| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 10, October 2024, pages 287-296
Role of Continuous Drainage of Tense Ascites in Peritoneal Dialysis: Mehandru/Masud Technique
Sushil K. Mehandrua, c, Supreet Kaura, Avais Masuda, Kyrillos Rezkallaa, Qalb Khana, Prit Paul Singha, Eric Constanzoa, Walid Abbouda, Tushar Vachharajanib, Arif Asifa
aDepartment of Medicine, Division of Nephrology and Hypertension, The Mehandru Center for Innovation in Nephrology, Jersey Shore University Medical Center, Hackensack Meridian School of Medicine, Neptune, NJ, USA
bDepartment of Nephrology and Hypertension, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Glickman Urological & Kidney Institute, Cleveland Clinic, Cleveland, OH, USA
cCorresponding Author: Sushil K. Mehandru, Department of Medicine, Division of Nephrology and Hypertension, The Mehandru Center for Innovation in Nephrology, Jersey Shore University Medical Center, Hackensack Meridian School of Medicine, Neptune, NJ, USA
Manuscript submitted March 5, 2024, accepted August 14, 2024, published online September 20, 2024
Short title: Super Large Volume Ascites Managed by PD
doi: https://doi.org/10.14740/jmc4056
| Abstract | ▴Top |
Insertion of a peritoneal dialysis (PD) catheter in end-stage renal disease (ESRD) patients with cirrhosis and tense ascites remains a challenge for nephrologists. Ascitic fluid leak at the surgical site, a common postoperative occurrence, leads to the disqualification of many patients who could be otherwise great candidates for PD. The ascitic fluid leak has been described to occur during or immediately after surgery even after the entire volume of ascitic fluid has been drained. In this study, we report a case study of three patients with ESRD, liver cirrhosis, and tense ascites on hemodialysis. The patients required super large volume paracentesis (SLVP), draining 9,000 - 15,000 cc of ascitic fluid twice weekly in an interventional radiology setup. Besides ascitic fluid drainage, the patients needed in-center hemodialysis (ICHD) 3 days a week, leading to their engagement in procedures 5 days a week. In addition, intradialytic symptomatic hypotension, hypoalbuminemia, and other adverse effects of hemodialysis lead to their poor lifestyle. To improve their lifestyle, all patients desired to switch to PD from ICHD. Upon the PD catheter insertion and drainage of the entire ascitic fluid, leaks developed at the insertion site within a few hours. To overcome these leaks, PD catheters of all three patients were attached via a transfer set to a bag for continuous drainage of ascitic fluid for about 2 weeks. No leak or complication was noted, leading to complete healing of insertion site. We recommend, for the patients with tense ascites requiring SLVP, approximately 2 weeks of healing period continuously be performed till initiation of PD training,.
Keywords: Continuous drainage; End-stage renal disease; Liver cirrhosis; Peritoneal dialysis; Super large volume paracentesis; Tense ascites
| Introduction | ▴Top |
Liver disease, including cirrhosis, is one of the primary causes of morbidity and mortality, and is the third leading cause among the population of adults aged 40 - 59 years [1]. Cirrhosis occurs during advanced stages of liver disease, due to irreversible fibrosis. This fibrosis in addition to portal hypertension leads to ascites, the accumulation of fluid in abdominal cavity. Various techniques for drainage of peritoneal dialysis (PD) solution and ascites have been devised. In the study by Bajo et al, the authors drained an extra 20% over the infused volume in their PD patients, to drain ascites with each exchange [2]. As reported by Chaudhary et al, during initiation of PD in an inpatient setting, 1 L of peritoneal fluid was drained, followed by infusion of 1.5% of dextrose PD solution and drainage of 1.5 L with a dwell time of 1 - 2 h [3]. This is continued until the complete drainage of PD fluid [3]. These studies also reflect that the PD catheter should not be removed if patients wished to switch back to hemodialysis (HD) modality. Literature suggests that even in patients on in-center hemodialysis (ICHD), the PD catheter can be maintained for episodic drainage of ascitic fluid, eliminating the need for frequent paracentesis [4]. The successful employment of PD in managing end-stage renal disease (ESRD) patients with ascites secondary to cirrhosis or heart failure [5, 6] and in patients with fulminant hepatic failure and kidney failure (KF) is well documented [7]. Cirrhotic ascites, pathologically manifested as accumulation of fluid in the peritoneal cavity, is observed in about 85% of cirrhosis patients as a complication. Ascites is considered as a marker of decompensated cirrhosis and is the most common cause for hospitalization and sign for orthotopic liver transplantation [6]. Portal hypertension is the leading cause in the formation of ascites which could in turn causes splanchnic vasodilation, triggering further renal sodium retention [8]. Intermittent HD in ESRD patients with severe liver failure is associated with a risk of encephalopathy [4]. Figure 1 depicts the path chosen by our team compared with Rajora et al for the similar patient population.
