| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 8, August 2023, pages 282-288
Primary Hepatic Other Iatrogenic Immunodeficiency-Associated Lymphoproliferative Disorders After Methotrexate Therapy
Yasuki Hatayamaa, Harutoshi Sugiyamaa, Daisuke Murakamia, Hirotaka Ouraa, Yukiko Shimaa, Miho Shiratoa, Takayoshi Nishinoa, Tadao Nakazawab, Kenichi Suehiroc, Makoto Araia, d
aDepartment of Gastroenterology, Tokyo Women’s Medical University Yachiyo Medical Center, Chiba 276-8523, Japan
bDepartment of Pathology, Tokyo Women’s Medical University Yachiyo Medical Center, Chiba 276-8523, Japan
cCenter for Rheumatic Diseases, Chibaken Saiseikai Narashino Hospital, Chiba 275-8580, Japan
dCorresponding Author: Makoto Arai, Department of Gastroenterology, Tokyo Women’s Medical University Yachiyo Medical Center, Chiba 276-8523, Japan
Manuscript submitted July 5, 2023, accepted August 7, 2023, published online August 28, 2023
Short title: Primary Hepatic OIIA-LPDs After MTX Therapy
doi: https://doi.org/10.14740/jmc4135
| Abstract | ▴Top |
Prior reports described cases of lymphoproliferative diseases occurring after methotrexate (MTX) administration, which are called methotrexate-associated lymphoproliferative disorders (MTX-LPDs). It has become clear that these lymphoproliferative diseases also occur following treatment with other immunosuppressive drugs, and they have been termed as other iatrogenic immunodeficiency-associated lymphoproliferative disorders (OIIA-LPDs). In most of these cases, the duration of immunosuppressive drugs is very long, on the order of years. In the present study, we evaluated the development of lymphoproliferative disease despite the short duration of immunosuppressive treatment and determined the tumor doubling time. A 71-year-old woman was diagnosed with adult-onset Still’s disease. The patient was administered prednisone 30 mg per day starting on February 25, 2022 and MTX 6 mg per week starting 2 weeks later. Because she was a hepatitis B virus (HBV) carrier, nucleic acid analog therapy was also started to prevent HBV activation. Eight weeks later, biweekly tocilizumab was started. After 5 months of MTX administration, a solitary liver tumor measuring 37 × 32 mm2 was detected. Three months later, repeat computed tomography revealed that the liver tumor had grown rapidly to 7 cm in diameter. We considered the possibility of OIIA-LPDs and stopped MTX therapy. Biopsy specimens of the liver tumor exhibited lymphocyte proliferation, which was consistent with OIIA-LPDs. The doubling time for tumor growth was 33 days. Despite withdrawing MTX for 6 weeks, the tumor continued to grow, and thus, the patient was referred to the hematology unit. In previously reported cases of MTX-LPDs of hepatic origin, the average duration of MTX administration was 7.3 (2 - 13) years. This report describes a primary hepatic OIIA-LPDs-associated tumor that rapidly increased in size after an extremely short period of MTX administration.
Keywords: Methotrexate; Hepatic lymphoma; Methotrexate-associated lymphoproliferative disorders; Tumor doubling time
| Introduction | ▴Top |
Methotrexate (MTX) is the most widely used drug globally for rheumatoid arthritis (RA). It has displayed high efficacy, persistence rates, and effectiveness in inhibiting the progression of bone destruction and improving quality of life and life expectancy, making it the first-line drug and a central player in the treatment of RA. Although MTX is a relatively safe immunosuppressive drug, common side effects in the early stages of oral administration include gastrointestinal symptoms, such as stomatitis, nausea, and diarrhea, and liver damage as the dosage increases. In addition, infections, anemia attributable to myelosuppression and interstitial pneumonia can also occur during MTX administration. Methotrexate-associated lymphoproliferative disorders (MTX-LPDs) represent the phenomenon of lymphoproliferative disease or lymphoma induced by MTX [1, 2]. In recent years, reports of tumor necrosis factor alpha inhibitor-induced LPDs have also been published, and therefore, MTX-LPDs are included among the four subtypes of other iatrogenic immunodeficiency-associated LPDs (OIIA-LPDs) in the World Health Organization classification [3]. The most frequent background disease is chronic RA, and the high number of reports from Japan is one characteristic of this disease. It is also not certain how long administration of immunosuppressive drugs increases the risk of developing OIIA-LPDs. Previous reports have shown that OIIA-LPD often develops after several years’ administration of immunosuppressive drugs [4]. But in some cases, OIIA-LPD develops after a longer period of administration, while in others, it develops after a shorter period of administration. Primary hepatic OIIA-LPD is rare. Accumulation of imaging findings on computed tomography (CT) or magnetic resonance imaging (MRI) of primary hepatic OIIA-LPD is still in process. Differentiation from hepatocellular carcinoma (HCC) is sometimes difficult, especially when the background liver is cirrhotic or viral hepatitis. The clinical characteristics of OIIA-LPD, such as whether it grows rapidly or metastasizes quickly to other organs, have not been clarified.
