| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 14, Number 9-10, October 2023, pages 307-316
Pancreatic Vasoactive Intestinal Peptide-Producing Tumor as a Rare Cause of Acute Diarrhea and Severe Hypokalemia
Vasilios Giampatzisa, e , Christina Kotsiarib, Prodromos Bostantzisb, Alexandra Chrisoulidouc, Aimilia Fotiadouc, Soultana Lotid, Stefanos Papantonioub, Persefoni Papadopouloub
aDepartment of Emergency Medicine, General Hospital of Kavala, Greece
bDepartment of Internal Medicine, General Hospital of Kavala, Greece
cEndocrinology Department, Theageneio Anticancer Hospital, Alexandrou Symeonidi 2, Thessaloniki 54639, Greece
dDepartment of Radiology, General Hospital of Kavala, Greece
eCorresponding Author: Vasilios Giampatzis, Department of Emergency Medicine, General Hospital of Kavala, Kavala 65500, Greece
Manuscript submitted July 24, 2023, accepted August 19, 2023, published online October 13, 2023
Short title: Acute Diarrhea in Pancreatic VIPoma
doi: https://doi.org/10.14740/jmc4141
| Abstract | ▴Top |
Pancreatic vasoactive intestinal peptide-producing tumor (VIPoma) is a rare functional neuroendocrine tumor most commonly presenting with watery diarrhea and electrolyte abnormalities that include hypokalemia, hypercalcemia and metabolic acidosis. This type of tumor has usually insidious clinical behavior that is characterized by chronic secretory diarrhea, lasting usually from months to years before diagnosis, not responsive to usual medical or dietary treatment approaches. Given the resemblance of VIPoma with other more common causes of chronic watery diarrhea, the final diagnosis is often delayed and the tumors are usually large and metastatic at the time of detection. Our case of pancreatic VIPoma demonstrates an unusual clinical course for this type of tumor with acute refractory diarrhea and rapid deterioration of patient’s clinical and biochemical status that required emergent in-hospital diagnosis and treatment. Our patient is a 45-year-old woman who presented with abrupt, watery diarrhea during the past 24 h before admission accompanied with severe hypokalemia as well as hyponatremia, hyperglycemia and hypercalcemia. Despite aggressive management with fluid administration and electrolyte replenishment, no significant improvement in patient’s symptoms and electrolyte imbalance was observed. After exclusion of other causes of acute diarrhea from the medical history and the laboratory tests, the clinical suspicion of a functional neuroendocrine tumor was raised. After the establishment of final diagnosis of pancreatic VIPoma with biochemical tests and magnetic resonance imaging (MRI), somatostatin analogues were prescribed and the patient underwent distal pancreatectomy and splenectomy with no signs of lymph node and splenic metastases. Few days after the surgical resection of the tumor, the patient readmitted to our hospital with tarry stools and severe anemia. The abdominal computed tomography (CT) revealed a retroperitoneal cystic lesion. The gastrointestinal bleeding gradually recessed after endoscopic hemostasis of duodenal ulcer lesions whereas the cystic lesion (postoperative lymphocele) was successfully drained under CT-guidance before discharge. After almost 10 years postoperatively, the patient is still asymptomatic with no signs of relapse or metastasis of the disease in the periodic laboratory and imaging follow-up. In conclusion, pancreatic VIPoma can sometimes manifest symptoms of abrupt onset and rapid progression that require high clinical suspicion, appropriate diagnostic workup and aggressive management.
Keywords: Neuroendocrine tumors; Pancreatic VIPoma; Acute diarrhea; Hypokalemia; Somatostatin analogues; Pancreatectomy
| Introduction | ▴Top |
Neuroendocrine tumors of the pancreas (pNETs) are rare tumors with an annual estimated incidence of 0.22 cases per 100,000 [1]. Pancreatic vasoactive intestinal peptide-producing tumor (VIPoma) is a rare neuroendocrine tumor that accounts approximately for less than 0.6 cases/million/year [2]. Most of the cases are sporadic whereas almost 5% of them are encountered as part of the multiple endocrine neoplasia type 1 (MEN1) syndrome [3]. Like other neuroendocrine tumors, VIPoma is often characterized by slow progression and malignant clinical course [4]. The typical clinical features of VIPoma are associated with the excessive secretion of the vasoactive intestinal peptide (VIP) and include watery diarrhea, hypokalemia and metabolic acidosis [5].
In the majority of patients, the diarrhea is chronic and secretory [5, 6] and the prompt diagnosis is difficult as more common causes of watery diarrhea should be excluded in the first place [5]. Many of these patients are diagnosed after months or years from symptoms initiation and often in the outpatient setting after multiple laboratory and imaging investigations [5].
We present a case of pancreatic VIPoma in a 45-year-old woman that initially manifested with acute diarrhea, a finding very unusual for this type of tumor. Given other more common diseases for patient’s age and gender, especially acute infectious diarrhea, pancreatic VIPoma was not among the predominant causes in our differential diagnosis. The severe electrolyte abnormalities and the resistance to initial therapy, though, made the prompt diagnosis and treatment during hospitalization necessary.
In this article, we analyze the unusual clinical presentation of our pancreatic VIPoma case along with the diagnostic steps, treatment approach and follow-up course of this patient (Table 1). The need for high clinical suspicion and prompt diagnostic and therapeutic management are highlighted.
 Click to view | Table 1. Timeline |
| Case Report | ▴Top |
Investigations
A 45-year-old woman presented to the emergency department of our hospital with multiple episodes of watery diarrhea, i.e., almost 9 - 10 episodes during the past 24 h, accompanied with few, self-limited episodes of vomiting. She did not report any symptoms or diarrhea in the preceding weeks or months. The loose stools did not contain any blood or signs of melena. The patient complained about mild abdominal discomfort with no fever, chills or sweats. She also reported loss of appetite and extreme fatigue. Her past medical history was unremarkable and she did not make systemic use of any medical substances. Her past surgical history was remarkable only for resection of uterus leiomyoma 5 years before presentation. Her family medical history did not provide evidence of any neoplastic diseases.
