| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 4, April 2023, pages 141-147
A Case Series of Non-Tuberculous Mycobacterial Pulmonary Disease Masquerading as Malignancy From a Community-Based Hospital
Eltaib Saada, d, Maria Abunseira, Mohammed S. Abdallaa, Abdurrahman Mustafaa, Mohammed Elamin Farisa, Harvey Friedmanb, c
aDepartment of Internal Medicine, Ascension Saint Francis Hospital, Evanston, IL, USA
bDepartment of Critical Care and Pulmonology, Ascension Saint Francis Hospital, Evanston, IL, USA
cUniversity of Illinois College of Medicine, IL, USA
dCorresponding Author: Eltaib Saad, Department of Internal Medicine, Ascension Saint Francis Hospital, Evanston, IL, USA
Manuscript submitted April 10, 2023, accepted April 20, 2023, published online April 30, 2023
Short title: NTM-PD Masquerading as Malignancy
doi: https://doi.org/10.14740/jmc4098
| Abstract | ▴Top |
Non-tuberculous mycobacteria (NTM) are ubiquitous organisms in the environment that can potentially cause a range of pulmonary and extrapulmonary infections in humans. Epidemiological risk factors and the host’s immune status determine the susceptibility to various clinical syndromes caused by different NTM species. Non-tuberculous mycobacteria pulmonary disease (NTM-PD) is primarily reported in patients with underlying lung disease. These infections often pose a significant disease burden on affected patients as they are often chronic, difficult to treat, and necessitate long-term multi-drug therapy. Mycobacterium avium complex (MAC) is the most common causative pathogen of NTM-PD in the USA, followed by Mycobacterium kansasii (M. kansasii). Less common species in the USA include Mycobacterium xenopi (M. xenopi), Mycobacterium abscessus, and others, largely depending upon the geographic location and exposure to species-specific predisposing risks. In this case series, the authors report on three elderly patients with chronic lung diseases who had pulmonary NTM disease caused by M. xenopi and MAC. The patients were encountered in both inpatient and outpatient settings from a community-based hospital in the midwestern USA. The clinical and radiological features of NTM-PD masqueraded as malignancy and posed a diagnostic dilemma. The epidemiology, clinical and radiological features, diagnosis, and management of NTM-PD are reviewed in this report.
Keywords: Non-tuberculous mycobacteria pulmonary disease; Mycobacterium avium complex; Mycobacterium xenopi; Chronic lung disease; Multi-drug therapy
| Introduction | ▴Top |
Non-tuberculous mycobacteria (NTM) are a heterogeneous group of free-living organisms that have the potential to cause pulmonary and less commonly extrapulmonary infections in humans [1, 2]. NTM infections exhibit a wide geographical variation worldwide [1-4]. Mycobacterium avium complex (MAC) is the most reported species causing pulmonary disease globally, followed variably by Mycobacterium kansasii (M. kansasii), Mycobacterium xenopi (M. xenopi), and other NTM, largely depending on geographic location [3, 4]. M. xenopi is the second most pathogen of pulmonary NTM infections in Canada, southwest Europe, and UK [4], while M. kansasii is only behind MAC as an isolated organism of non-tuberculous mycobacteria pulmonary disease (NTM-PD) in the USA, predominately Southwest and Midwest regions of the USA [5]. The usual infection route is inhaling airborne particles from an environmental source [1-3]. NTM infections are universally not contagious [1, 3].
The majority of NTM-PD were reported in hosts with underlying lung diseases, mostly chronic obstructive pulmonary disease (COPD), cystic fibrosis, bronchiectasis, and prior mycobacterial tuberculosis [6]. Despite immune deficiency being considered a risk factor for NTM lung infections, immunocompromised patients comprise only a small population of those with pulmonary disease, in contrast to typical mycobacterial tuberculosis [6]. The clinical features of NTM lung disease are variable and usually mimic the symptoms of pre-existing lung diseases [4, 6, 7].
The diagnosis of NTM-PD is challenging, partly because of the nonspecific nature of their clinical features and radiological characteristics [1, 4, 6]. The diagnosis typically requires specific microbiological criteria in the presence of pulmonary symptoms and suggestive radiological findings [2, 6]. The microbiological criteria entail either two positive sputum cultures, a positive culture from a bronchial wash or lavage, or a lung biopsy with histologic findings of mycobacterial infection in the presence of a positive culture [7].
