| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 1, January 2024, pages 7-14
Epidural Abscess Complicating Tunneled Caudal Epidural Catheter in an Infant for Postoperative Pain Management of Open Abdominal Surgery
Amr Elhamrawya, c , Savannah Aeplia, Grant Heydingera, b, Joseph D. Tobiasa, b, Ralph J. Beltrana, b
aDepartment of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH, USA
bDepartment of Anesthesiology & Pain Medicine, The Ohio State University College of Medicine, Columbus, OH, USA
cCorresponding Author: Amr Elhamrawy, Department of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital, Columbus, OH 43205, USA
Manuscript submitted December 1, 2023, accepted January 10, 2024, published online January 28, 2024
Short title: Epidural Abscess in an Infant
doi: https://doi.org/10.14740/jmc4180
| Abstract | ▴Top |
Regional anesthesia is being used more frequently in pediatric anesthesia practice, including the perioperative care of neonates and infants. Adverse effects may be encountered during epidural needle placement, with catheter advancement, or subsequently during infusion of local anesthetic agents. Despite applying standard practice of care regarding placement of epidural catheter, epidural catheter-related infections may still occur. We present the rare occurrence of an epidural abscess in a 4-month-old infant after placement and subsequent use of a tunneled caudal epidural catheter for postoperative pain management following abdominal surgery. Magnetic resonance imaging (MRI) was the gold standard diagnostic imaging modality and was used to identify the abscess. Management included intravenous antibiotic therapy as well as hemilaminectomy with evacuation of the epidural abscess and hematoma. The patient continued to progress well with no deficits noted on neurological examination. There were no other postoperative concerns. When there is a concern for epidural catheter-related infection, the catheter should be removed immediately. The epidural catheter tip as well as any purulent discharge from the insertion site should be sent for culture and sensitivity. Urgent neurosurgical and infectious disease consultation is suggested to provide opinions regarding surgical intervention and antibiotic therapy.
Keywords: Epidural anesthesia; Caudal block; Epidural abscess; Infection
| Introduction | ▴Top |
Epidural analgesia is an essential component of a multimodal approach to perioperative pain management in neonates, infants, and children [1]. Although opioids are commonly administered to prevent or treat severe postoperative pain, their use may be associated with adverse effects including respiratory depression and delayed gastrointestinal motility [2-4]. Despite its efficacy in controlling postoperative pain, adverse effects may still occur during epidural anesthesia related to catheter placement or its subsequent use and the agents used to provide analgesia [5]. Although relatively uncommon, adverse effects during epidural anesthesia may include failure to correctly identify the epidural space, inadvertent dural puncture, epidural hematoma, neurological injuries, medication errors, catheter malfunction, catheter migration, infection, or local anesthetic toxicity [6-8]. We present a 4-month-old infant who developed an epidural abscess after placement and use of a tunneled caudal epidural catheter.
| Case Report | ▴Top |
Investigations
Review of this case and presentation in this format followed the guidelines of the Institutional Review Board approval at Nationwide Children’s Hospital (Columbus, OH). A 5.2 kg, 4-month-old full-term infant presented for laparoscopic Ladd procedure, hiatal hernia repair, and insertion of a gastrostomy tube. Past medical history was notable for gastroschisis, previously managed with silo placement and bedside closure. She had been an inpatient in the neonatal intensive care unit (NICU) since birth. Family history was negative. On physical examination, the patient was in no acute distress. Preoperative vital signs revealed temperature 36.7 °C (98 °F), pulse 173 beats/min (normal range: 100 - 160 beats/min), respiration 56 breaths/min (normal range: 30 - 60 breaths/min), blood pressure 79/35 mm Hg (normal range: 60 - 80/30 - 45 mm Hg), and oxygen saturation 100% (normal range: 