied abdominal pain, fever, or chills. She stated that no other family members at the gathering showed signs of diarrhea or vomiting. On examination she was pale, her mucous membranes were dry, the abdomen was soft and nondistended, and there was no abdominal tenderness. The patient was noted to have a mild elevation of transaminases, elevated total bilirubin, and mild leukocytosis. Other laboratory results are shown in Table 2. An abdominal ultrasound was negative for acute abnormalities. She was given IV Zofran 4 mg, 500 mL normal saline (NS) bolus, and subsequently was able to tolerate oral intake. She was discharged with supportive management.
 Click to view | Table 2. Laboratory ED Results, First Day |
Three days later, the patient came back to the emergency room due to profuse diarrhea, lethargy, persistent nausea, and vomiting. She was not able to eat solid food. She denied any blood in emesis or stools. Physical examination was normal except for dry mucous membranes and lethargy.
Diagnosis
A chest X-ray showed slight increased parenchymal stranding in the left lower lobe. A repeat abdominal ultrasound showed no abnormalities. Computed tomography (CT) of the abdomen and pelvis without IV contrast showed increased density of the liver and colonic diverticulosis with no signs of diverticulitis or bowel obstruction. Abdominal Doppler showed no evidence of thrombus in the portal venous system. Viral hepatitis panel was negative. Acetaminophen levels were less than 10 µg/mL (normal range: 10 - 30 µg/mL). A test for antinuclear antibodies was negative. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Clostridioides difficile (C. difficile) PCR with reflex to toxin were negative as well. Other laboratory results are shown in Table 3. Having markedly elevated liver enzymes, altered mental status, and prolonged international normalized ratio (INR), ALF was diagnosed. Given negative results of other causes of ALF, amiodarone was presumed to be the culprit.
 Click to view | Table 3. Laboratory Results, Second Admission |
Treatment
Amiodarone was discontinued and the patient was treated with supportive care. Liver enzymes trended down (Fig. 1). The patient had a couple of episodes of asymptomatic atrial fibrillation with RVR and required IV metoprolol once, then was placed back on her home dose of propranolol, with good heart rate control.
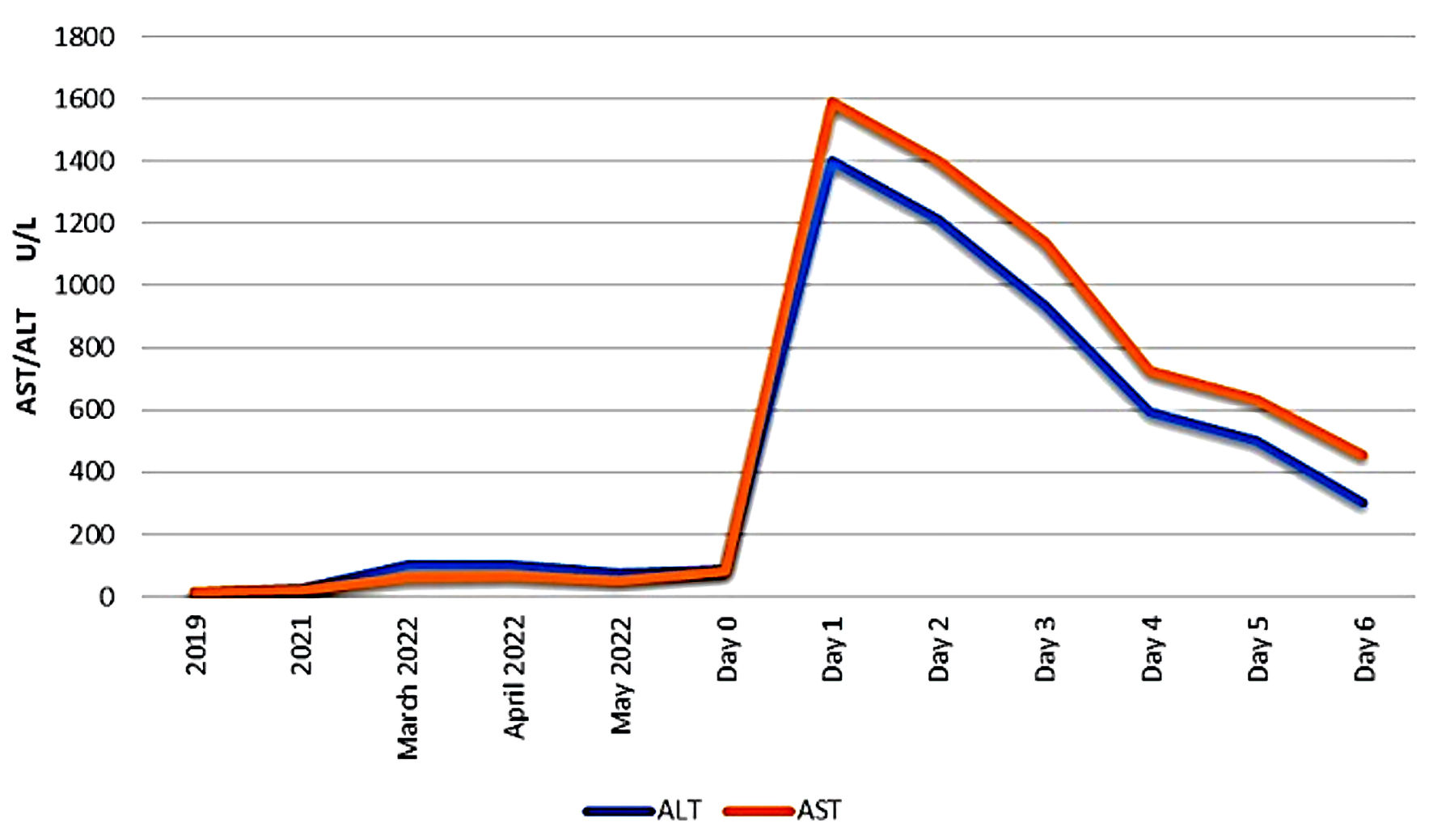 Click for large image | Figure 1. Liver function test (LFT) trending before and after admission. AST: aspartate aminotransferase; ALT: alanine aminotransferase. |
Follow-up and outcomes
The initial symptomatology improved, the patient became hemodynamically stable, and she was transferred to a skilled nursing facility.
| Discussion | ▴Top |
ALF is a potentially fatal loss of liver function with a high mortality rate. It is characterized by elevated liver enzymes and may progress to hepatic encephalopathy and elevated prothrombin time [1]. According to O’Grady, ALF can be classified as hyperacute, acute, or subacute depending on the onset of encephalopathy. If the onset of encephalopathy is within 7 days, ALF is considered hyperacute; within 8 to 28 days, it is acute, and if it presents after 28 days, it is classified as subacute ALF [2]. In our patient ALF was considered hyperacute, given encephalopathy developed in less than 7 days.