Herein, the authors present a series of three elderly patients with chronic lung diseases, who were diagnosed with NTM-PD from a community-based hospital in the midwestern USA. The infections were caused by M. xenopi in two patients and MAC in the third patient. The NTM-PD masqueraded clinically and radiologically as lung malignancy, which posed a diagnostic dilemma. The current literature reporting on the epidemiology, clinical and radiological features, diagnosis, and management of NTM-PD is briefly reviewed in this communication.
| Case Reports | ▴Top |
Case 1
Investigations
A 67-year-old male with a past medical history of heavy smoking (a 55-pack-year), emphysema, and occupational asbestos exposure, presented initially to the pulmonary outpatient clinic for abnormal findings on a low-dose computed tomography (CT) scan for lung cancer screening. The patient was asymptomatic, and the physical exam was only notable for a hyperinflated chest wall with decreased breath sounds bilaterally. The low-dose chest CT scan showed diffuse emphysema with large bulla/cavities most significantly in the upper lobes and bilateral small lung nodules. A follow-up CT was recommended within 3 months for close monitoring of the nodular lesions, but the patient lost to follow-up. After 2 years, the patient presented to the pulmonary clinic with exertional shortness of breath and a productive cough for several weeks. The patient’s symptoms only partially improved with courses of oral antibiotic therapy and bronchodilator inhalers. A CT scan of the chest redemonstrated a similar appearance of severe emphysema and thick-walled apical bullae with an interval progression in the number and size of left-sided pulmonary nodules (Fig. 1a). Positron emission tomography (PET) demonstrated only a hyper-metabolic uptake in the prostate region. He was referred to the urology service, and further workup ruled out prostate cancer. The patient’s complaints continued to progress despite regular compliance with the prescribed inhalers, and he lost significant weight over 3 months’ time.
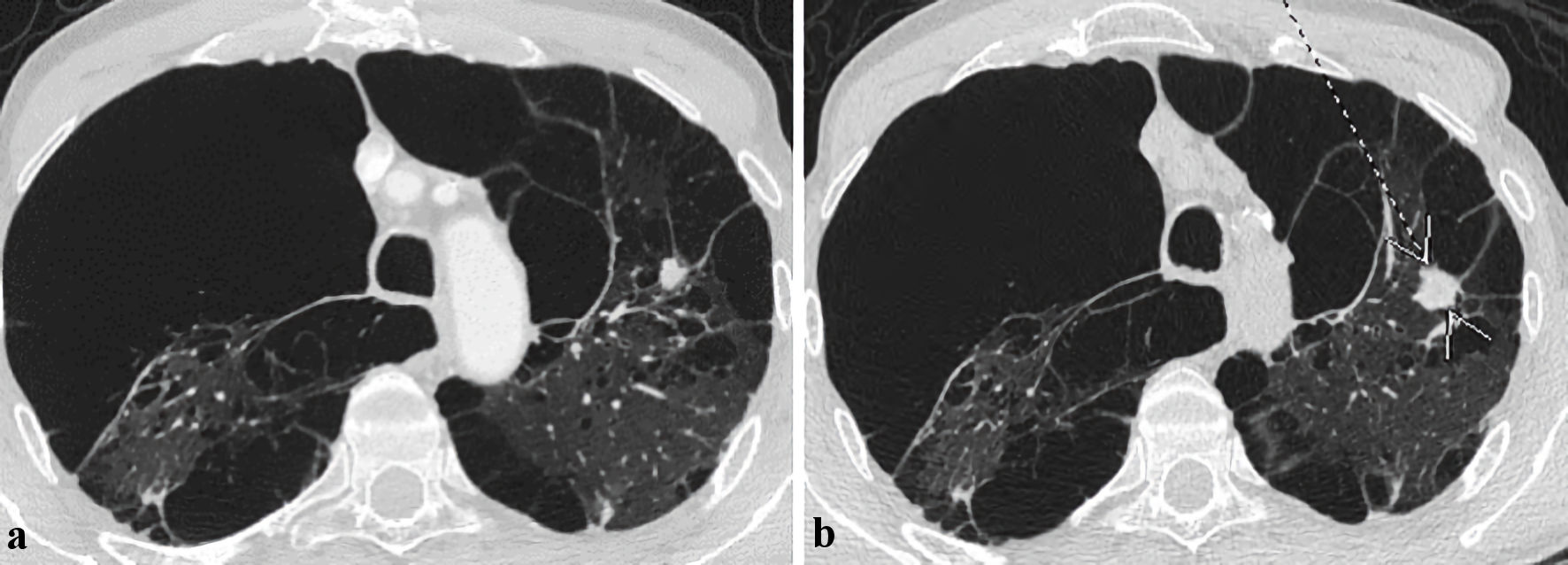 Click for large image | Figure 1. Axial images of computed tomography (CT) scans of the chest demonstrating interval progression of the left-sided lung nodules in the upper lobe over a period. (a) Previous CT chest scan 2 years prior to presentation. (b) CT chest scan on index presentation (arrow and pointer showing interval nodule size progression). |
Diagnosis
A repeat CT chest scan depicted an interval progression of the left lung nodular processes in the left upper lobe (Fig. 1b) without significant mediastinal lymphadenopathy. Other lung pathologies were fairly stable. Sputum Gram stain and culture were negative for any growth. Urine antigens for fungal pathogens were unrevealing. Serial expectorant morning sputum samples were positive for many acid-fast bacilli (AFB), and cultures grew M. xenopi, which was further identified by the direct matrix-associated laser desorption (MALDI) technique. Sputum polymerase chain reaction (PCR) was negative for Mycobacterium tuberculosis (M. tuberculosis) and MAC. A diagnosis of an atypical mycobacterial infection with M. xenopi was made based on a combination of a suggestive clinical history with possible radiological findings and confirmatory microbiology.