The initial clinical examination revealed signs of dehydration with a normal level of consciousness. The arterial blood pressure and the body temperature were within normal range. The abdomen was non-tender and painless on examination and the bowel sounds were normal in frequency. The electrocardiogram at admission presented sinus tachycardia and repolarization abnormalities in some leads.
Diagnosis
The initial laboratory investigations in the emergency department revealed severe hypokalemia accompanied with hyponatremia, hypercalcemia and hyperglycemia as well as findings of acute renal failure. The laboratory results of the patient at presentation are depicted in Table 2. The arterial blood gas analysis showed severe metabolic acidosis (pH = 7.25) with very low bicarbonate levels (HCO3- = 5.3 mmol/L). The X-ray images of the patient on admission were normal, whereas the abdominal ultrasonography revealed only an enlarged gallbladder.
 Click to view | Table 2. Laboratory Results of the Patient |
The patient was transferred immediately to the department of internal medicine where further diagnostic and therapeutic investigations were performed. The initial treatment of the patient included fluid replacement, intravenous administration of potassium and antibiotic prescription. Despite of fasting during the first few days of hospital stay, the episodes of diarrhea persisted at a rate of 8 - 9 episodes daily. The first step in our diagnostic pathway was to define the characteristics of acute diarrhea. Even if measurement of fecal osmolarity was not performed, the watery diarrhea was characterized as secretory based on the large daily volume of diarrheic stools, the non-responsiveness to fasting and the accompanied serum electrolyte abnormalities.
The differential diagnosis of acute diarrhea in this case included various infectious and non-infectious causes (Table 3). The dietary, drug-related and iatrogenic causes were initially excluded based on the past and recent medical history of the patient. Given the predominance of infectious causes in the context of acute diarrhea, the further diagnostic steps included stool samples for microscopic examination for ova and parasites, stool cultures and stool testing for Clostridium difficile toxin, which were all negative. The serologic tests WIDAL and WRIGHT for the diagnosis of possible salmonellosis and brucellosis, respectively, were negative as well as the serologic tests for hepatitis B, hepatitis C and human immunodeficiency virus. Moreover, the levels of thyroid-stimulating hormone (TSH) and intact parathormone (PTH) were also normal excluding, thus, common endocrinopathies as the culprit of acute diarrhea.
 Click to view | Table 3. Differential Diagnosis of Acute Diarrhea |
The presence of specific gastrointestinal diseases was also less likely in our case based on clinical judgement and routine stool examination. Accordingly, the age and comorbidities of our patient were not suggestive of ischemic colitis, whereas the absence of abdominal pain on clinical examination rendered ischemic bowel disease, diverticulitis and other abdominal inflammatory conditions less likely. Even if endoscopic assessment of the lower gastrointestinal tract was not feasible during that period, the existence of inflammatory bowel disease was not supported. Indeed, the acute presentation of symptoms of our patient, the absence of blood and leucocytes in routine stool analysis and the non-elevated serum inflammatory biomarkers were not in accordance with the chronic, intermittent, bloody diarrhea of inflammatory bowel disease. The absence of any gastrointestinal surgery also excluded the possibility of short bowel syndrome or postoperative diarrhea.
Despite the management of the patient with fluid and electrolyte replacement, the serum potassium levels remained low (K+ = 2.2 mEq/L) after 7 days of treatment. The other biochemical values at the seventh day are depicted in Table 2. The metabolic acidosis at admission was partially restored (pH = 7.31) because of respiratory compensation (pCO2 = 14 mm Hg) but the levels of bicarbonate remained low (HCO3- = 7.0 mmol/L). In turn, the levels of potassium excretion on 24-h urine collection were found to be very low (7.20 mmol/24 h, normal range 25 - 100 mmol/24 h). Therefore, the sustained hypokalemia did not result from concomitant urinary potassium wasting and was attributed exclusively to the gastrointestinal losses.
Due to persistence of symptoms and electrolyte abnormalities, the clinical suspicion of a functional neuroendocrine tumor was raised. Even if this type of tumors is not often characterized by acute diarrhea, the exclusion of other pathological entities in our case, the refractory diarrhea and the urgency towards a prompt diagnosis in view of life-threatening hypokalemia, all were factors that determined our next diagnostic steps. Given its high diagnostic value in this context, we decided to perform an urgent magnetic resonance imaging (MRI) of the abdomen to test our hypothesis. The unenhanced MRI findings demonstrated a lesion of 63 × 51 mm at the level of the pancreatic tail that showed heterogeneous uptake of the contrast medium in the contrast-enhanced images. These findings were consistent with a tumor of the islet cells of the pancreas (Figs. 1 and 2). The remaining MR images demonstrated bilateral pleural effusion, significant enlargement of the gallbladder (122 × 73 mm) as well as diffused, remarkable enlargement of the small (almost 120 mm diameter) and large intestine (almost 96 mm diameter of ascending colon). No focal lesions were found in the liver, spleen or kidneys and no pathologically enlarged pelvic or retroperitoneal lymph nodes were pinpointed.