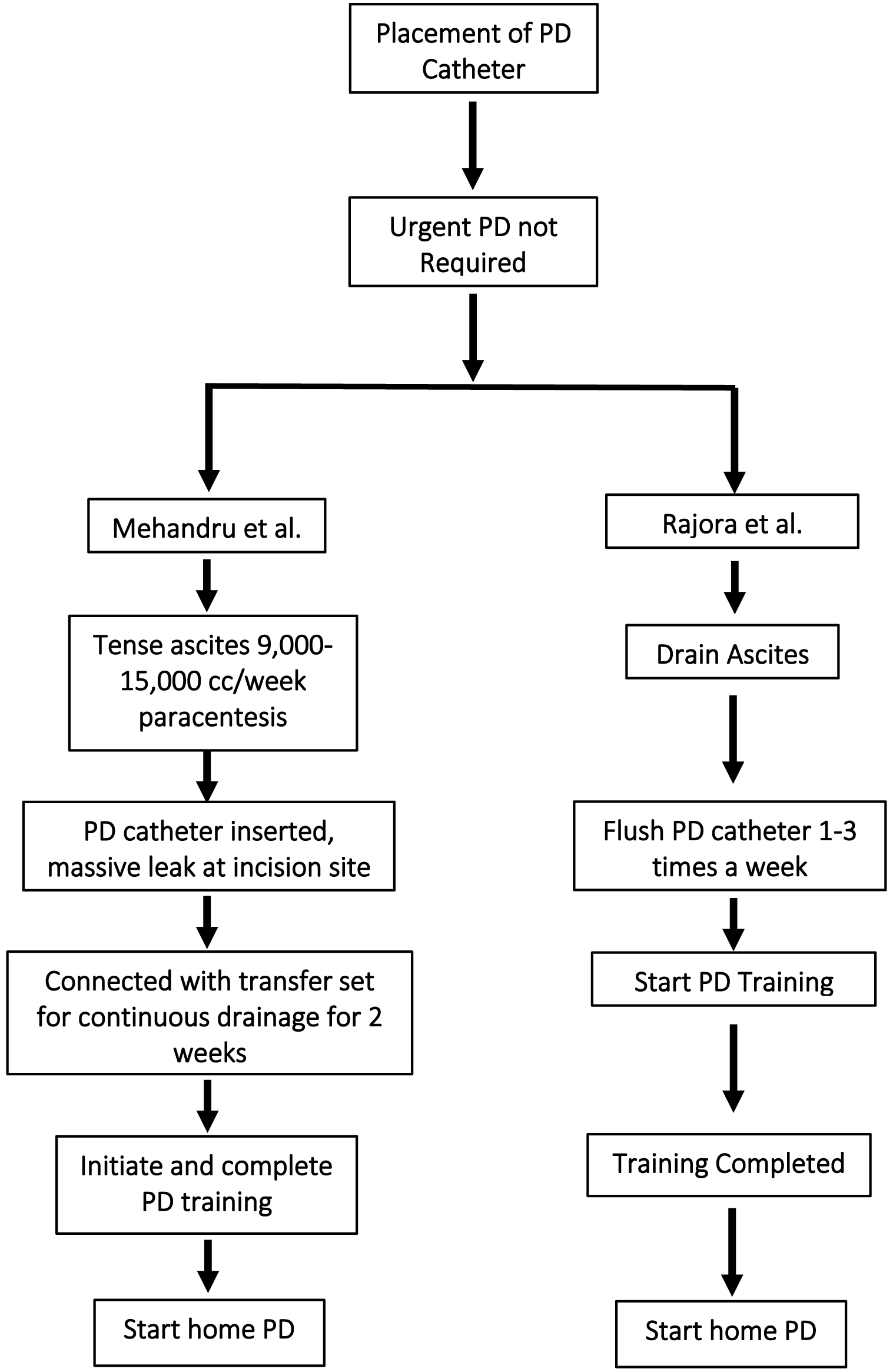 Click for large image | Figure 1. Comparison between Mehandru et al (this study) and Rajora et al study. |
The risk of encephalopathy increases with rapid osmosis, and electrolyte shift secondary to intermittent HD due to increased brain water content [8]. PD offers great advantages over HD in patients with ESRD and co-existing cirrhosis, both with and without ascites [3]. Advantages and disadvantages of PD in patients with kidney disease and ascites secondary to liver cirrhosis are discussed in Figure 2.
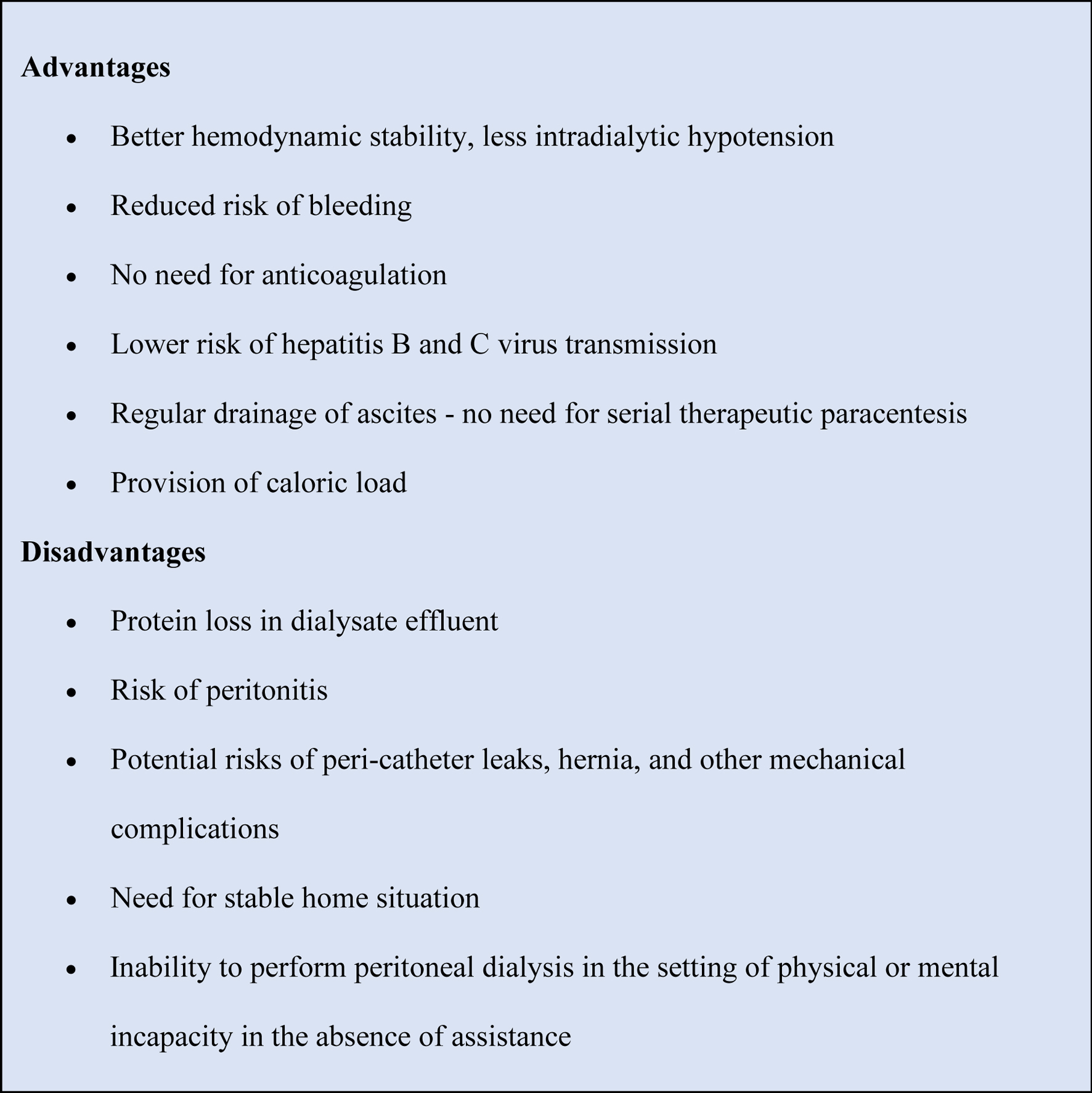 Click for large image | Figure 2. Potential advantages and disadvantages of PD in patients with kidney failure and ascites. PD: peritoneal dialysis. |
Only a few patients with advanced cirrhosis have “refractory ascites” and do not respond to conventional treatment [1]. Frequent paracentesis to ease ascites is the only treatment of choice unless the patient is on PD. A large proportion of such patients have associated chronic kidney disease which may require dialysis therapy. PD may offer a better choice than HD. Several reports in the literature describe using newly placed PD catheter for initial large volume paracentesis (LVP), for symptomatic relief, thereafter, conducting flushes or small volume PD.
| Method | ▴Top |
For this case study, three patients had super large volume paracentesis (SLVP) requiring twice weekly draining of 9,000 - 15,000 cc, accumulating over 2,000 - 3,000 cc ascitic fluid daily. A continuous drainage was a safe way to prevent leakage from the incision site in patients with newly placed PD catheter. Patients with co-existing ascites and KF pose a significant management challenge for the nephrologists [9]. Inclusion criteria are the following: between the ages of 18 and 90, gender neutral, and the ability to train in PD technique. Participants were recruited from an ICHD unit. The presence of ascites in patients with KF poses numerous challenges, including volume management due to poor hemodynamics [10-12]. Co-existence of encephalopathy, coagulopathies, and malnutrition creates a perfect storm, hence making HD an inadequate treatment modality [4, 8]. PD creates a better choice due to the consistency of the treatment by providing hemodynamic stability and better volume management. PD eliminates the need for LVP by draining ascites with each treatment [13-16]. However, the sensitivity in employment of PD continues to exist due to its association with a higher risk of infection, excessive albumin loss, and overall poor outcomes, leading to a low prevalence of PD in patients with KF and ascites [2, 13, 14].