Treatment
The patient commenced triple therapy comprising a multi-drug regimen of rifampin, ethambutol, and azithromycin after the diagnosis was made.
Follow-up and outcomes
The patient’s symptoms started to gradually improve with the triple therapy and a repeat CT chest revealed an interval reduction in the size of the left upper lobe lesion.
Case 2
Investigations
A 73-year-old female with a medical history significant for heavy smoking (a 25-pack-year), daily marijuana use, long-standing COPD with bullous emphysema, and prior unprovoked pulmonary embolism on anticoagulation, presented to the pulmonary outpatient clinic with increasing tiredness and worsening productive cough for a few months. The patient endorsed regular compliance with her medications, but she admitted to continuing active smoking. Notably, the patient has a history of cavitary and nodular lung disease under yearly surveillance. Her last PET scan 7 years prior to her index presentation was concerning for abnormal hyper-metabolic uptake in an upper right lobe cavitary lesion. Subsequently, she underwent endobronchial ultrasound (EBUS) which ruled a neoplastic process but revealed a non-caseating granuloma. Extensive infectious workup at that time (i.e., AFB stains, and serial cultures of the sputum, bronchial lavage, and lung biopsy) did not yield any infectious culprit for the granulomatous pathology, particularly, mycobacterial, and fungal etiologies. The patient remained relatively asymptomatic for the interval 6-year period with fairly stable cavitary and nodular lesions appearances over the serial scans. The physical examination revealed a low body mass index (BMI) of 16.5, which was significantly reduced from the prior visit 1 year ago. The respiratory examination showed reduced breathing sounds bilaterally with diffuse expiratory wheezes. An acute exacerbation of COPD was suspected, and she was commenced on an albuterol inhaler, a short course of prednisone, and oral antibiotics. However, the patient’s symptoms were not adequately controlled.
Diagnosis
A CT chest demonstrated an interval significant progression of the right upper lobe cavity lesion (3.2 × 1.7 cm) (Fig. 2a) from the prior exam (1.1 × 0.8 cm) which was nearly 1 year ago (Fig. 2b). The bilateral nodular lesions and advanced emphysematous bullous disease were relatively unchanged. A PET scan depicted no significant hyper-metabolic activity in the right upper cavitary lesion at this time. An interdisciplinary review of the serial CT images suggested a possibility of a chronic infectious process given the low metabolic activity of the lesion. Nevertheless, the possibility of lung malignancy remained a concern considering the patient’s high-risk status and the patient has been counseled about further invasive workup if needed. Sputum cultures grew M. xenopi, which was identified by the MALDI technique. Sputum PCR was negative for M. tuberculosis and MAC.