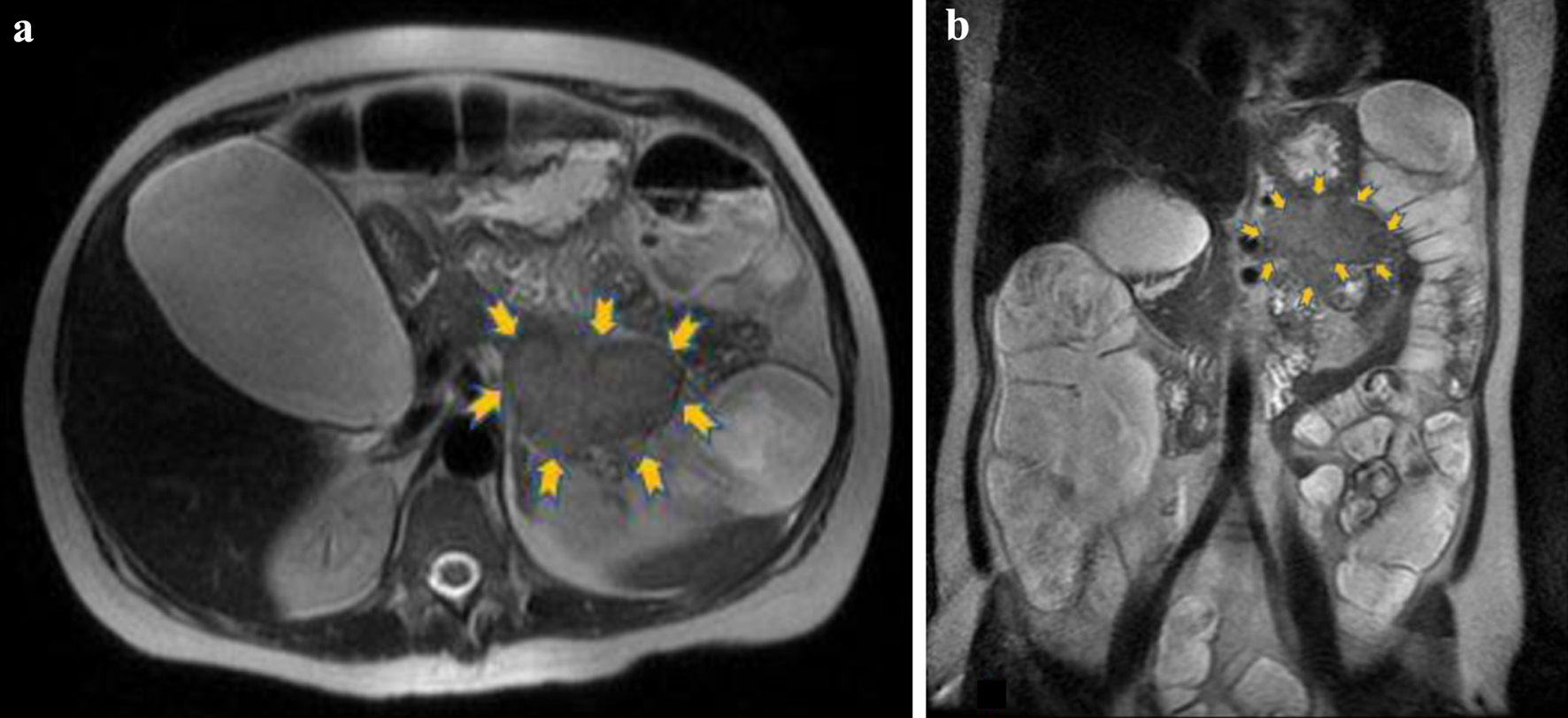 Click for large image | Figure 1. The lesion (6.3 × 5.1 cm) is located in the anatomic position of the pancreatic tail (yellow arrows) and presents intermediate signal intensity relative to pancreas. (a) Axial T2 and (b) coronal T2 magnetic resonance images. |
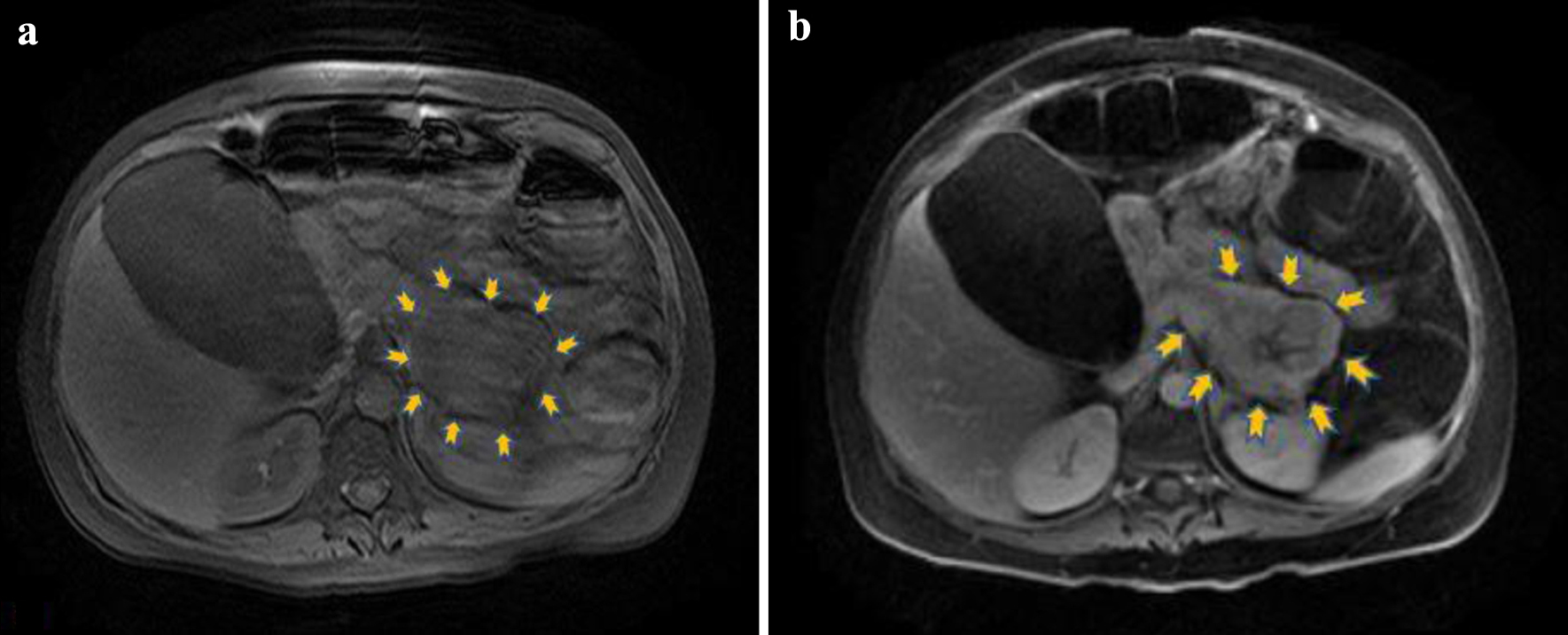 Click for large image | Figure 2. (a) Unenhanced axial T1 magnetic resonance image depicting the lesion in the pancreatic tail (yellow arrows) with mildly hypointense signal relative to pancreas (motion artifacts are present). (b) Axial T1 image after intravenous contrast medium administration showing heterogeneous uptake in the lesion with central necrosis (yellow arrows). |
Moreover, we performed further imaging assessment to determine if this pancreatic tumor was sporadic or as a part of the MEN1 syndrome. The brain CT did not reveal any anatomic abnormalities in the area of sella turcica and the ultrasound imaging of thyroid and parathyroid glands was normal. Despite the existence of pleural effusion in the abdominal MRI, the chest CT did not identify any specific lesions in the pulmonary parenchyma or any pathologic lymph nodes in the mediastinum. In the context of the concomitant hypercalcemia, the X-rays of certain skeletal compartments did not yield any pathological images.