We experienced a primary hepatic OIIA-LPD-associated tumor that rapidly increased in size after an extremely short period of treatment with MTX and other immunosuppressive drugs and succeeded in measuring the tumor doubling time for the first time.
| Case Report | ▴Top |
Investigations
A 71-year-old woman who had undergone a thorough examination for fever of unknown origin since November 2021 was diagnosed with adult-onset Still’s disease (AOSD). She had diabetes mellitus, for which she was receiving insulin injections and oral medication. The patient was administered prednisone (PSL) 30 mg/day starting on February 25, 2022 and MTX 6 mg/week 2 weeks later. She was diagnosed as an inactive hepatitis B virus (HBV) carrier because HBV-DNA and hepatitis B s antigen (HBsAg) were persistently positive but liver function was normal. Nucleic acid analog (tenofovir alafenamide fumarate) therapy was also started to prevent HBV activation. CT and 18F-fluorodeoxyglucose positron emission tomography (PET) revealed no obvious tumors in the liver or other organs. Eight weeks later, biweekly tocilizumab 8 mg/kg was started. PSL was administered in gradually decreasing doses to 5 mg. After 5 months of MTX administration, a solitary liver tumor measuring 37 × 32 mm2 was detected (Fig. 1). Contrast-enhanced CT revealed that the tumor margins were somewhat irregular with a thick contrast effect. Conversely, the contrast effect in the center was poor. MRI revealed a pale low signal on T1-weighted images and a slightly heterogeneous high signal on T2-weighted images. This finding was suspicious for an abscess. However, there was no fever, and blood tests revealed no inflammatory reaction, dismissing the possibility of abscess. Upper and lower endoscopy revealed no abnormalities and no lesions other than the liver. Because the level of the tumor marker carbohydrate antigen 19-9 (CA19-9) was 178 IU/L (reference level: 0 - 37 IU/L) and no liver tumor was detected before MTX treatment, a malignant tumor was suspected. She was referred to our hospital for close examination. Because of the examinations at the previous hospital and for her own reasons, 3 months passed between detection of the tumor and her first visit to our hospital (Table 1). Repeat CT revealed that the liver tumor had grown rapidly to 7 cm in diameter. Her soluble interleukin-2 receptor level increased from 623 to 1,060 U/mL in the 3 months before she visited our hospital.