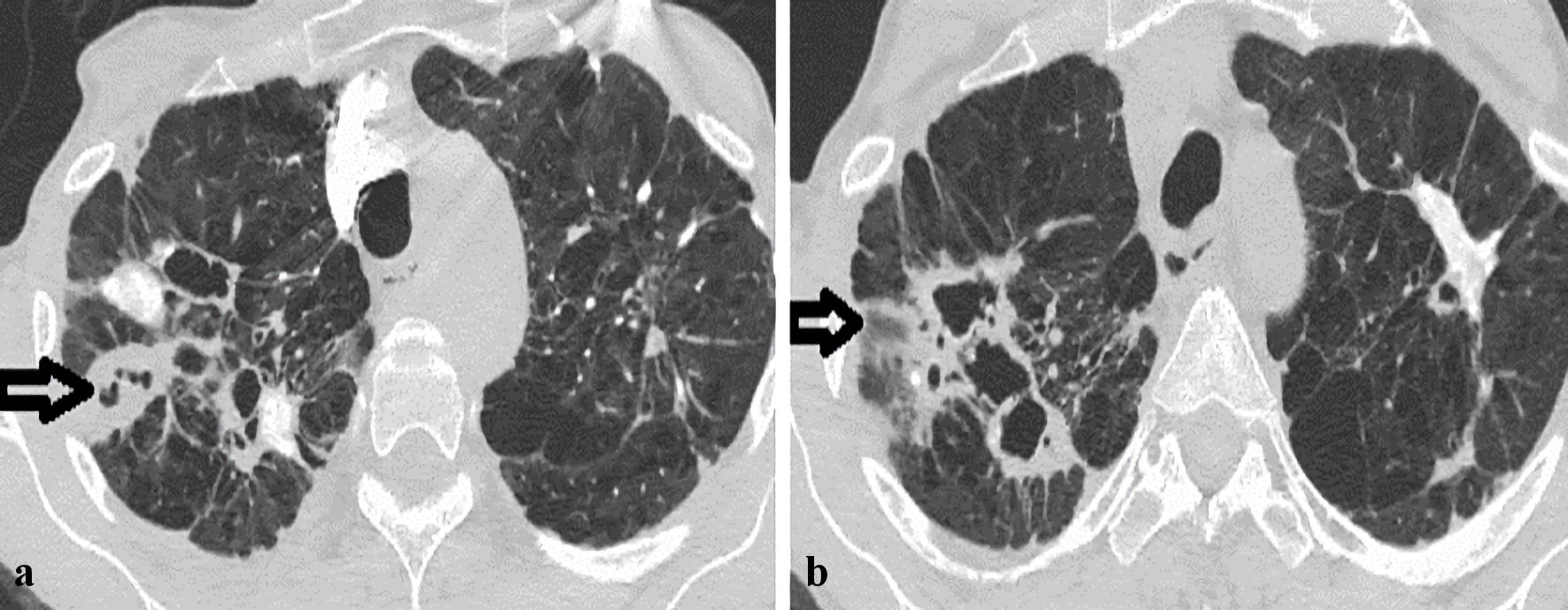 Click for large image | Figure 2. Axial images of computed tomography (CT) scans of the chest demonstrating interval progression of the right upper cavitary over 1 year (horizontal arrows). (a) CT chest scan on presentation. (b) Previous CT chest scan over 1 year prior to presentation. |
Treatment
After the diagnosis was made, triple therapy (azithromycin, rifampin, and ethambutol) was started.
Follow-up and outcomes
A repeat CT chest imaging was planned after 8 weeks from initiating anti-tuberculous therapy to evaluate the clinical and radiological response.
Case 3
Investigations
A 74-year-old male patient with a past medical history of heavy smoking (a 60-pack-year), advanced emphysema on 3 L of oxygen at home, bronchiectasis, remote history of bladder cancer status post-surgical resection and chemotherapy, presented to our emergency department with exertional dyspnea and fatigue for 1 day. He also reported a chronic cough and intermittent sputum production. The patient was discharged from our hospital 2 days prior to his index presentation following a short-coursed admission with an acute exacerbation of COPD due to coronavirus disease 19 (COVID-19) infection. The patient had no increase in supplemental oxygen requirement at that admission. The patient improved with supportive management with analgesics, albuterol nebulizers, dexamethasone, and COVID-directed therapy (remdesivir). Notably, the patient had several admissions to the medical floor with acute flare-ups of COPD in the past few months. The patient is originally from Iraq and immigrated to the USA 10 years ago, but he denied any recent traveling outside the USA. Initial evaluation revealed a frail and underweight elderly patient (BMI of 16.5) with signs of acute respiratory distress. He was afebrile with sinus tachycardia (heart rate of 110 beats per minute), tachypnea (respiratory rate of 34 breaths per minute), and stable blood pressure. The lung examination was significant for decreased breathing sounds bilaterally and mild diffuse expiratory wheezing.