Due to the above findings and the severity of clinical status, the patient was transferred to the endocrinology department of the collaborating oncology hospital for further diagnostic and therapeutic management. Given the severe hypokalemia and the radiological imaging of paralytic ileus, the patient was managed conservatively at the initial phase. The treatment approach consisted of antibiotic treatment, fluid and electrolyte prescription through central venous line, parenteral feeding and decompression of the intestine with nasogastric tube in the context of ileus management. The specific laboratory investigations during the first 3 days at the oncology hospital are depicted in Table 4. The levels of the 24-h urine hydroxyindoleacetic acid (HIAA) were into the normal range at 9.6 mg/24 h (normal values < 15 mg/24 h). The plasma levels of VIP were elevated to more than 120 pmol/L (normal values < 30 pmol/L), confirming the diagnosis of pancreatic VIPoma.
 Click to view | Table 4. Specific Laboratory Investigations in the Endocrinology Department |
Treatment
Despite the abovementioned therapeutic interventions, the clinical and biochemical status of the patient did not improve significantly after 3 days of stay at the endocrinology department. The diarrhea persisted with almost 4 - 5 diarrheal episodes daily with serum K+ levels remaining low at a range from 2.0 to 2.44 mEq/L. As a result, the surgical management of the tumor was chosen after multidisciplinary decision. Administration of somatostatin acetate was initiated perioperatively. Distal pancreatectomy with splenectomy was successfully performed with concomitant decompression of the gallbladder and the intestine. The histological examination of the dissected pancreatic tissue revealed neoplastic, polygonal cells that were stained immunohistochemically positive for cytokeratin-19 receptor, synaptophysin, CD56, calcitonin, chromogranin and Ki67 (15%). The peripancreatic and perisplenic lymph nodes as well as the dissected spleen were negative for metastasis. The histological findings were compatible with a well-differentiated neuroendocrine tumor of the pancreas grade 2, based on the World Health Organization (WHO) classification [7].
Immediately after surgery, the patient was transferred to the intensive care unit for further respiratory and hemodynamic support. Mechanical ventilation was implemented and vasoactive/inotropic drugs were prescribed due to the hemodynamic instability of the patient. The daily infusion of somatostatin acetate was halted on the fifth postoperative day. After 7 days of stay in the intensive care unit, the patient returned to the department of surgery of the collaborating oncology hospital where she remained clinically stable and discharged home. The diarrhea had subsided and hypokalemia and hypercalcemia were restored back to normal (K+ = 3.5 mEq/L, Ca2+ = 7.46 mg/dL).
Eight days after hospital discharge, the patient was admitted again to the emergency department of our hospital reporting fatigue and tarry stools during the next few days following surgery. The full blood count on admission demonstrated severe anemia (hematocrit = 15.4%, hemoglobin = 5 g/dL) as a result of upper gastrointestinal bleeding. The patient was supported with fluid administration, fresh frozen plasma and blood transfusions, whereas an urgent endoscopic assessment of the upper gastrointestinal tract was performed in order to determine the cause of bleeding. The esophagogastroduodenoscopy detected the presence of esophagitis grade C (Los Angeles Classification) [8] and ulcer lesions in the duodenal bulb. Endoscopic hemostasis of the culprit duodenal ulcer lesions was performed. The urgent CT of the abdomen demonstrated a retroperitoneal, cystic lesion in the anatomic position of the pancreatic tail (Fig. 3). The rest of the tomographic images did not reveal any pathological findings. Given the recent medical history of the patient and the preceded surgical procedure, the patient was transferred to the department of surgery of the collaborating oncology hospital for further evaluation. The subsequent esophagogastroduodenoscopy at the fourth day of stay confirmed the presence of ulcerative grade C esophagitis and ulcer lesions in the duodenal bulb without active bleeding. Due to relapse of melena the following day, though, a new emergent esophagogastroduodenoscopy was performed with similar findings. However, fresh blood was observed in the descending part of the duodenum, probably derived from the major duodenal papilla (hemosuccus pancreaticus). In view of the duodenal ulcer lesions and the nodal appearance of the mucosa, biopsies of the descending part of the duodenum were received and the levels of serum gastrin hormone were measured. The bioptic sample was negative for malignancy and the serum gastrin was not elevated (gastrin = 68 pg/mL, normal values < 110 pg/mL).