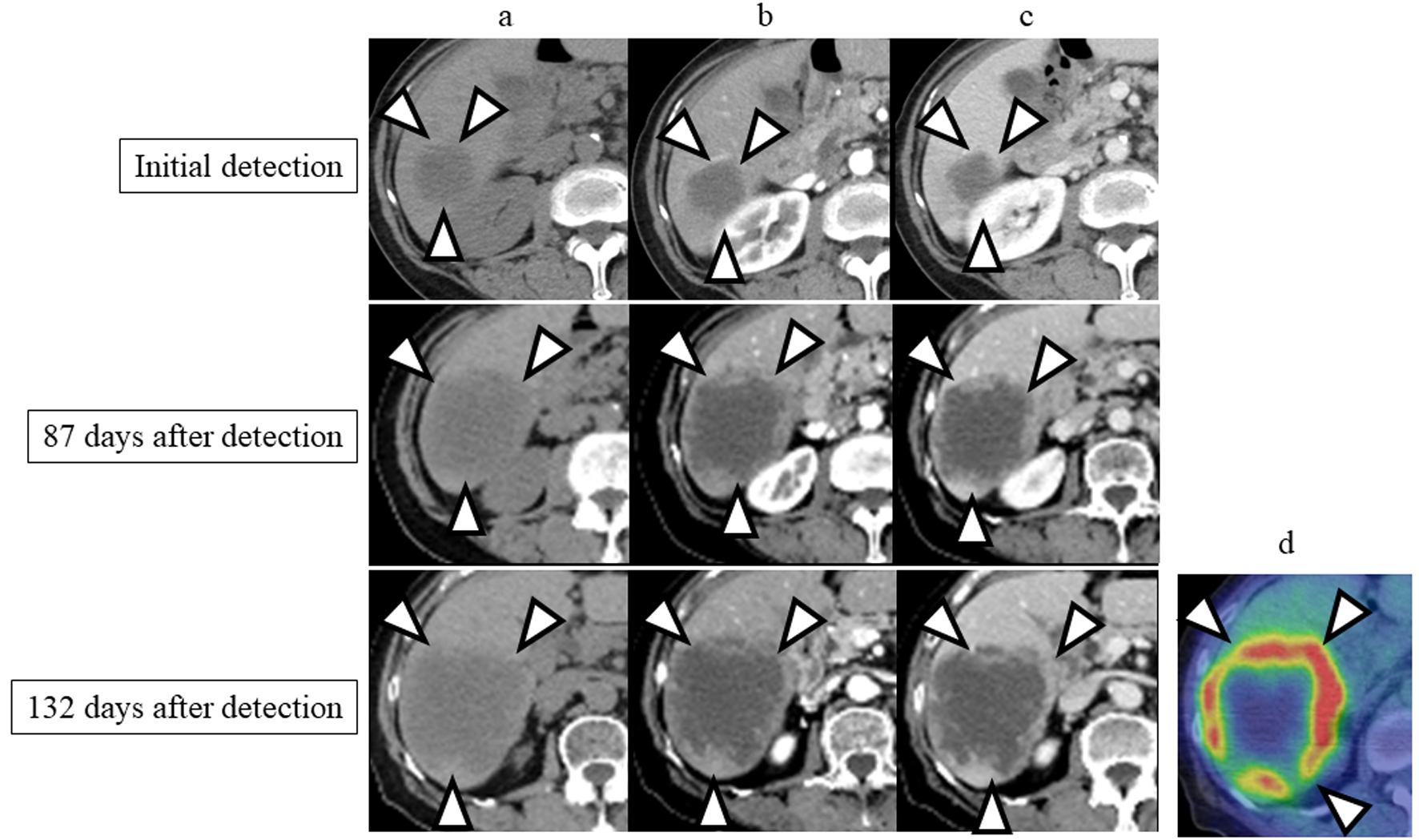 Click for large image | Figure 1. Enhanced computed tomography (CT) images performed at the time of detection and 87 and 132 days after the initial detection. (a) Precontrast phase; (b) arterial phase; (c) portal phase; (d) 18F-fluorodeoxyglucose positron emission tomography (PET)/CT performed at 139 days after the initial detection. The tumor is indicated by arrowheads. |
 Click to view | Table 1. Laboratory Data on First Visit to Our Hospital |
Diagnosis
We considered the possibility of OIIA-LPDs and stopped MTX. Liver tumor biopsy was performed with a 21-G needle, but only necrotic material was obtained. Therefore, another liver tumor biopsy targeting the tumor margins was performed with an 18-G needle because the center of the tumor was expected to be necrotic. Biopsy specimens of the liver tumor demonstrated lymphocyte proliferation and a wide variety of forms (Fig. 2). Immunostaining revealed the following findings for the tumor: CD20 (+), CD3 (partly +), CD5 (-), and CD10 (-). In situ hybridization disclosed partial positivity for Epstein-Barr virus (EBV)-encoded small RNAs (EBERs). The findings were consistent with OIIA-LPDs. Despite withdrawing MTX for 7 weeks, the tumor continued to grow. The tumor volume was calculated using CT images. The tumor doubling time was 33 days at 3 months after initial detection of the tumor. In the following 7 weeks, the tumor doubling time was 58 days. The tumor doubling time over the entire 132-day course from initial detection was 38 days.