Diagnosis
Chest X-ray (CXR) showed stable reticulonodular and emphysematous changes with a volume loss in the left upper lung from scarring (Fig. 3a). CT chest demonstrated diffuse random-sized nodules with an ill-defined cavitary lesion in the posterior aspect of the left upper lobe, extending inferiorly into a more solid component along the pleura, which was seen in a previous CT chest that was performed 1 month ago (Fig. 3b, c). Further review of prior images revealed that the cavitary lesion was visualized 1.5 years ago on imaging performed at an outside hospital to rule out pulmonary embolism, but it has progressed in size during the index imaging. The patient was started on empirical cefepime and vancomycin, nebulizer, and dexamethasone for possible worsening bronchiectasis. An infectious workup revealed positive sputum for Pseudomonas aeruginosa, but serial blood cultures remained negative. Urine antigens for histoplasmosis, and blastomycosis were also negative. Consecutive sputum cultures grew many AFB, which was identified as MAC. Sputum PCR was negative for M. tuberculosis.
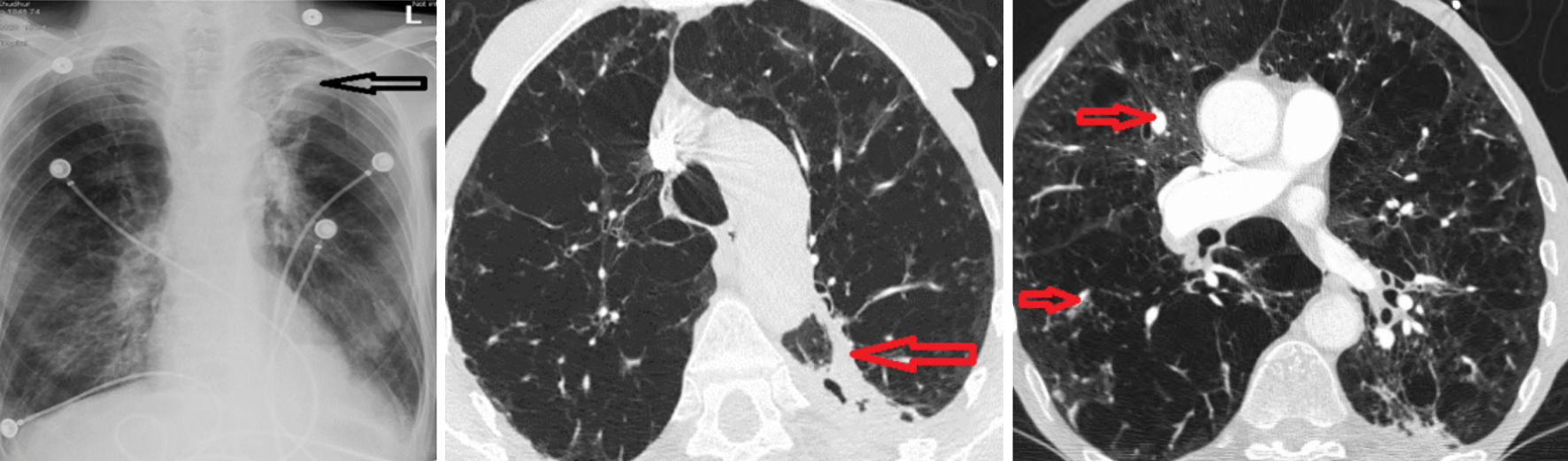 Click for large image | Figure 3. (a) Chest X-ray with the reticulonodular process and a loss of volume in the left upper lobe (horizontal arrow). (b) Contrast-enhanced CT chest revealed a cavitary lesion in the posterior aspect of the left upper lobe (LUL) with a solid component (horizontal arrow). (c) Diffuse nodular lesions (small horizontal arrows). |
Treatment
A multi-drug therapy regime of rifampicin, azithromycin, and ethambutol was started for MAC. The parenteral antibiotic therapy was transitioned to levofloxacin upon discharge to complete a 4-week course.