In this study, we determined the impact of continuous drainage of ascitic fluid immediately following insertion of a PD catheter to prevent leaks at the incision site in an ESRD patient with tense ascites, weekly SLVP of 9,000 - 15,000 cc. All three patients in this study had low plasma albumin (< 1.6 g/dL) while on ICHD, whereas at 6 months after initiation of PD, their albumin improved (> 3.5 g/dL). There was no incident of peritonitis or leaks. Blood pressure that was fluctuating widely on ICHD rendering patients symptomatic, was stabilized within a matter of few weeks after PD training. Concomitant cirrhosis and other causes remain unclear. Smaller studies conducted in Asia have observed a 4-6% occurrence of cirrhosis in KF, although the incidence of ascites has not been reported in most studies [17-20]. The presence of cirrhosis adds to overall mortality risk in KF patients. In a study conducted in Taiwan, cirrhosis was found to be an independent predictor of mortality in dialysis patients [20]. Similarly in a Korean study, cirrhotic patients had significantly lower 1-, 3-, and 5-year survival rates compared with non-cirrhotic KF patients [17]. Studies have investigated on the effects of renal replacement therapy (RRT) in the setting of acute KF and results suggested that early initiation of RRT in patients with co-existing liver failure and KF might be beneficial. In a study by Wilkinson et al (1977) [12] involving 25 KF and cirrhotic patients, the overall outcome was uniformly negative in both groups as all 25 patients died.
Cirrhosis increases risk of bleeding, leading to instability with HD, especially in patients with arterial venous access. Similarly, patients with central venous catheters have also faced this problem, including the inherent risk of infection as well as catheter malfunction. Although minimum use of anticoagulants is implemented in ESRD patients with cirrhosis, there is no evidence-based recommendation for this patient population.
Case Report
This study reports three patients with cirrhosis and ESRD on HD. These patients had tense ascites secondary to cirrhosis of the liver. Table 1 presents the demographics of these patients along with the etiology of disease, frequency of paracentesis, and hemodynamics. Despite being on lasix 80 mg twice daily and HD three times a week, all three patients required biweekly SLVP to ease tense ascites. They were also suffering from intradialytic hypotension, severe hypoalbuminemia, and poor quality of life. Table 2 depicts pre- and post-PD conditions. Although all patients were abstaining from consumption of alcohol, there was no improvement in health status due to overall complications. With above circumstances, PD appeared to be the most viable option in these patients, illustrating the positive changes.