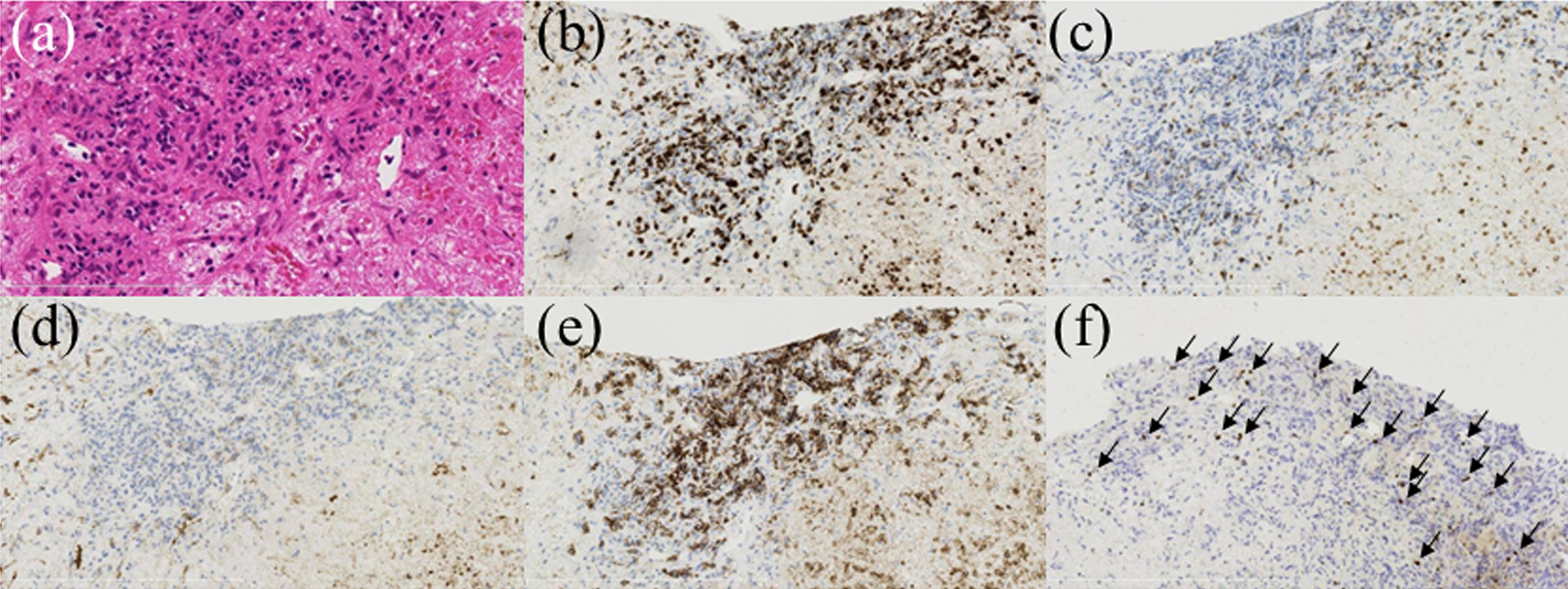 Click for large image | Figure 2. Pathological findings of the liver biopsy. Hematoxylin and eosin (H&E) staining revealed lymphocyte proliferation and a wide variety of forms. The tumor cells were positive for CD20 and CD3 and negative for CD5 and CD10. In situ hybridization revealed partial positivity for EBV-encoded small RNAs (EBERs). (a) H&E staining, × 400; (b) anti-CD3 staining, × 100; (c) anti-CD5 staining, × 100; (d) anti-CD10 staining, × 100; (e) anti-CD20 staining, × 100; (f) in situ hybridization EBERs, × 100 (arrows denote cells positive for EBERs on in situ hybridization). |
Treatment
The patient was referred to the hematology unit to receive treatment for the malignant lymphoma. We stopped tocilizumab, and chemotherapy (R-CHOP, rituximab, cyclophosphamide, adriamycin, vincristine, and prednisolone) with dose reduction to 70% was administered. She continues to take nucleic acid analogs, and her HBV-DNA level has remained below the limit of detection. In addition, the use of rituximab has not changed her symptoms related to AOSD.