The prognosis depends on the severity of the liver injury, and 30% of patients with ALF die without liver transplantation [3]. The incidence of ALF in patients hospitalized for jaundice is 3-5% in the United States [4].
One feature of ALF is hepatic encephalopathy which is graded from I to IV (Table 4) and can cause symptoms ranging from mild changes in behavior to coma [5]. Cerebral edema is present in up to 75% of those with grade IV encephalopathy and may lead to increased intracranial pressure [5]. The pathophysiology behind cerebral edema in ALF is multifactorial. Cytotoxic edema predominantly related to ammonia, plays a central role. Ammonia can cross the blood-brain barrier easily and is metabolized to glutamine within astrocytes. This amino acid has osmotic properties and causes astrocytic swelling. Other mechanisms include edema from proinflammatory cytokines, vasogenic edema, and impaired cerebral autoregulation [6].
 Click to view | Table 4. Grade of Hepatic Encephalopathy |
Additional clinical findings of ALF include jaundice, fever, right upper quadrant tenderness, ascites, hepatomegaly, and intravascular signs of volume depletion [1]. Laboratory findings can vary depending on the specific cause of ALF, but commonly include markedly elevated aminotransferases and high INR [7]. Acute kidney injury (AKI) can be seen in 30% to 70% of patients with ALF. The pathogenesis may be similar to hepatorenal syndrome. A retrospective analysis of more than 1,600 patients showed that AKI affects short- and long-term outcomes, but rarely results in chronic kidney disease [8].
Diagnostic imaging should include abdominal Doppler ultrasound to rule out hepatic and portal vein thrombosis, and to evaluate portal hypertension, presence of ascites, and liver size [9]. Abdominal CT may show diffuse low density of the liver, hepatomegaly, or ascites. Signs of specific causes can be also present, like evidence of malignant infiltration or hepatic vein occlusion [7]. CT of the brain can be useful as it can show signs of cerebral edema including a reduction in cerebral ventricle size, flattening of cerebral convolutions, or attenuation of the signal intensity of brain parenchyma [10].
ALF can result from a wide variety of causes. Acetaminophen overdose, viral hepatitis, idiosyncratic drug reactions, and hypoperfusion are the most common etiologies of ALF [1, 11]. In the United States, acetaminophen overdose is the leading cause of ALF [12], while in other countries, like Japan, more than half of cases of ALF are due to viral hepatitis [13]. The hepatotoxicity from acetaminophen is dose dependent and rarely occurs at therapeutic doses, except in patients who have underlying liver disease or those who are taking cytochrome P450 inducers [14]. Ischemic hepatitis is another cause of ALF, and can result from cardiac failure, sepsis, Budd-Chiari syndrome, or some drugs that have vasoconstricting properties like cocaine and methamphetamine [15].