Follow-up and outcomes
The patient continued MAC therapy with close follow-up with pulmonary and infectious disease services.
| Discussion | ▴Top |
The exact incidence of NTM-PD in the USA is not precisely known, as the disease is not mandatory reportable in most states [7].
There are four distinct clinical syndromes described with various NTM species in humans [8, 9]; pulmonary disease, especially in elderly patients with chronic lung disease, is caused primarily by MAC, both globally and in the USA [1, 2, 7], followed by M. kansasii, particularly in midwestern regions of the USA [5]. Less common species include M. xenopi, which is more prevalent in Canada, southwest Europe, and the UK [4]. Superficial lymphadenitis, commonly cervical lymphadenitis in the pediatric population, is largely caused by MAC and Mycobacterium scrofulaceum (M. scrofulaceum) [9]. Mycobacterium marinum (M. marinum) and Mycobacterium ulcerans (M. ulcerans) are the principal pathogens for skin and soft tissue infections that are acquired through direct inoculation, and they may also cause surgical site infections [9]. The disseminated disease is typically noted in severely immunocompromised patients (mostly caused by MAC) [9].
Our case series demonstrated NTM-PD in three elderly patients with COPD and significant smoking history, consistent with the literature reported above [1, 6, 7, 9]. Nevertheless, M. xenopi was isolated in two out of three patients in this series, a finding that is not much in keeping with the epidemiological pattern of NTM-PD in the midwestern USA, where those patients were diagnosed [5]. The reported patients had no history of recent traveling to regions where M. xenopi is more prevalent.
The clinical presentation of MAC and M. xenopi lung disease of our patients is similar to those in the literature [1, 9, 10]. The symptoms of NTM lung diseases are nonspecific (i.e., dry or productive cough, fatigue, dyspnea, and occasionally hemoptysis) [1, 9]. They closely resemble the symptoms of the underlying lung disease, thus making clinical suspicion quite difficult [9].
The clinical course of MAC is generally less progressive compared to mycobacterial tuberculosis [10]. Extensive lung parenchymal destruction is often evident at the time of MAC diagnosis, partly because of the indolent nature of the disease [9, 10]. Disseminated disease is usually associated with severe cell-mediated immunity deficiency [9]. A less frequent variant of MAC lung disease is notably reported in older women without prior overt lung disease or smoking history, which is characterized by a right middle lobe or lingular infiltrate; referred to as the Lady Windermere syndrome [11]. The least common MAC variant is described in relatively younger immunocompetent hosts with significant exposure to hot tubs [9]. The latter variant is considered a hypersensitivity pneumonitis-like reaction to aerosolized MAC from hot tubs [9].
M. xenopi is a low-virulent NTM that emerged as an opportunistic pathogen for pulmonary disease and less commonly extrapulmonary infections (mostly osteoarticular infections) [12]. A comprehensive review of 136 cases of M. xenopi lung disease from a single tertiary center in France described three disease patterns; cavitary disease in patients with chronic lung disease, mostly COPD (as observed in our reported patients), acute infiltrates in immunocompromised hosts, and rarely solitary nodular disease in immunocompetent patients [13].
A retrospective study of 74 patients from a single institution in Canada by Carrillo et al [2] compared the radiological characteristics of the lung disease caused by MAC and M. xenopi [2]. The study noted that patients with M. xenopi were relatively younger and more often had advanced emphysema and lung malignancy [2]. MAC patients mostly had a nodular bronchiectatic pattern, followed by a cavitary pattern (which was noted in our MAC patient) [2]. While the cavitary pattern tended to predominately occur in M. xenopi infection, the majority of patients had variable patterns of random nodules, consolidation, bronchiectasis, tree-in-bud appearance, and ground-glass opacities [2]. However, these imaging patterns were not statistically significant and were neither pathognomonic for MAC nor M. xenopi [2]. The cavitation process of M. xenopi typically involves the upper lobes, as observed in the second patient with M. xenopi infection [7].