 Click to view | Table 1. Age, Gender, Etiology of Liver and Kidney Disease, Frequency of Paracentesis, and Systolic Blood Pressure Before and After Initiation of PD |
 Click to view | Table 2. Conditions of the Patients in This Study With ESRD and Cirrhosis Before and After PD |
Due to deteriorating conditions of these patients secondary to frequent ascites and HD, PD was thought to be a better option. After educating the patients, a collective decision to switch dialysis modality from HD to PD was made. During catheter insertion, all patients had their newly inserted PD catheter attached to a transfer set and a bag for continuous drainage to avoid further leaks and promote adequate healing of the surgical site. Once the incision site healed in 14 days, PD training was initiated. Table 3 presents the treatment timeline for the patients on PD. Table 4 depicts complications immediately after insertion of PD catheter as well as post-establishment of PD. No complication such as peritonitis or leakage was noted. These patients remained compliant with the prescribed therapy and treatment regimen. Patients were followed for over a 2-year period with successful continuation of PD modality and great improvement in lifestyle, even allowing these patients to lead their lives independently.
 Click to view | Table 3. Treatment Timeline for the Patients on Peritoneal Dialysis |
 Click to view | Table 4. Peritoneal Leak After PD Catheter Insertion (Immediately, During PD Training and 2 Years After PD Initiation) |
Patients were educated on both modalities, risks and benefits were discussed, and consents were obtained to initiate PD with insertion of PD catheter. General surgery was consulted, and PD catheter was inserted in the peritoneal cavity in a hospital setting. A few hours after surgical intervention, profuse leakage of ascitic fluid was noted at the incision site in all patients. Patients were trained on PD, and they were able to remain free of ascites, hemodynamically stable with normal albumin levels a few months and 2 years after PD initiation.
| Discussion | ▴Top |
Several reports in the literature describe the use of newly placed PD catheter for initial LVP, for symptomatic relief, thereafter, conducting flushes or small volume PD [21]. The patients in our study had SLVP requiring twice weekly draining of 9,000 - 15,000 cc, accumulating over 2,000 - 3,000 cc ascitic fluid daily. Continuous drainage was a safe way to prevent leakage from the incision site in patients with ESRD. Patients with co-existing ascites and KF pose a significant management challenge for the nephrologists [9]. PD is relatively contraindicated in cirrhotic patients with ascites in several publications. Despite the consensus, available clinical report suggests that patients with cirrhosis can be successfully managed on PD.
Ascites presents numerous challenges in the overall management of KF. These patients usually have poor hemodynamics, which makes volume management very difficult [10-12]. Furthermore, the presence of coagulopathy, malnutrition, and encephalopathy compounds the management complexity, therefore, such patients do not tolerate HD well [4, 8]. PD by offering steady-state treatment may provide hemodynamic stability and better volume management. Additionally, PD eliminates the need for LVP due to drainage of ascites with each treatment [13-16]. However, there is a perception that use of PD is associated with higher risk of infection, excessive albumin loss, and overall poor outcomes, and therefore should not be offered in patients with KF and ascites [2, 13, 14].
Prevalence of ascites in KF due to cirrhosis and other causes remains uncertain. Small studies from Asia have observed a 4-6% incidence of cirrhosis in KF, but the incidence of ascites has not been reported in most studies [17-20]. The process of cirrhosis increases the overall mortality in KF patients. In a Taiwan National Cohort, cirrhosis was found to be an independent predictor of mortality in dialysis patients [20]. A study by Wilkinson et al (1977) investigated on 25 such patients, where HD was used in five patients, PD was used in 18 patients, and two patients used both modalities [12]. The patients receiving PD had lower risk of bleeding and hypotensive episodes who have renal disease. The outcome was uniformly negative in both graphs as all 25 patients eventually died. Dialysis technology and management of acute KF have improved considerably since Wilkinson’s publication progresses to ESRD, requiring RRT. The prevalence of patients with comorbid ESRD and cirrhosis secondary to liver disease is not known. However, with increasing prevalence of chronic diseases, the population with co-existence of KF and liver disease may continue to grow. Although they may co-exist, the causes of kidney and liver disease might be unrelated. Diabetes mellitus and hypertension are the most common causes of renal disease, whereas liver disease is most often a result of viral infection or alcohol-related. Another population at risk for KF is patients with liver transplant: up to 18% of such patients require dialysis 5 years after transplantation [3, 22]. Due to hemodynamic instability, recent interest is focused on managing KF with continuous RRT.
PD patients who need dialysis in association with liver disease show a lower overall mortality rate than HD. Patients’ comorbidities play an important role in the mortality rate as well as severity of liver cirrhosis and serum albumin levels. In patients with ascites and CKD stage 5 requiring dialysis, PD may be the treatment of choice [23]. The dropout rates in the study by De Vecchi et al were similar in the cirrhotic and non-cirrhotic groups, where leaks and mechanical complications leading to technique failure were similar in both groups [14]. Adequate solute clearance as well as dialysis adequacy can be accomplished along with evacuation of ascites with employment of PD [3]. In patients with non-fatal liver disease, PD can be used successfully to treat ascites and KF. This treatment modality may be better tolerated than HD and should be the RRT of choice especially in these particular patient populations [24]. The presence of liver cirrhosis does not increase the risk of bacterial peritonitis or other dialysis-related complications [14]. Clinical symptoms of sclerosing encapsulating peritonitis were observed in two cirrhotic patients on PD therapy, therefore, it represents a frequent patient complication in the series by De Vecchi et al [14], compared with the rate reported in a larger series of PD patients. However, sclerosing encapsulating peritonitis was also described in three cirrhotic patients that were never treated with PD [24, 25].