Follow-up and outcomes
Her general condition was good and she received six courses of chemotherapy, leading to partial remission.
| Discussion | ▴Top |
We experienced a case of lymphoproliferative disease of hepatic origin occurring only 5 months after initiating MTX therapy. HCC was first suspected because the patient was an HBV carrier, but tumor biopsy provided the correct diagnosis and led to appropriate treatment. In addition, the tumor doubling time was measured. In an analysis of 48 cases of MTX-LPDs, the mean duration of MTX therapy before onset was reported to be 54 months [4]. Most cases develop after long-term MTX administration. In the 11 previously reported cases of MTX-LPDs of hepatic origin, the average duration of MTX administration was 7.3 (range, 2 - 13) years (Table 2) [5-15]. In our case, a primary hepatic OIIA-LPDs-associated tumor rapidly increased in size after an extremely short period of MTX administration. One reason why MTX-LPDs are attracting attention is that spontaneous tumor regression can be achieved by MTX discontinuation alone [16, 17]. However, there are cases in which the tumor does not regress despite MTX discontinuation, necessitating chemotherapy. In a review of 121 cases of MTX-LPDs, 59 cases (48.8%) improved with MTX discontinuation alone, 49 cases (40.5%) required chemotherapy without improvement despite discontinuation, and 13 cases (10.7%) required second-line chemotherapy [18].
 Click to view | Table 2. Summary of 11 Reported Cases of Hepatic Methotrexate-Associated Lymphoproliferative Disorders |
Lymphoma of hepatic origin is rare [19]. It was important to differentiate this malignancy from HCC and other malignancies. In this case, the tumor margins were somewhat irregular with a thick contrast effect. The central part of the tumor had a poor contrast effect, a finding that was suspected to be complicated by necrosis. These findings were suspicious for liver abscess. On the contrary, HCC exhibits a strong contrast effect inside the tumor in the early phase. In the late phase, the contrast effect inside the tumor is lost, whereas the membrane is strongly contrasted. Both tumors exhibit contrast effects, and their differentiation can sometimes be difficult. To differentiate liver tumors, the first step is identifying the aforementioned characteristic imaging findings on contrast-enhanced CT. In addition, the presence of liver cirrhosis or viral hepatitis should be checked. If present, HCC should be initially suspected. In addition, tumor markers are useful for differentiating liver tumors. HCC is generally typified by increases in the levels of tumor markers such as α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II). Meanwhile, the presence of fever and a strong inflammatory response is suspicious for liver abscess. Diagnosis by liver tumor biopsy is also recommended when it can be performed safely without adversely affecting subsequent surgery or other treatments. In fact, some cases of liver MTX-LPDs were misdiagnosed as HCC and treated with surgery or transcatheter arterial chemo embolization (cases 7 and 11, Table 2). Routine imaging of the liver was not performed in most cases of MTX-LPDs. Therefore, most tumors were massive or multiple upon diagnosis. In this case, because of the patient’s own circumstances and other reasons, we performed the imaging evaluation 3 months after the liver tumor was first noted. For the first time, we successfully measured the doubling time of a hepatic OIIA-LPDs-associated tumor. In a meta-analysis, the tumor doubling time for HCC was 4.6 months (95% confidence interval (CI): 3.9 - 5.3) [20]. On the contrary, the tumor doubling time at 3 months from the initial detection of the tumor in this case was 33 days, and after MTX discontinuation, the tumor doubling time increased to 58 days. Compared to the growth of HCC, the OIIA-LPDs-associated tumor in this case grew extremely rapidly. Interestingly, the tumor doubling time decelerated after MTX was discontinued. Of course, it is possible that the tumor doubling time slowed because the tumor diameter was already large, but it is also possible that the decrease was partially attributable to the discontinuation of MTX. In a previous report of hepatic MTX-LPDs, multiple tumors, the largest being 55 mm in diameter, were found even though imaging tests 4 months earlier had not disclosed any abnormality in a patient with autoimmune hepatitis (case 11, Table 2). Considering this finding and those of our case, hepatic OIIA-LPDs-associated tumors might grow at an extremely rapid rate compared to normal HCC. We were unable to obtain tumor tissue from the first liver biopsy, as the only tissue obtained was necrotic material. The rapid rate of tumor growth and necrosis of the central part of the tumor could be characteristics of OIIA-LPDs-associated tumors.