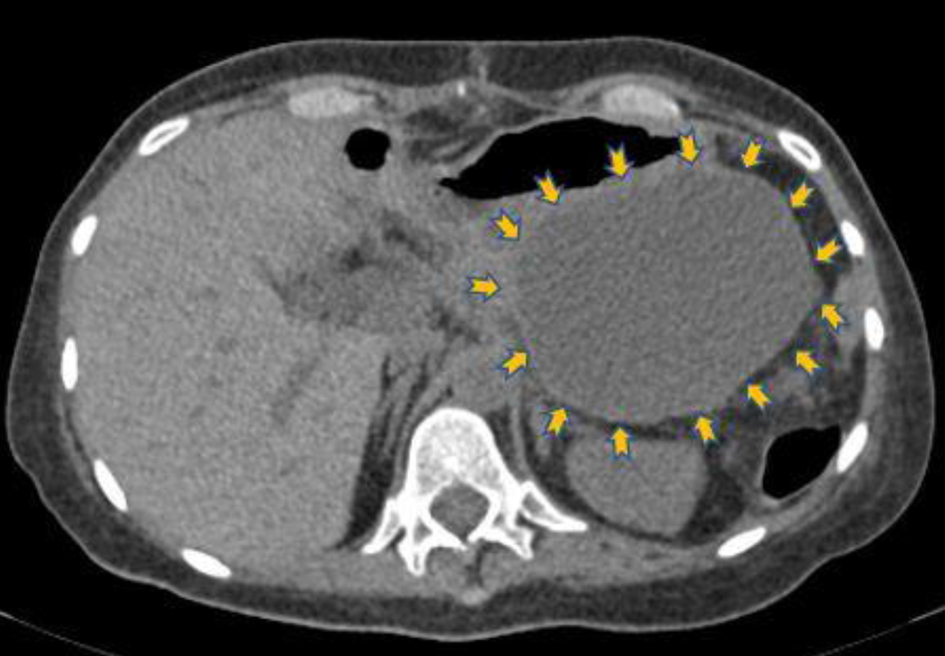 Click for large image | Figure 3. Transverse non-contrast computed tomography image that shows hypodense lesion (9 × 9 cm) in the anatomic position of the pancreatic tail (yellow arrows), which has been attributed to postoperative complication (lymphocele). |
The patient received supportive care with blood transfusions, fresh frozen plasma and octreotide administration. The cystic lesion in the retroperitoneal region was drained successfully under CT imaging guidance during hospitalization. The patient remained hemodynamically stable, melena gradually recessed and the cystic lesion shrank significantly in size, as confirmed by a repetitive abdominal CT before discharge. The cystic lesion was considered to be a postoperative complication (lymphocele).
Follow-up and outcomes
One month after discharge the patient was reassessed clinically by the department of endocrinology and department of surgery of the oncology hospital. She was clinically stable with no symptoms, no episodes of diarrhea and no tarry stools.
Two months after discharge, the patient was hospitalized in the endocrinology department of the collaborating oncology hospital for follow-up examination. The laboratory investigations included measurement of hormonal blood markers, i.e., morning cortisol, PTH, TSH, T4, calcitonin as well as specific antigens, i.e., carcinoembryonic antigen (CEA), chromogranin A (CgA) and neuron-specific enolase (NSE). All of the above tests were into the normal range. The abdominal MRI did not show any signs of metastasis or relapse of the disease.
The next three follow-up meetings, arranged during the next 14 months after the initial hospitalization, showed normal levels of thyroid hormones, CgA, NSE and calcitonin. Furthermore, the two abdominal CTs performed during these visits did not reveal any local relapse of the disease or any metastatic lesions. Moreover, the whole-body somatostatin receptor scintigraphy with radiolabeled octreotide, performed after 18 months from surgical resection of the tumor, did not identify any abdominal or extra-abdominal sites of possible malignant activity.
After almost 10 years from the initial diagnosis and management, the patient’s clinical condition is stable with no signs of relapse of the disease or metastatic foci in the periodic clinical, laboratory and imaging follow-up investigations performed once yearly (Table 1).
| Discussion | ▴Top |
The case of pancreatic VIPoma presented herein differs from other previously reported cases [9-16] in terms of the short duration and rapid progression of patient’s symptoms before admission. The patient reported no similar symptoms in the past few months or intake of medications that could be responsible for diarrhea.
Despite the abrupt onset of patient’s symptoms, our case presented with severe hypokalemia, hypercalcemia, hyperglycemia and metabolic acidosis, all typical findings of VIPoma [5, 17]. Overproduction of VIP can lead to severe potassium depletion due to fecal losses as well as due to stimulation of the renin-angiotensin-aldosterone system [18]. Hypercalcemia in VIPoma can be encountered in conjunction with the MEN1 syndrome or can be attributed to the direct effects of VIP on osteoclasts activity [19]. Moreover, the observed hyperglycemia in VIPoma has been associated with the capacity of VIP to induce glycogenolysis [20].
After exclusion of other common causes of acute diarrhea and in view of the unchanged clinical and biochemical status of the patient despite initial supportive treatment, the suspicion of a functional neuroendocrine tumor was raised as the culprit of acute diarrhea. The decision of an urgent abdominal MRI was taken due to the high sensitivity and specificity of this imaging modality that are estimated to be over 85% and 75%, respectively, for the diagnosis of neuroendocrine tumors [21]. Besides, MRI has proven to be more effective in detecting small pNETs (less than 2 cm) and liver metastases than contrast-enhanced abdominal CT [21, 22]. The MRI confirmed our clinical suspicion and localized the tumor at the level of the pancreatic tail, a finding that is in accordance with previous reports [22]. However, we should not undervalue the important role of CT imaging in the diagnosis of neuroendocrine tumors as it often constitutes the initial imaging modality due to its lower cost and higher availability compared to MRI [21]. Moreover, contrast-enhanced CT is quite accurate in diagnosing relatively larger pNETs (more than 2 cm) with sensitivity of over 63% and specificity of over 83% [21].