In addition, the presence of malnutrition in cirrhotic patients does not complicate PD outcomes. However, hypoalbuminemia might be complicated due to large daily peritoneal protein loss during the first month of PD therapy, decreasing with time [26]. The process of counterpressure exerted by dialysate might be responsible for reduction in formation of ascitic fluid [14]. It is theorized that counterpressure due to increased intraabdominal pressure opposes portal pressure and leads to decreased ascites formation, hence decreasing protein losses [3]. Improvement in albumin levels was clearly observed in all three patients. The loss of albumin was approximately 25 g/day for the first month of PD, thereafter, decreasing until it was minimal at the end of 2 years on PD in all patients. This was evident by increasing serum albumin levels. Table 5 shows serum and ascitic fluid albumin levels before and after PD in these patients, demonstrating an enormous reduction in loss of albumin after initiation of PD.
 Click to view | Table 5. Serum and Ascitic Fluid Albumin Before and After Peritoneal Dialysis |
Some studies have demonstrated that PD provides hemodynamic stability and adequate volume management compared with HD. Moreover, because PD obviates the need for therapeutic paracentesis by facilitating continuous drainage of ascites, the need for paracentesis is eliminated. Furthermore, small studies have suggested that outcomes such as peritonitis and mechanical complications are comparable to those in PD patients without ascites [9]. Although the urgent start of PD is a popular procedure, a minimum of a 2-week period should be allowed after the insertion of PD catheter in ESRD [9].
“Controlled paracentesis” has been described as slow and safe decompression of abdomen, after 2-L dialysis infusion and dwell, with drain restricted to 2,300 - 2,400 cc [2, 13]. This pattern of drainage-restricted exchanges allows eventual slow removal of ascites, although it might not remove adequate ascitic fluid. In three cases used in this study, however, patients had massive ascites, 1,800 - 2,200 cc/24 h and approximately 15,000 cc weekly, and SLVP was required twice weekly. We discovered that continuous drainage with a transfer set after insertion of PD catheter along with total drainage of ascitic fluid during surgery proved beneficial in avoiding leaks. All leakage stopped after establishing connection for continuous drainage. No incidence of peritonitis occurred. All three patients continued receiving ICHD three times weekly until 2 weeks after PD catheter insertion. Symptomatic hypotensive episodes occurring on each HD session ceased to occur after initiating PD. Suleyman et al infused pre-operative intravenous fresh frozen plasma (FFP) 15 mL/kg in a case with massive ascites. After the placement of a PD catheter, 1,000 cc of ascitic fluid was drained. Thereafter, catheter was flushed every day, draining 500 - 700 cc each time. The amount of dialysis exchanges was gradually increased to 4 per day [27].
Dialysis treatment can be a grueling process, as the presence of ascites further adds to challenges in these patients. Due to presence of ascites, hypovolemia is common secondary to low effective arterial blood volume in these patients. Rapid removal of fluid from the intervascular compartment during HD may produce severe hypovolemia and hypotension leading to hemodynamic instability [10-12]. This hemodynamic instability further leads to shortened treatments that compromises solute clearance and adequate fluid removal, increasing uremia and worsening ascites. This uremic state along with rapid osmotic shift during HD treatment can alter cerebral water content and predispose patients to encephalopathy [4, 8].
Patients with cirrhosis due to comorbid conditions have multiple abnormalities of hemostatic function increasing risk of bleeding as well as thrombosis [28-30]. Platelets can increase the risk of bleeding disorders. The risk of gastrointestinal hemorrhage and excessive bleeding at the site of dialysis access also increases due to use of anticoagulation in HD. Even in light of these deficiencies, use of PD remains nominal while HD continues to be the most prevalent dialysis modality among KF patients with ascites [9]. In a study using a nationwide inpatient sample, less than 1% of ESRD patients with cirrhosis and accompanying ascites were initiated on PD [31]. Inadequate research as well as the unwillingness of nephrologists to take risk for better outcomes can also be blamed for these poor statistics. PD provides hemodynamic stability through treatment that is slow and steady leading to continuous drainage of ascites with daily exchanges [31]. PD eliminates the need for anticoagulation hence, reducing the risk of hemorrhage [9]. Furthermore, PD may serve as a much needed source of calories in malnourished KF patients with ascites [32].
PD also provides better management of uremia. In a study by De Vecchi et al, weekly creatinine clearance was higher in patients with cirrhosis compared to non-cirrhotic ESRD patients [14].
Continuous drainage of ascites, as observed in our patients, obviates the need for significant glucose load during dialysis by using glucose concentration solutions as this minimized the adverse effects of hypertonic dextrose solution. Nevertheless, using glucose solutions, although in a lower concentration, provides an additional source of calories for patients with malnutrition and poor appetite. Hence, PD should be considered a preferred and most beneficial option in KF patients with ascites. Patients suffering from advanced cirrhosis and accompanying ascites are significantly malnourished due to inadequate dietary intake, impaired digestion and absorption, as well as altered metabolism of nutrients [33]. Additionally, cirrhosis is associated with decreased production of creatine, so creatinine levels remain low. These lower serum creatinine levels in patients with cirrhosis result in overestimation of the glomerular filtration rate. This delays diagnosis of KF and initiation of dialysis, resulting in a prolonged uremic state leading to further worsening of the patient’s nutritional status [34, 35]. Hypoalbuminemia is a known predictor of mortality in KF patients and an independent risk factor for peritonitis in PD [36-38]. Continued loss of albumin in PD treatments may exacerbate hypoalbuminemia in KF patients with ascites. This may lead to poor outcomes as well as morbidity and mortality. Nonetheless, majority of the literature contradicts this concept [9]. Our study supports this observation in all three of the patients. Similarly, a study involving 11 KF patients with cirrhosis observed no change in serum albumin at 6 and 12 months after PD initiation [14].