ALF can be induced by amiodarone, the drug of choice for both supraventricular and ventricular arrythmias [1, 11]. It is a class III antiarrhythmic that works predominantly by blocking potassium channels, which increases the cardiac action potential duration [16]. Amiodarone is metabolized in the liver and its full antiarrhythmic effect occurs after 10 weeks of therapy. This drug is highly lipophilic and accumulates easily in the hepatocytes, with a half-life of 25 - 100 days [16].
Side effects from oral amiodarone include photosensitivity, thyroid, pulmonary or hepatic dysfunction, cardiac arrhythmias, and optic neuropathy [1, 17]. Hepatotoxicity associated to oral amiodarone administration is documented in 15-20% of patients, with mild elevation of transaminases (aspartate aminotransferase/alanine aminotransferase (ALT/AST)), but with minimal increase of gamma glutamyl transferase (GGT) and no change in bilirubin levels or alkaline phosphatase. This injury is thought to be caused by amiodarone accumulation of lipid-rich material in lysosomes and direct damage to mitochondria [18]. The incidence of significant liver injury remains under 1% [19, 20]. ALF induced by amiodarone is more common with IV infusion than oral administration. IV formulation contains polysorbate-80; hypersensitivity reaction or idiosyncratic reactions to this component may explain ALF. It is also known that polysorbate-80 has hypotensive effects that can lead to impaired hepatic [21].
Treatment of drug-induced ALF is mainly supportive, but a good percentage of patients may require liver transplant. Management also includes treating the specific underlying ALF cause, such as transjugular intrahepatic portosystemic shunt placement, surgical decompression, or thrombolysis in the case of acute Budd-Chiari [22]. Patients can be managed in the intensive care unit or on a general medical ward, depending on the grade of encephalopathy. On a general ward, frequent neurologic checks are required to assure mental status monitoring. If the encephalopathy worsens, intensive care may be indicated [23].
Frequent laboratory monitoring is recommended, including serum aminotransferases, bilirubin, coagulation studies, complete blood counts, metabolic panels, and arterial blood gasses. An improvement on aminotransferase levels may indicate spontaneous recovery or loss of hepatocyte mass. Glucose and electrolytes abnormalities are common in this type of patients. Ammonia levels should be followed as well, given numbers above 150 µg/dL has been associated with increased risk of cerebral herniation [24].
N-acetylcysteine has been shown to improve survival in patients with early-stage liver failure caused by acetaminophen intoxication. In patients with severe failure, emergency liver transplant is necessary [17].
Some reports describe the beneficial effects of corticosteroids in moderate/severe drug-induced liver injury, but more studies are required [25].
In conclusion, despite oral amiodarone is usually associated with mild elevation of liver enzymes, acute hepatotoxicity should be considered in a patient presenting with acute encephalopathy and elevation of liver enzymes and prothrombin time who is taking this drug. The medication should be stopped immediately, and supportive treatment should be provided.
Learning points
ALF is a rare but potentially fatal side effect of oral amiodarone. In our patient, with multiple comorbidities and a history of paroxysmal atrial fibrillation, the use of oral amiodarone was needed in the first place to control her arrhythmia. It caused elevation of transaminases, elevated total bilirubin, and mild leukocytosis. After assessment of other etiologies, ALF after chronic oral intake of amiodarone was diagnosed. The discontinuation of amiodarone led to a lowering of the liver function enzymes. If there are signs of hepatic injury, the drug should be stopped immediately, and supportive treatment should begin. American and European guidelines do not recommend regular monitoring of liver function as it has not been proven to be effective. However, they suggest that it is reasonable to monitor serum liver biochemistries during the initial 6 months of treatment with a potentially hepatotoxic agent (conditional recommendation, very low quality of evidence) [4, 26].
Acknowledgments
None to declare.
Financial Disclosure
This is a self-financed manuscript without funding source.
Conflict of Interest
The authors do not have any conflict of interest.
Informed Consent
Verbal informed consent was obtained from the patient for publication of this case report.