In this case, tocilizumab was also administered. Tocilizumab is an immunosuppressive drug used to treat RA, juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and AOSD. It is a humanized monoclonal antibody against the interleukin-6 receptor. Interleukin-6 is a cytokine that plays an important role in immune responses. The overall risk of cancer among patients with RA treated with tocilizumab does not differ substantially from that of biologic drug-naive patients or those treated with conventional systemic disease-modifying antirheumatic drugs [21]. Regarding the possibility that AOSD can increase the risk of malignant tumors such as lymphoma, this is difficult to determine because of the small number of patients with AOSD and the limited number of patients receiving long-term treatment with tocilizumab. Although MTX-LPDs have been reported to be associated with EBV, Balandraud et al found tocilizumab significantly decreased the EBV load, and MTX was concomitantly used in some cases without altering the EBV load [22]. Therefore, we speculate that tocilizumab administration was unlikely to be associated with this case.
It is unclear why this patient developed lymphoproliferative disease after a short treatment course. Although no previous reports described the association between hepatitis virus and OIIA-LPDs, we cannot dismiss the possibility that being an HBV carrier resulted in immunological differences that affected the case. In this case, glucocorticoid and tocilizumab were also administered, and the possibility that the concomitant use of multiple immunosuppressive drugs influenced the development of OIIA-LPDs cannot be dismissed. According to a report summarizing cases of MTX-LPDs [4], one patient developed MTX-LPD after only 2 months of MTX administration, although the primary source was not determined. In addition, detailed course of the disease was not described, and it is unknown whether the absence of a tumor was properly confirmed by imaging or other means prior to the start of treatment.
We experienced a case of lymphoproliferative disease, which is usually considered to be caused by long-term immunosuppressive treatment, after only 5 months of MTX administration. With the correct diagnosis, the patient received optimal treatment. When tumors are detected in patients receiving MTX or other immunosuppressive drugs, the possibility of lymphoproliferative disease should be considered regardless of the duration of treatment. And we measured the tumor doubling time and observed rapid growth compared to that of normal HCC.
Learning points
Hepatic OIIA-LPDs can develop even with short-term (fewer than 6 months) MTX administration, and its tumor growth is faster than that of HCC. When there is chronic disease in background liver, it may be misdiagnosed as HCC.
Acknowledgments
We deeply appreciate the staffs of Yachiyo Medical Center.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The possibility of presenting case reports at academic conferences and papers is posted in the hospital.
Author Contributions
YH, HS, DM, HO, YO, TN (Nishino) and MA treated her as a practice group and YH and MA compiled a paper report. TN (Nakazawa) evaluated the case as an expert in pathology. KS was responsible for the treatment of AOSD.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
AOSD: adult-onset Still’s disease; EBERs: Epstein-Barr virus (EBV)-encoded small RNAs; HBV: hepatitis B virus; MTX: methotrexate; MTX-LPDs: methotrexate-associated lymphoproliferative disorders; OIIA-LPDs: other iatrogenic immunodeficiency-associated lymphoproliferative disorders; PSL: prednisone; RA: rheumatoid arthritis
| References | ▴Top |
- Kamel OW, van de Rijn M, Weiss LM, Del Zoppo GJ, Hench PK, Robbins BA, Montgomery PG, et al. Brief report: reversible lymphomas associated with Epstein-Barr virus occurring during methotrexate therapy for rheumatoid arthritis and dermatomyositis. N Engl J Med. 1993;328(18):1317-1321.
doi pubmed - Hoshida Y, Tomita Y, Zhiming D, Yamauchi A, Nakatsuka S, Kurasono Y, Arima Y, et al. Lymphoproliferative disorders in autoimmune diseases in Japan: analysis of clinicopathological features and Epstein-Barr virus infection. Int J Cancer. 2004;108(3):443-449.
doi pubmed - Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO classification of tumors of haematopoietic and lymphoid tissues, 4th ed, 2008; IARC, Press, Lyon, France.
- Hoshida Y, Xu JX, Fujita S, Nakamichi I, Ikeda J, Tomita Y, Nakatsuka S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
pubmed - Soubrier M, Arrestier S, Bouloudian S, Dubost JJ, Ristori JM. Epstein-Barr virus infection associated hepatic lymphoma in a patient treated with methotrexate for rheumatoid arthritis. Joint Bone Spine. 2006;73(2):218-219.
doi pubmed - Fujita T, Tanabe M, Iida E, Okimoto T, Matsunaga N. Multi-modality imaging findings of methotrexate-related Epstein-Barr virus-associated hepatic tumor. Clin Imaging. 2013;37(5):962-964.