However, a minimal, insignificant drop in serum albumin from baseline was noted in patients with KF, cirrhosis, and ascites, with mean follow-up times of 4.5 and more than 6 years, respectively [16, 23]. Remarkably, Selgas et al described disproportionate protein losses (more than 30 g/dL) in the PD fluid initially, but this drastically reduced to 7 - 15 g/dL among eight KF patients with cirrhosis and accompanying ascites [13]. Similarly, the serum albumin level increased after an initial drop in an inverse relation to the peritoneal protein loss [13]. Improvement in uremia also led to improved appetite and better calorie intake, eventually increasing serum albumin levels. Majority of patients tolerate PD well, with minimal change in serum albumin [14, 16, 23]. Other studies have also mentioned some protein loss daily in PD fluid, but a notable change in serum albumin from the baseline value is not observed [13, 14, 16].
Cirrhosis enables translocation of gut microorganisms, predominantly Escherichia coli and other gram-negative bacteria, into the peritoneum that reduces function of peritoneal phagocytes and complement deficiency causing spontaneous bacterial peritonitis [39-42]. Apprehension of introducing PD in cirrhotic patients includes the concern that PD might increase the risk of peritonitis by adding PD catheter-related peritonitis risks to the inherent risk of spontaneous bacterial peritonitis in these patients. However, this is contradicted by most of the recent data. Peritonitis rates in cirrhotic and non-cirrhotic PD patients with ascites have been reported to be comparable by several studies [14, 16, 20, 24, 43].
In a series of nine KF patients with cirrhosis and ascites, an 8-year survival was observed, with six of the nine patients surviving more than 18 months with adequate control of uremia and fluid volume [24]. Comparable results were observed in two series of five and eight KF patients with cirrhosis, which noted hemodynamic stability with PD modality [2, 13]. Three of the patients in this study were switched from HD due to continuous hemodynamic instability [2, 13]. In majority of the studies, mortality was related to complications of cirrhosis and not from PD-associated issues. Ranganathan et al recommended initiation can be delayed promoting healing of the surgical site and reduction in risks of catheter complications by 2 - 4 weeks. However, even if PD initiation is delayed, the ascitic fluid should be drained and the catheter concomitantly flushed to ensure patency and monitor any leaks [44].
There is no firm opinion on the amount of ascitic fluid to be initially drained. During initial visits, drainage of 5 - 6 L of ascitic fluid has been described [13]. On successive visits, various strategies have been applied to determine the volume to be drained. Some centers have drained 10% to 20% extra fluid over the instilled fill volume (FV) with every exchange [2, 13]. Others have drained 400 - 600 mL above the instilled FV until the ascitic fluid is completely drained [3, 14, 45]. Chaudhary et al reported use of a purse string suture in the posterior fascia to control the leakage [3]. Despite the purse string suture, profuse leak may occur for several days after insertion of catheter in cirrhotic patients with ascites. Some centers have infused albumin while draining ascites [2, 3, 13, 14, 16, 45]. There is no consensus on the initial volume of dialysate to be instilled [9]. While Selgas et al [13] initiated with 2 L FV from the outset, De Vecchi et al [14] started with 1,000 mL, and Lee et al [45] used 500 mL with stepwise increase to 2 L. In general, a typical starting FV is 500 - 750 mL. FV should be gradually increased every 3 - 4 days or as tolerated, while being vigilant for leaks and overfills, until the patient reaches a maximum prescribed FV, usually 1.5 - 2.5 L within a few weeks. Supine position is best described to perform for complete healing of catheter site [9].
PD offers several advantages over HD in patients with ESRD and cirrhosis with concomitant ascites, such as permanent drainage of ascitic fluid, elimination of anticoagulation, and increased hemodynamic stability [46]. Intradialytic hypotension in cirrhotic patients remains a major issue. Slow removal of fluid in PD eliminates the concern of hypotension. HD-related hypotension has been associated with increased risk of overall mortality [47]. In cirrhotic patients who received dialysis, cardiovascular mortality was a major cause of death (52.4% in HD and 48% in PD patients) [23].
Intradialytic hypotension is a major concern with use of HD in cirrhotic patients, leading to shortened treatments and frequent volume overload. Cirrhotic patients with ascites have decreased peripheral vascular resistance due to high circulating levels of nitric oxide [10, 48]. To avoid hypotension, cirrhotic patients conserve fluid and salt and increase cardiac output; the abrupt changes in intravascular volume by ultrafiltration during HD exacerbate hypotension [3]. HD can also result in higher levels of nitric oxide, further complicating this [11, 49]. In summary, PD has proven to be a safe and practical dialysis modality in patients with KF and cirrhosis with ascites by providing hemodynamic stability and simplified volume management compared with HD. Continuous drainage of ascites eliminates the need for SLVP. Reduced use or elimination of anticoagulants in PD patients diminishes hemorrhagic complications. Peritonitis and other mechanical complications in these patients are comparable to the general PD population. Although an initial drop in serum albumin level is noted, this is temporary and does not affect the result of stability provided by PD.
Conclusion
In patients with ESRD and tense ascites requiring SLVP, this study suggests continuous drainage immediately after PD catheter insertion to prevent massive leak. Continuous drainage of 2 - 3 weeks results in adequate healing of the surgical site and prepares patient for successful PD modality. Switching from HD to PD in these complicated cases can be much easier with deployment of this technique. This opens the path to provide quality treatment options for patients with kidney and liver failure despite of cirrhosis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None of the authors declare any conflict of interest.