Another important diagnostic tool for pNETs that should be highlighted is the endoscopic ultrasound (EUS). More specifically, EUS-fine-needle aspiration (EUS-FNA) or fine-needle biopsy (EUS-FNB) are diagnostic methods quite accurate in determining the nature of a pancreatic mass as well as in defining grading of pNETs with comparable concordance with surgical grading [23]. Their diagnostic yield for tissue sampling becomes even greater with the use of specific type and size of needles according to a recent meta-analysis [24]. Unfortunately, there was lack of experience and proper equipment for EUS-guided tissue sampling in our region when our patient was firstly diagnosed with the pancreatic mass 10 years ago.
The subsequent diagnostic steps in the endocrinology department of the oncology hospital were focused on determining the nature of the pancreatic mass. Specific blood markers, i.e., CgA, NSE and synaptophysin, have been correlated with the presence of neuroendocrine tumors [25]. The elevated serum CgA in our case confirmed the neuroendocrine nature of the tumor whereas the high levels of plasma VIP, i.e., more than four times the laboratory’s reference normal value, established the final diagnosis. The levels of calcitonin were initially elevated possibly because of the co-secretion of calcitonin by the tumor cells [5]. The normal values of 24-h urine HIAA and serum gastrin excluded the presence of a carcinoid tumor and gastrinoma, respectively. Moreover, the laboratory and radiological investigations performed did not provide evidence of the existence of MEN1 syndrome in our patient.
The primary step in managing patients with secretory diarrhea caused by VIPoma is the stabilization of the patient with fluid and electrolyte replacement [26]. Somatostatin analogues play a fundamental role in reducing diarrheic episodes in patients with primary or metastatic VIPoma whereas, in parallel, seem to exert antiproliferative properties and impede tumor growth [27].
Surgical resection of the tumor remains the treatment of choice in patients with pancreatic VIPoma [4], even in those with metastatic lesions [28]. Our patient underwent distal pancreatectomy with splenectomy. Histologically, the resected spleen and the lymph nodes did not present metastatic lesions. The resected tumor was stained positive for synaptophysin, cytokeratin and chromogranin, all of which constitute typical immunohistochemical characteristics of neuroendocrine tumors [10].
Chemotherapeutic treatment regimens consisting of streptozocin or platinum have also been tested for patients with advanced pNETs with various results [29]. However, the majority of functional pNETs are resistant to chemotherapy whereas serious adverse events can also occur [29].
Two molecular-targeted agents, everolimus and sunitinib malate, have been approved for the treatment of metastatic or locally advanced pNETs with promising results [27]. Other treatment options include peptide receptor radionuclide therapy with radiolabeled somatostatin analogues as adjuvant treatment or in cases of advanced metastatic disease, debulking surgery for inoperable metastatic tumors and radiofrequency ablation or cryoablation for small metastases [27].
EUS-guided radiofrequency ablation (EUS-RFA) is an emerging minimally invasive option for patients with functional and non-functional pNETs [30]. Recent observational data showed similar efficacy and better safety of EUS-RFA compared with surgery in patients with pancreatic insulinoma [31]. However, the decision to proceed with either EUS-RFA or surgery for patients with pNETs requires a multidisciplinary approach taking into consideration all the relevant patient- and procedural-related factors and outcomes [30].
The follow-up of our patient was based on repetitive CT and MR scanning, measurement of specific tumor markers (CgA, NSE) and octreoscan scintigraphy, all of which play primary roles in detecting intra- and extra-abdominal areas of malignancy and defining prognosis [32].
To the best of our knowledge, there is paucity of data regarding cases of pancreatic VIPoma with acute onset and rapid progression. Our search of the literature revealed only two similar case reports in which the patients had acute diarrhea, i.e., duration of symptoms up to 2 weeks [33, 34]. In the first report [33], the patient was stabilized with fluid and electrolyte replacement and managed with distal pancreatectomy and splenectomy in agreement with our study. No report on the administration of somatostatin analogues was given, either pre- or postoperatively. In contrast to the relapse of the disease in the above case, our patient remains asymptomatic almost 10 years after surgical removal of the tumor without any signs of recurrence or metastasis. In the second report [34], the clinical course of the patient deteriorated to the extent that intensive care management became necessary. Despite the gradual improvement in clinical status after the administration of octreotide, the patient eventually died of severe respiratory infection. The concomitant congenital myopathy might have partially contributed to the poor outcome in the latter case. Regarding our patient, the surgical resection of the tumor was successful in spite of the unfavorable clinical and biochemical profile of the patient before surgery. The initiation of somatostatin treatment, though, was done just perioperatively. The use of a somatostatin analogue earlier during the preoperative period could have controlled more efficiently fluid losses and electrolyte abnormalities [35]. What is more, the earlier use of a somatostatin analogue might have helped in the restoration of bowel motility and recession of paralytic ileus [36].
Our case underlines the importance of early over delayed diagnosis in patients with VIPoma, irrespective of duration of symptoms or clinical presentation. Previous reports have highlighted the complications of delayed diagnosis in cases of VIPoma associated with chronic diarrhea [37]. Our patient presented with treatment-resistant diarrhea of acute onset accompanied with life-threatening hypokalemia, factors that rendered the situation time-sensitive and the need for early diagnosis urgent. Even in cases like ours, clinical suspicion of VIPoma and early diagnosis can prevent complications such as dehydration, metabolic acidosis, electrolyte imbalance and acute kidney injury, all of which were initially present in our patient. The time delay between initial presentation and MRI diagnosis of tumor was 7 days and the respective delay till surgery was 10 days in our case. Earlier detection of VIPoma in our patient, even just few days earlier, could have led to earlier surgery and control of symptoms as well as surgical operation under more stable conditions (in terms of dehydration, nutritional status, hypokalemia, paralytic ileus, etc.).