Interestingly, M. xenopi infection was reportedly associated with lung malignancy [14]. This suggested association was postulated by scar tissues that may potentially undergo a malignant transformation [2, 14]. Heavy smoking, as a risk factor for lung cancer, is also prevalent among M. xenopi patients, which is an observation that may partly explain the coincidence of M. xenopi and malignancy [14]. In the review of Carrillo et al [2], about one-fourth (25%) of patients with M. xenopi had prior or concurrent lung cancer [2]. The concern of lung malignancy was evident in our M. xenopi patients, considering their suggestive clinical history and the suspicious radiological findings on initial presentation. A similar case reported by Fogla et al [15] described an elderly, heavy-smoker, male patient who had an upper lobe cavitary lesion on imaging. Percutaneous needle biopsy and bronchial lavage did not yield neoplastic cells but necrotic tissues. The patient underwent a wedge resection because of a high malignancy suspicion; histology demonstrated caseating granulomas with positive AFP, and further molecular studies with 16S RNA confirmed M. xenopi infection and ruled out typical tuberculosis [15]. Nevertheless, the small possibility of concurrent lung cancer cannot be entirely ruled out in our presented patients.
Microbiological confirmation remains the gold standard to diagnose NTM-PD, as their clinical course and radiological findings greatly mimic typical mycobacterial, fungal, and neoplastic lung diseases [3, 8]. Specific diagnostic criteria were established by the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA) [7]. These criteria require at least two positive expectorated sputum cultures, a positive sputum culture from a bronchial wash or lavage, and a lung biopsy with histologic findings of mycobacterial infection (granulomatous inflammation or AFB) in the presence of a positive NTM culture [7].
Nevertheless, positive sputum results should be interpreted cautiously as these NTM have variable virulence, therefore, they can be only transiently recovered from the respiratory tract without causing a progressive infection [9]. In addition, NTMs may contaminate laboratory specimens, for instance, tap water (liquid or frozen) may contain NTMs that can potentially contaminate these specimens [9]. Fortunately, highly accurate nucleic acid probes are becoming readily available for faster identification of NTM, including MAC and M. xenopi [16].
Management of NTM-PD remains challenging. As the infection is usually chronic, a multi-drug regimen is warranted, and there are no randomized comparative trials that evaluated the currently employed regimens [17]. The ATS/IDSA treatment guidelines from 2007 include a combination of a macrolide with rifampin and ethambutol, which should be continued until sputum cultures remain negative for 12 months for MAC [7], with the same guidelines being currently recommended for M. xenopi [7]. In contrast, the British Thoracic Society (BTS) guidelines from 2017 recommend initial treatment with four medications including a macrolide, rifampin, and ethambutol combined with either a fluoroquinolone or isoniazid for NTM infections [18]. These guidelines are based on limited evidence obtained from non-randomized controlled trials [19].
Surgical intervention for NTM-PD is currently limited only to patients who failed medical therapy (i.e., remained sputum positive after 6 months of continued anti-tuberculous therapy), those who cannot tolerate various medications, and hosts with macrolide-resistant strains [20].
Conclusions
This case series highlights NTM-PD as an uncommon etiology of cavitary and nodular lung disease in three elderly patients with underlying lung conditions. Interestingly, two of the NTM infections in our series were caused by M. xenopi, a less frequent culprit of NTM-PD in the midwestern USA. The NTM infections masqueraded clinically and radiologically as lung malignancy, which posed a diagnostic dilemma. Physicians should be aware of NTMs as causative pathogens for chronic pulmonary diseases, especially in certain high-risk patients. The diagnostic criteria for NTM-PD established by the AST/IDSA should be followed to achieve an accurate and timely diagnosis to start anti-tuberculous therapy.
Learning points
NTM-PD is an uncommon etiology of cavitary and nodular lung disease in three elderly patients with underlying lung conditions.
NTM-PD can greatly mimic lung malignancy both clinically and radiologically, which may pose a diagnostic dilemma.
Physicians should be aware of NTMs as causative pathogens for chronic pulmonary diseases, especially in certain high-risk patients.
Acknowledgments
The authors would like to acknowledge the Department of Infectious Disease at Ascension Saint Francis Hospital for providing valuable input to this case series.
Financial Disclosure
The authors confirm that there is no funding to declare regarding the publication of this submitted work.
Conflict of Interest
All authors declare that they have no conflict of interest regarding the publication of this case series.