Informed Consent
Informed consents were obtained from the patients.
Author Contributions
Sushil K. Mehandru: conceptualization, project administration, investigation methodology, and original draft; Supreet Kaur: investigational research and original draft; Avais Masud: investigational research and validation; Kyrillos Rezkalla: data collection and analysis, data curator; Qalb Khan: data collection, analysis, and validation; Prit Paul Singh: data collection and analysis; Walid Abboud: literature research and resources; Tushar Vachharajani: review and editing; Arif Asif: final review and editing.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
CKD: chronic kidney disease; ESRD: end-stage renal disease; FFP: fresh frozen plasma; FV: fill volume; HD: hemodialysis; ICHD: in-center hemodialysis; KF: kidney failure; LVP: large volume paracentesis; PD: peritoneal dialysis; RRT: renal replacement therapy; SLVP: super large volume paracentesis
| References | ▴Top |
- Ros Ruiz S, Gutierrez Vilchez E, Garcia Frias TP, Martin Velazquez TM, Blanca Martos L, Jimenez Salcedo T, Hernandez Marrero D. The role of peritoneal dialysis in the treatment of ascites. Nefrologia. 2011;31(6):648-655.
doi pubmed - Bajo MA, Selgas R, Jimenez C, Del Peso G, Fernandez-Reyes MJ, Dapena F, De Alvaro F. CAPD for treatment of ESRD patients with ascites secondary to liver cirrhosis. Adv Perit Dial. 1994;10:73-76.
pubmed - Chaudhary K, Khanna R. Renal replacement therapy in end-stage renal disease patients with chronic liver disease and ascites: role of peritoneal dialysis. Perit Dial Int. 2008;28(2):113-117.
pubmed - Donovan JP, Schafer DF, Shaw BW, Jr., Sorrell MF. Cerebral oedema and increased intracranial pressure in chronic liver disease. Lancet. 1998;351(9104):719-721.
doi pubmed - Rubin J, Kiley J, Ray R, McFarland S, Bower J. Continuous ambulatory peritoneal dialysis. Treatment of dialysis-related ascites. Arch Intern Med. 1981;141(8):1093-1095.
pubmed - Gluck Z, Nolph KD. Ascites associated with end-stage renal disease. Am J Kidney Dis. 1987;10(1):9-18.
doi pubmed - Mactier RA, Dobbie JW, Khanna R. Peritoneal dialysis in fulminant hepatic failure. Perit Dial Bull. 1986;6:199-202.
- Winney RJ, Kean DM, Best JJ, Smith MA. Changes in brain water with haemodialysis. Lancet. 1986;2(8515):1107-1108.
doi pubmed - Rajora N, De Gregorio L, Saxena R. Peritoneal dialysis use in patients with ascites: a review. Am J Kidney Dis. 2021;78(5):728-735.
doi pubmed pmc - Martin PY, Gines P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339(8):533-541.
doi pubmed - Yokokawa K, Mankus R, Saklayen MG, Kohno M, Yasunari K, Minami M, Kano H, et al. Increased nitric oxide production in patients with hypotension during hemodialysis. Ann Intern Med. 1995;123(1):35-37.
doi pubmed - Wilkinson SP, Weston MJ, Parsons V, Williams R. Dialysis in the treatment of renal failure in patients with liver disease. Clin Nephrol. 1977;8(1):287-292.
pubmed - Selgas R, Bajo MA, Del Peso G, Sanchez-Villanueva R, Gonzalez E, Romero S, Olivas E, et al. Peritoneal dialysis in the comprehensive management of end-stage renal disease patients with liver cirrhosis and ascites: practical aspects and review of the literature. Perit Dial Int. 2008;28(2):118-122.
pubmed - De Vecchi AF, Colucci P, Salerno F, Scalamogna A, Ponticelli C. Outcome of peritoneal dialysis in cirrhotic patients with chronic renal failure. Am J Kidney Dis. 2002;40(1):161-168.
doi pubmed - Tse KC, Li FK, Tang S, Chan TM, Lai KN. Peritoneal dialysis in patients with refractory ascites. Perit Dial Int. 2001;21(6):626-627.
pubmed - Jones RE, Liang Y, MacConmara M, Hwang C, Saxena R. Peritoneal dialysis is feasible as a bridge to combined liver-kidney transplant. Perit Dial Int. 2018;38(1):63-65.
doi pubmed - Kim AJ, Lim HJ, Ro H, Jung JY, Lee HH, Chung W, Chang JH. Liver cirrhosis leads to poorer survival in patients with end-stage renal disease. Korean J Intern Med. 2016;31(4):730-738.
doi pubmed pmc - Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC, Taiwan Society of Nephrology. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant. 2010;25(8):2616-2624.
doi pubmed - Chien CC, Wang JJ, Sun YM, Sun DP, Sheu MJ, Weng SF, Chu CC, et al. Long-term survival and predictors for mortality among dialysis patients in an endemic area for chronic liver disease: a national cohort study in Taiwan. BMC Nephrol. 2012;13:43.
doi pubmed pmc - Huang ST, Chuang YW, Cheng CH, Wu MJ, Chen CH, Yu TM, Shu KH. Outcome of peritoneal dialysis in cirrhotic patients with end-stage renal disease: a 24-years’ experience in Taiwan. Clin Nephrol. 2011;76(4):306-313.
doi pubmed - Minino AM, Heron MP, Murphy SL, Kochanek KD, Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics System. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55(19):1-119.
pubmed - Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931-940.
doi pubmed - Chou CY, Wang SM, Liang CC, Chang CT, Liu JH, Wang IK, Hsiao LC, et al. Peritoneal dialysis is associated with a better survival in cirrhotic patients with chronic kidney disease. Medicine (Baltimore). 2016;95(4):e2465.