In terms of treatment, the prompt surgical resection of the tumor and the effective pre- and postoperative supportive care (fluid and electrolyte replenishment, somatostatin analogues) remain the therapeutic management of choice, even in cases of pancreatic VIPoma with acute onset and severe clinical picture like ours. Given the time-sensitive condition of these cases with the concomitant severe electrolyte abnormalities, the diagnosis and final management should be completed in inpatient settings and preferably during the first days from admission for the optimal outcome. Other therapeutic options should be considered in cases of inoperable or metastatic disease or for control of tumor growth.
The prognosis of patients with VIPoma varies and depends largely on the size of the tumor, the grading and staging of the tumor at the initial diagnosis, the effective management of fluid and electrolyte abnormalities pre-operatively and the surgical operability of the tumor [27]. Despite the persistent hypokalemia of our patient pre-operatively and the large size of the tumor at initial diagnosis, the surgical removal of the tumor proved to be effective and lifesaving for the patient. Even if there is paucity of literature data on prognosis of VIPoma cases with acute onset and rapid progression, the relatively earlier diagnosis in such cases followed by prompt surgical tumor resection may result in favorable long-term prognosis and survival, as observed in our case. The lower time frame for tumor expansion and metastases in these cases compared with insidious VIPoma cases with delayed diagnosis, might partially contribute to a possible positive prognosis.
In conclusion, our case report highlights the fact that pancreatic VIPoma can occasionally have acute presentation that requires high clinical suspicion. After exclusion of common causes of secretory diarrhea, the persistence of clinical symptoms accompanied with the specific electrolyte abnormalities are suggestive of pancreatic VIPoma irrespective of the duration of symptoms. Similarly to the slow progressing cases of pancreatic VIPoma, the timely surgical resection of the tumor in combination with the optimal pre- and postoperative supportive care should be the first priority in cases with acute onset.
Learning points
Pancreatic VIPoma is a rare neuroendocrine tumor manifested mainly with chronic secretory diarrhea and electrolyte abnormalities, the most important of which is hypokalemia. The abrupt onset of symptoms with multiple diarrheal episodes accompanied with severe and persistent hypokalemia is extremely rare for this type of tumor but warrants high clinical suspicion. Thus, pancreatic VIPoma should be considered in the differential diagnosis of acute diarrhea, especially after negative or inconclusive initial investigations for common causes of diarrhea and/or persistent symptoms despite initial supportive treatment. The diagnosis should be as prompt as possible and include a combination of laboratory and imaging investigations. After stabilization of the patient, the prompt and extensive surgical resection of the tumor remains the treatment of choice whereas other treatment options are also available for advanced or metastatic disease.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
No potential conflict of interest relevant to this article.
Informed Consent
Informed consent was obtained from the patient for publication of this case report. All the images have been anonymized and there are no patient identifiers.
Author Contributions
All authors were involved in diagnosis, treatment, and management of the patient. Giampatzis V. drafted the report. All authors read and critically reviewed the manuscript, and then approved the final submitted version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CEA: carcinoembryonic antigen; CgA: chromogranin A; CT: computed tomography; EUS: endoscopic ultrasound; EUS-FNA: EUS-fine-needle aspiration; EUS-FNB: EUS-fine-needle biopsy; EUS-RFA: EUS-radiofrequency ablation; HIAA: hydroxyindoleacetic acid; MEN1: multiple endocrine neoplasia type 1; MRI: magnetic resonance imaging; NSE: neuron-specific enolase; pNETs: neuroendocrine tumors of the pancreas; PTH: parathormone; TSH: thyroid-stimulating hormone; VIP: vasoactive intestinal peptide; VIPoma: vasoactive intestinal peptide-producing tumor
| References | ▴Top |
- Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19(10):1727-1733.
doi pubmed pmc - Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15(2):409-427.
doi pubmed pmc - Bode J. VIPoma. In: Lang F, editor. Encyclopedia of molecular mechanisms of disease. Springer Berlin Heidelberg; 2009. p. 2206-2207.
- Ro C, Chai W, Yu VE, Yu R. Pancreatic neuroendocrine tumors: biology, diagnosis,and treatment. Chin J Cancer. 2013;32(6):312-324.
doi pubmed pmc - Ghaferi AA, Chojnacki KA, Long WD, Cameron JL, Yeo CJ. Pancreatic VIPomas: subject review and one institutional experience. J Gastrointest Surg. 2008;12(2):382-393.
doi pubmed - Nikou GC, Toubanakis C, Nikolaou P, Giannatou E, Safioleas M, Mallas E, Polyzos A. VIPomas: an update in diagnosis and management in a series of 11 patients. Hepatogastroenterology. 2005;52(64):1259-1265.
pubmed - Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707-712.
doi pubmed - Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172-180.
doi pubmed pmc - Johnson JB, Marsden L, Samadder NJ. A rare cause of diarrhea: pancreatic VIPoma. Endoscopy. 2013;45(Suppl 2 UCTN):E311-E312.
doi pubmed - Abu-Zaid A, Azzam A, Abudan Z, Algouhi A, Almana H, Amin T. Sporadic pancreatic vasoactive intestinal peptide-producing tumor (VIPoma) in a 47-year-old male. Hematol Oncol Stem Cell Ther. 2014;7(3):109-115.
doi pubmed - Adam N, Lim SS, Ananda V, Chan SP. VIPoma syndrome: challenges in management. Singapore Med J. 2010;51(7):e129-132.