Informed Consent
Informed consent was obtained from the patients to write and publish their cases with all accompanying clinical and radiological data. No personal identifying information has been used in this article.
Author Contributions
ES and MA contributed to conceptualizing and writing the first manuscript. MSA, AM, and MEF contributed to writing the first draft. ES edited the first draft. HF performed the critical review of the final draft and acted as a senior author for this submission. All authors agreed to the final draft submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36(1):13-34.
doi pubmed pmc - Carrillo MC, Patsios D, Wagnetz U, Jamieson F, Marras TK. Comparison of the spectrum of radiologic and clinical manifestations of pulmonary disease caused by Mycobacterium avium complex and Mycobacterium xenopi. Can Assoc Radiol J. 2014;65(3):207-213.
doi pubmed - Diel R, Ringshausen F, Richter E, Welker L, Schmitz J, Nienhaus A. Microbiological and Clinical Outcomes of Treating Non-Mycobacterium Avium Complex Nontuberculous Mycobacterial Pulmonary Disease: A Systematic Review and Meta-Analysis. Chest. 2017;152(1):120-142.
doi pubmed - Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 2002;23(3):553-567.
doi pubmed - Bittner MJ, Horowitz EA, Safranek TJ, Preheim LC. Emergence of Mycobacterium kansasii as the leading mycobacterial pathogen isolated over a 20-year period at a midwestern Veterans Affairs hospital. Clin Infect Dis. 1996;22(6):1109-1110.
doi pubmed - Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997 2003. Thorax. 2007;62(8):661-666.
doi pubmed pmc - Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416.
doi pubmed - Iseman MD, Marras TK. The importance of nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med. 2008;178(10):999-1000.
doi pubmed - Erasmus JJ, McAdams HP, Farrell MA, Patz EF, Jr. Pulmonary nontuberculous mycobacterial infection: radiologic manifestations. Radiographics. 1999;19(6):1487-1505.
doi pubmed - Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Brown MR, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178(10):1066-1074.
doi pubmed pmc - Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest. 1992;101(6):1605-1609.
doi pubmed - Falkinham JO, 3rd, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl Environ Microbiol. 2001;67(3):1225-1231.
doi pubmed pmc - Andrejak C, Lescure FX, Pukenyte E, Douadi Y, Yazdanpanah Y, Laurans G, Schmit JL, et al. Mycobacterium xenopi pulmonary infections: a multicentric retrospective study of 136 cases in north-east France. Thorax. 2009;64(4):291-296.
doi pubmed - Souilamas R, Danel C, Chauffour X, Riquet M. Lung cancer occurring with Mycobacterium xenopi and Aspergillus. Eur J Cardiothorac Surg. 2001;20(1):211-213.
doi pubmed - Fogla S, Pansare VM, Camero LG, Syeda U, Patil N, Chaudhury A. Cavitary lung lesion suspicious for malignancy reveals Mycobacterium xenopi. Respir Med Case Rep. 2018;23:83-85.
doi pubmed pmc - Tortoli E, Mariottini A, Mazzarelli G. Evaluation of INNO-LiPA MYCOBACTERIA v2: improved reverse hybridization multiple DNA probe assay for mycobacterial identification. J Clin Microbiol. 2003;41(9):4418-4420.
doi pubmed pmc - Wallace RJ, Jr., Brown BA, Griffith DE, Girard WM, Murphy DT, Onyi GO, Steingrube VA, et al. Initial clarithromycin monotherapy for Mycobacterium avium-intracellulare complex lung disease. Am J Respir Crit Care Med. 1994;149(5):1335-1341.
doi pubmed - Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, et al. British Thoracic Society Guideline for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). BMJ Open Respir Res. 2017;4(1):e000242.
doi pubmed pmc - Zaheen A, Hirama T, Mehrabi M, Brode SK, Marras TK. Clinical outcomes in Mycobacterium xenopi versus Mycobacterium avium complex pulmonary disease: A retrospective matched cohort study. Respir Med. 2020;167:105967.
doi pubmed - Watanabe M, Hasegawa N, Ishizaka A, Asakura K, Izumi Y, Eguchi K, Kawamura M, et al. Early pulmonary resection for Mycobacterium avium complex lung disease treated with macrolides and quinolones. Ann Thorac Surg. 2006;81(6):2026-2030.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