doi pubmed pmc - Marcus RG, Messana J, Swartz R. Peritoneal dialysis in end-stage renal disease patients with preexisting chronic liver disease and ascites. Am J Med. 1992;93(1):35-40.
doi pubmed - Carbonnel F, Barrie F, Beaugerie L, Houry S, Chatelet F, Gallot D, Malafosse M, et al. [Sclerosing peritonitis. A series of 10 cases and review of the literature]. Gastroenterol Clin Biol. 1995;19(11):876-882.
pubmed - Selgas R, Bajo MA, Jimenez C, Sanchez C, Del Peso G, Cacho G, Diaz C, et al. Peritoneal dialysis in liver disorders. Perit Dial Int. 1996;16(Suppl 1):S215-S219.
pubmed - Koz S, Sahin I, Terzi Z, Koz ST. Course of encephalopathy in a cirrhotic dialysis patient treated sequentially with peritoneal and hemodialysis. Case Rep Med. 2015;2015:375456.
doi pubmed pmc - Intagliata NM, Argo CK, Stine JG, Lisman T, Caldwell SH, Violi F, Faculty of the 7th International Coagulation in Liver Disease. Concepts and controversies in haemostasis and thrombosis associated with liver disease: Proceedings of the 7th International Coagulation in Liver Disease Conference. Thromb Haemost. 2018;118(8):1491-1506.
doi pubmed pmc - Marks PW. Hematologic manifestations of liver disease. Semin Hematol. 2013;50(3):216-221.
doi pubmed - Northup PG, Friedman LS, Kamath PS. AGA clinical practice update on surgical risk assessment and perioperative management in cirrhosis: expert review. Clin Gastroenterol Hepatol. 2019;17(4):595-606.
doi pubmed - Nader MA, Aguilar R, Sharma P, Krishnamoorthy P, Serban D, Gordon-Cappitelli J, Shen W, et al. In-hospital mortality in cirrhotic patients with end-stage renal disease treated with hemodialysis versus peritoneal dialysis: a nationwide study. Perit Dial Int. 2017;37(4):464-471.
doi pubmed - Kotla SK, Saxena A, Saxena R. A model to estimate glucose absorption in peritoneal dialysis: a pilot study. Kidney360. 2020;1(12):1373-1379.
doi pubmed pmc - Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol. 2012;10(2):117-125.
doi pubmed - Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10(2):301-309.
doi pubmed - Asrani SK, Jennings LW, Trotter JF, Levitsky J, Nadim MK, Kim WR, Gonzalez SA, et al. A model for glomerular filtration rate assessment in liver disease (GRAIL) in the presence of renal dysfunction. Hepatology. 2019;69(3):1219-1230.
doi pubmed - Wang Q, Bernardini J, Piraino B, Fried L. Albumin at the start of peritoneal dialysis predicts the development of peritonitis. Am J Kidney Dis. 2003;41(3):664-669.
doi pubmed - Fox L, Tzamaloukas AH, Murata GH. Metabolic differences between persistent and routine peritonitis in CAPD. Adv Perit Dial. 1992;8:346-350.
pubmed - Sharma AP, Gupta A, Sharma RK, Agarwal DK, Sural S, Wardhe DJ. Does serum albumin at start of continuous ambulatory peritoneal dialysis (CAPD) or its drop during CAPD determine patient outcome? Adv Perit Dial. 2000;16:119-122.
pubmed - Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, Rodes J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35(1):140-148.
doi pubmed - Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27(4):669-674.
doi pubmed - Niu B, Kim B, Limketkai BN, Sun J, Li Z, Woreta T, Chen PH. Mortality from spontaneous bacterial peritonitis among hospitalized patients in the USA. Dig Dis Sci. 2018;63(5):1327-1333.
doi pubmed pmc - Runyon BA. Patients with deficient ascitic fluid opsonic activity are predisposed to spontaneous bacterial peritonitis. Hepatology. 1988;8(3):632-635.
doi pubmed - Chow KM, Szeto CC, Wu AK, Leung CB, Kwan BC, Li PK. Continuous ambulatory peritoneal dialysis in patients with hepatitis B liver disease. Perit Dial Int. 2006;26(2):213-217.
pubmed - Ranganathan D, John GT, Yeoh E, Williams N, O'Loughlin B, Han T, Jeyaseelan L, et al. A randomized controlled trial to determine the appropriate time to initiate peritoneal dialysis after insertion of catheter (timely PD study). Perit Dial Int. 2017;37(4):420-428.
doi pubmed - Lee SM, Son YK, Kim SE, An WS. Clinical outcomes of peritoneal dialysis in end-stage renal disease patients with liver cirrhosis: a propensity score matching study. Perit Dial Int. 2017;37(3):314-320.
doi pubmed - Burguera Vion V, Sosa Barrios RH, Cintra Cabrera M, Sofia O, Marta P, Yael C, Cristina C, et al. FP604 peritoneal dialysis (PD) in end-stage renal disease patients (ESRD) with liver cirrhosis: one center experience. Nephrol Dial Transpl. 2019;34(Supplement_1):gfz106.FP604.
doi - Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66(3):1212-1220.
doi pubmed - Chin-Dusting JP, Rasaratnam B, Jennings GL, Dudley FJ. Effect of fluoroquinolone on the enhanced nitric oxide-induced peripheral vasodilation seen in cirrhosis. Ann Intern Med. 1997;127(11):985-988.
doi pubmed - Kang ES, Acchiardo SR, Kang AH. Implications for the role of endogenous nitric oxide inhibitors in hemodialysis hypotension. Free Radic Res. 2001;35(4):341-365.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