Author Contributions
ECM, GK contributed to writing of the manuscript. The paper was drafted by SLN and ECM. Critical revisions and final approval were made by JC.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ALF: acute liver failure; CAD: coronary artery disease; CKD: chronic kidney disease; IV: intravenous; CT: computed tomography; AKI: acute kidney injury; INR: international normalized ratio
| References | ▴Top |
- Essrani R, Mehershahi S, Essrani RK, Ravi SJK, Bhura S, Sudhakaran A, Hossain M, et al. Amiodarone-induced acute liver injury. Case Rep Gastroenterol. 2020;14(1):87-90.
doi pubmed - O'Grady JG. Acute liver failure. Postgrad Med J. 2005;81(953):148-154.
doi pubmed - Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ, 2nd, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856-864.e851.
doi pubmed - Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ, Practice Parameters Committee of the American College of G. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966.
doi pubmed - Gill RQ, Sterling RK. Acute liver failure. J Clin Gastroenterol. 2001;33(3):191-198.
doi pubmed - Scott TR, Kronsten VT, Hughes RD, Shawcross DL. Pathophysiology of cerebral oedema in acute liver failure. World J Gastroenterol. 2013;19(48):9240-9255.
doi pubmed - Shakil AO, Jones BC, Lee RG, Federle MP, Fung JJ, Rakela J. Prognostic value of abdominal CT scanning and hepatic histopathology in patients with acute liver failure. Dig Dis Sci. 2000;45(2):334-339.
doi pubmed - Tujios SR, Hynan LS, Vazquez MA, Larson AM, Seremba E, Sanders CM, Lee WM, et al. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol. 2015;13(2):352-359.
doi pubmed - Rajaram P, Subramanian R. Acute liver failure. Semin Respir Crit Care Med. 2018;39(5):513-522.
doi pubmed - Chavarria L, Alonso J, Rovira A, Cordoba J. Neuroimaging in acute liver failure. Neurochem Int. 2011;59(8):1175-1180.
doi pubmed - Lewis JH, Ranard RC, Caruso A, Jackson LK, Mullick F, Ishak KG, Seeff LB, et al. Amiodarone hepatotoxicity: prevalence and clinicopathologic correlations among 104 patients. Hepatology. 1989;9(5):679-685.
doi pubmed - Lee WM, Squires RH, Jr., Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47(4):1401-1415.
doi pubmed - Oketani M, Ido A, Tsubouchi H. Changing etiologies and outcomes of acute liver failure: A perspective from Japan. J Gastroenterol Hepatol. 2011;26(Suppl 1):65-71.
doi pubmed - Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89(2):193-199.
doi pubmed - Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol. 2017;33(3):158-163.
doi pubmed - Hamilton D, Sr., Nandkeolyar S, Lan H, Desai P, Evans J, Hauschild C, Choksi D, et al. Amiodarone: A Comprehensive Guide for Clinicians. Am J Cardiovasc Drugs. 2020;20(6):549-558.
doi pubmed - Verhovez A, Elia F, Riva A, Ferrari G, Apra F. Acute liver injury after intravenous amiodarone: a case report. Am J Emerg Med. 2011;29(7):843.e845-846.
doi pubmed - Jaiswal P, Attar BM, Yap JE, Devani K, Jaiswal R, Wang Y, Szynkarek R, et al. Acute liver failure with amiodarone infusion: A case report and systematic review. J Clin Pharm Ther. 2018;43(1):129-133.
doi pubmed - Paudel R, Dogra P, Suman S, Acharya S, Matta J. Acute Liver and Renal Failure: A Rare Adverse Effect Exclusive to Intravenous form of Amiodarone. Case Rep Crit Care. 2016;2016:5232804.
doi pubmed - Huang CH, Lai YY, Kuo YJ, Yang SC, Chang YJ, Chang KK, Chen WK. Amiodarone and risk of liver cirrhosis: a nationwide, population-based study. Ther Clin Risk Manag. 2019;15:103-112.
doi pubmed - Hirama S, Tatsuishi T, Iwase K, Nakao H, Umebayashi C, Nishizaki Y, Kobayashi M, et al. Flow-cytometric analysis on adverse effects of polysorbate 80 in rat thymocytes. Toxicology. 2004;199(2-3):137-143.
doi pubmed - Klein AS, Molmenti EP. Surgical treatment of Budd-Chiari syndrome. Liver Transpl. 2003;9(9):891-896.
doi pubmed - Polson J, Lee WM, American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41(5):1179-1197.
doi pubmed - European Association for the Study of the Liver. Clinical practice guidelines panel, Wendon J, Panel m, Cordoba J, Dhawan A, Larsen FS, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047-1081.
doi pubmed - Bjornsson ES, Vucic V, Stirnimann G, Robles-Diaz M. Role of corticosteroids in drug-induced liver injury. A systematic review. Front Pharmacol. 2022;13:820724.
doi pubmed - European Association for the Study of the Liver. Clinical Practice Guideline Panel Chair, Panel members, Easl Governing Board representative. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. 2019;70(6):1222-1261.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