doi pubmed - Tatsumi G, Ukyo N, Hirata H, Tsudo M. Primary hepatic lymphoma in a patient with rheumatoid arthritis treated with methotrexate. Case Rep Hematol. 2014;2014:460574.
doi pubmed pmc - Nonami A, Takenaka K, Harada N, Kono K, Kamezaki K, Numata A, Karube K, et al. Primary hepatic lymphoma 1 year after resection of hepatocellular carcinoma. J Clin Oncol. 2006;24(36):5784-5786.
doi pubmed - Kawahara A, Tsukada J, Yamaguchi T, Katsuragi T, Higashi T. Reversible methotrexate-associated lymphoma of the liver in rheumatoid arthritis: a unique case of primary hepatic lymphoma. Biomark Res. 2015;3:10.
doi pubmed pmc - Matsumoto R, Numata K, Doba N, Hara K, Chuma M, Fukuda H, Nozaki A, et al. A case of multiple hepatic lesions associated with methotrexate-associated lymphoproliferative disorder. J Med Ultrason (2001). 2016;43(4):545-551.
doi pubmed - Takei D, Abe T, Amano H, Hirano N, Kobayashi T, Ohdan H, Kondo T, et al. Methotrexate-associated primary hepatic malignant lymphoma following hepatectomy: A case report. Int J Surg Case Rep. 2017;31:5-9.
doi pubmed pmc - Tsukazaki Y, Shinohara T, Tanaka K, Naruse K, Iwahara Y, Inoue S. Hepatosplenic Hodgkin lymphoma without lymphadenopathy following reversible methotrexate-associated lymphoproliferative disorder. Mod Rheumatol. 2017;27(2):372-375.
doi pubmed - Ono R, Kumagae T, Uojima H, Teshima S, Kudo M, Kitagawa I, Yoshizawa M. Hepatic methotrexate-associated lymphoproliferative disorders identified by multiple liver tumors: a case report and review of the literature. J Med Case Rep. 2019;13(1):196.
doi pubmed pmc - Mizusawa T, Kamimura K, Sato H, Suda T, Fukunari H, Hasegawa G, Shibata O, et al. Methotrexate-related lymphoproliferative disorders in the liver: case presentation and mini-review. World J Clin Cases. 2019;7(21):3553-3561.
doi pubmed pmc - Tsuruoka M, Inoue J, Kakazu E, Ninomiya M, Iwata T, Sano A, Masamune A. Methotrexate-associated lymphoproliferative disorder in the liver resembling hepatocellular carcinoma treated with transarterial chemoembolization. Intern Med. 2020;59(18):2255-2260.
doi pubmed pmc - Rizzi R, Curci P, Delia M, Rinaldi E, Chiefa A, Specchia G, Liso V. Spontaneous remission of "methotrexate-associated lymphoproliferative disorders" after discontinuation of immunosuppressive treatment for autoimmune disease. Review of the literature. Med Oncol. 2009;26(1):1-9.
doi pubmed - Miyazaki T, Fujimaki K, Shirasugi Y, Yoshiba F, Ohsaka M, Miyazaki K, Yamazaki E, et al. Remission of lymphoma after withdrawal of methotrexate in rheumatoid arthritis: relationship with type of latent Epstein-Barr virus infection. Am J Hematol. 2007;82(12):1106-1109.
doi pubmed - Harada T, Iwasaki H, Muta T, Urata S, Sakamoto A, Kohno K, Takase K, et al. Outcomes of methotrexate-associated lymphoproliferative disorders in rheumatoid arthritis patients treated with disease-modifying anti-rheumatic drugs. Br J Haematol. 2021;194(1):101-110.
doi pubmed - Noronha V, Shafi NQ, Obando JA, Kummar S. Primary non-Hodgkin's lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207.
doi pubmed - Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, Marrero J, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut. 2021;70(2):401-407.
doi pubmed pmc - Wadstrom H, Frisell T, Askling J, Anti-Rheumatic Therapy in Sweden Study Group. Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a nationwide cohort study from Sweden. JAMA Intern Med. 2017;177(11):1605-1612.
doi pubmed pmc - Balandraud N, Texier G, Massy E, Muis-Pistor O, Martin M, Auger I, Guzian MC, et al. Long term treatment with abatacept or tocilizumab does not increase Epstein-Barr virus load in patients with rheumatoid arthritis - A three years retrospective study. PLoS One. 2017;12(2):e0171623.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