pubmed - Farina DA, Krogh KM, Boike JR. Chronic diarrhea secondary to newly diagnosed VIPoma. Case Rep Gastroenterol. 2019;13(1):225-229.
doi pubmed pmc - Chen C, Zheng Z, Li B, Zhou L, Pang J, Wu W, Zheng C, et al. Pancreatic VIPomas from China: case reports and literature review. Pancreatology. 2019;19(1):44-49.
doi pubmed - Mendes Filho O, Maues CAD, De Macedo F, Monteiro SAC, Araujo EFC, Rodriguez JER, Cauduro JF, et al. Neuroendocrine pancreatic tumor causing chronic diarrhea in young adult, a case report. AME Case Rep. 2020;4:13.
doi pubmed pmc - Ataallah B, Buttar BS, Kulina G, Kaell A. Hypercalcemia in a patient diagnosed with a vasoactive intestinal peptide tumor. Cureus. 2020;12(2):e6882.
doi pubmed pmc - Angelousi A, Koffas A, Grozinsky-Glasberg S, Gertner J, Kassi E, Alexandraki K, Caplin ME, et al. Diagnostic and management challenges in vasoactive intestinal peptide secreting tumors: a series of 15 patients. Pancreas. 2019;48(7):934-942.
doi pubmed - Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135(5):1469-1492.
doi pubmed pmc - Jensen R, Norton JA. Pancreatic endocrine tumors. In: M F, LS F, MH S, editors. Sleisenger and Fordtran’s gastrointestinal and liver diseases. Philadelphia: Saunders; 2002. p. 988-1016.
- Jiang J, Zhang L, Wu Z, Ai Z, Hou Y, Lu Z, Gao X. A rare case of watery diarrhea, hypokalemia and achlorhydria syndrome caused by pheochromocytoma. BMC Cancer. 2014;14:553.
doi pubmed pmc - Feliu JE, Marco J. Stimulatory effect of the intestinal peptide PHI on glycogenolysis and gluconeogenesis in isolated rat hepatocytes. Biochem J. 1983;214(3):999-1002.
doi pubmed pmc - Lee DW, Kim MK, Kim HG. Diagnosis of pancreatic neuroendocrine tumors. Clin Endosc. 2017;50(6):537-545.
doi pubmed pmc - Camera L, Severino R, Faggiano A, Masone S, Mansueto G, Maurea S, Fonti R, et al. Contrast enhanced multi-detector CT and MR findings of a well-differentiated pancreatic vipoma. World J Radiol. 2014;6(10):840-845.
doi pubmed pmc - Tacelli M, Bina N, Crino SF, Facciorusso A, Celsa C, Vanni AS, Fantin A, et al. Reliability of grading preoperative pancreatic neuroendocrine tumors on EUS specimens: a systematic review with meta-analysis of aggregate and individual data. Gastrointest Endosc. 2022;96(6):898-908.e823.
doi pubmed - Gkolfakis P, Crino SF, Tziatzios G, Ramai D, Papaefthymiou A, Papanikolaou IS, Triantafyllou K, et al. Comparative diagnostic performance of end-cutting fine-needle biopsy needles for EUS tissue sampling of solid pancreatic masses: a network meta-analysis. Gastrointest Endosc. 2022;95(6):1067-1077.e1015.
doi pubmed - Ehehalt F, Saeger HD, Schmidt CM, Grutzmann R. Neuroendocrine tumors of the pancreas. Oncologist. 2009;14(5):456-467.
doi pubmed - Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract Res Clin Gastroenterol. 2012;26(6):737-753.
doi pubmed pmc - Abdullayeva L. VIPoma: Mechanisms, clinical presentation, diagnosis and treatment (Review). World Acad Sci J. 2019;1(5):229-235.
- Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197(1):29-37.
doi pubmed - Zhou C, Zhang J, Zheng Y, Zhu Z. Pancreatic neuroendocrine tumors: a comprehensive review. Int J Cancer. 2012;131(5):1013-1022.
doi pubmed - Rossi G, Petrone MC, Healey AJ, Arcidiacono PG. Approaching small neuroendocrine tumors with radiofrequency ablation. Diagnostics (Basel). 2023;13(9):1561.
doi pubmed pmc - Crino SF, Napoleon B, Facciorusso A, Lakhtakia S, Borbath I, Caillol F, Do-Cong Pham K, et al. Endoscopic ultrasound-guided radiofrequency ablation versus surgical resection for treatment of pancreatic insulinoma. Clin Gastroenterol Hepatol. 2023.
doi pubmed - Vinik AI, Woltering EA, Warner RR, Caplin M, O'Dorisio TM, Wiseman GA, Coppola D, et al. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39(6):713-734.
doi pubmed - Singh HM, Teller T, Esrason KT, Abbas MA. VIPoma: A rare cause of acute diarrhea. Surgical Rounds. 2007;414:8.
- Schembri J, Sammut L, Ellul P. Vipoma: a rare tumour , a rarer cause of acute diarrhoea. JSM Clin Case Rep. 2013;1(1):1007.
- Akerstrom G, Falconi M, Kianmanesh R, Ruszniewski P, Plockinger U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: pre- and perioperative therapy in patients with neuroendocrine tumors. Neuroendocrinology. 2009;90(2):203-208.
doi pubmed - Koberstein B, Layer P, Balzer K, Muller MK, Singer MV, Goebell H. Paralytic ileus responding to somatostatin therapy: first manifestation of a VIPoma. Dig Dis Sci. 1989;34(11):1803-1804.
doi pubmed - Sanchez-Salazar SM, Torres-Alzate S, Mumoz-Cortes VM, Builes-Barrera CA, Gutierrez-Montoya JI, Roman-Gonzalez A. VIPoma: a rare cause of diarrhea. A case report. Revista Facultad de Medicina. 2021;69(3):e81603.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