95-100%). The airway, cardiac, and respiratory examinations were unremarkable. Preoperative laboratory evaluations including a complete blood count, electrolytes, and renal function were normal. The hemoglobin was 11.9 g/dL with a hematocrit of 34.9%. The anesthesia plan included general anesthesia with a rapid sequence induction. In addition, the parents were consented for caudal epidural catheter placement at the end of procedure if the surgical procedure switched to open instead of laparoscopic. The patient was held nil per os for 6 h and maintenance intravenous fluids were administered. The patient was transported to operating room where routine American Society of Anesthesiologists’ monitors were applied. Anesthesia was induced with propofol (2 mg/kg), fentanyl (1 µg/kg), and rocuronium (1.2 mg/kg). The patient’s trachea was intubated with a 3.0 mm cuffed endotracheal tube on the second attempt using a Miller 1 laryngoscope blade and direct laryngoscopy. Cefazolin was administered for prophylaxis against a surgical site infection. Maintenance anesthesia was continued with sevoflurane (expired concentration 2-2.5%) in air and oxygen. The procedure lasted approximately 4 h and conversion from laparoscopic to open was necessary due to intra-abdominal adhesions. Intraoperative analgesic agents included intravenous fentanyl (4 µg/kg) in four divided doses, and a single dose of intravenous acetaminophen (15 mg/kg). No intraoperative concerns were encountered. Total fluid intake included 10% dextrose (80 mL), normal saline (60 mL), and 5% albumin (50 mL). After completion of the procedure, a caudal catheter was inserted using standard sterile technique and draping, including a sterile field with personnel wearing sterile gowns, hat, mask, and gloves. The neonate was positioned in the left lateral decubitus position and a regional anesthetic time-out was performed. After the skin was cleaned with chlorhexidine, sterile towels were placed surrounding the insertion site of the Tuohy needle into the caudal space. After measuring the distance from the caudal inlet (sacrococcygeal ligament) to T9, the location of the sacral hiatus was identified. An 18-gauge Tuohy needle was inserted at a 45° to the skin and redirected when the posterior surface of the sacrum was contacted. After loss of resistance (approximately 2 cm from the skin) to identify the sacro-coccygeal ligament, the caudal epidural catheter was threaded through the Touhy needle until its tip was seen at T8-9 under ultrasound guidance. The length of the epidural tubing was 14 cm at exit site (the total length of caudal epidural set is 91.5 cm). After a negative test dose (0.5 mL of 1.5% lidocaine with epinephrine 1:200,000), the caudal epidural catheter was tunneled subcutaneously and fixed to the skin. The insertion site was covered by a chlorhexidine gluconate (CHG) dressing. After a bolus dose of 3 mL of 0.1% ropivacaine (slow incremental doses), a continuous epidural infusion was initiated with 1.5% chloroprocaine with clonidine 0.15 µg/mL at 3.5 mL/h. The catheter tip was confirmed to be at T8-9 by intraoperative ultrasound, and subsequent postoperative radiograph. Residual neuromuscular blockade was reversed with sugammadex (10 mg) and the patient’s trachea was extubated. She was transferred to the NICU. Her postoperative pain management plan included intravenous acetaminophen 48 mg every 6 h, intravenous morphine 0.24 mg every 3 h as needed, and the continuous epidural chloroprocaine-clonidine infusion at 3.5 mL/h. On postoperative day (POD) 3, she required three doses of intravenous morphine for a pain score > 4. The epidural catheter was removed on POD 4 without complications. On POD 5, the tunneled insertion site was noted to be erythematous with a purulent discharge (Fig. 1). The patient had a new onset of fever to 38.2 °C. A summary of the hospital course is listed in Table 1.
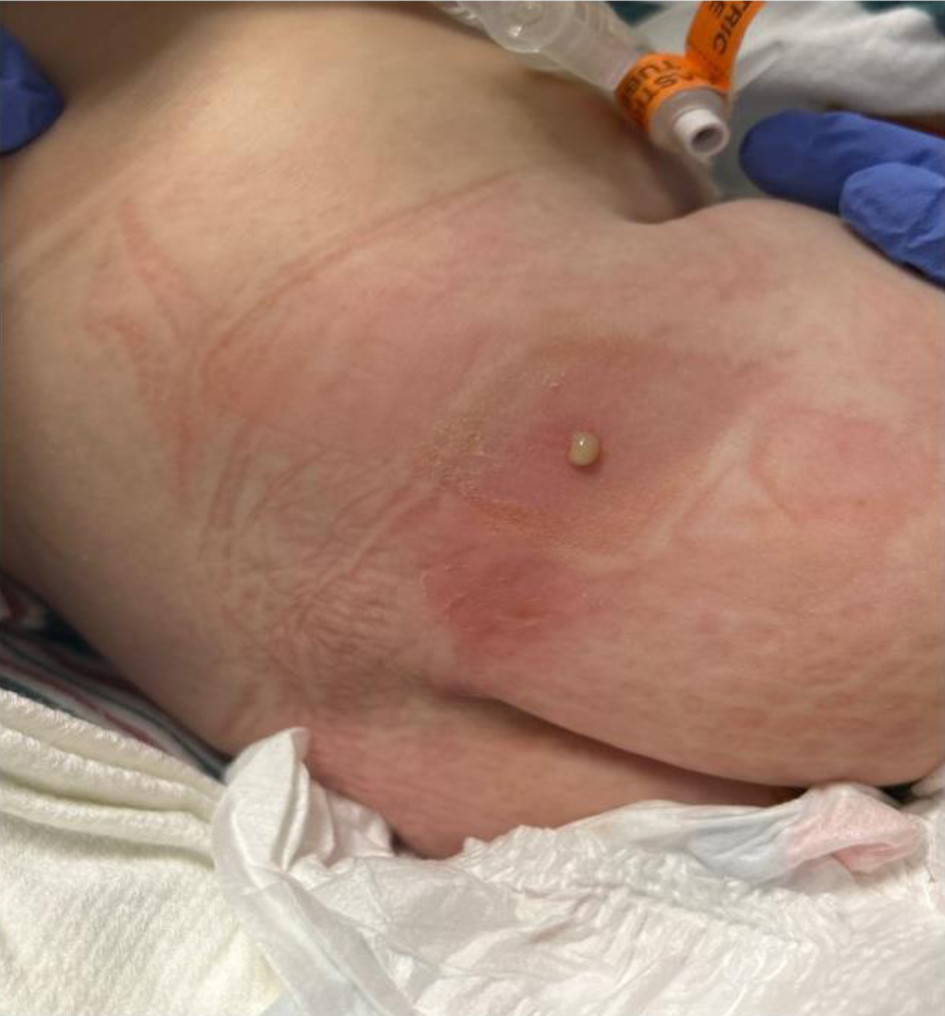 Click for large image | Figure 1. Postoperative photograph of our patient showing the insertion site and tunneled trach with a purulent discharge that was noted on postoperative day 5. |
 Click to view | Table 1. Hospital Course |
Diagnosis
Blood cultures were obtained and broad-spectrum antibiotics vancomycin (20 mg/kg/dose every 8 h), nafcillin (50 mg/kg/dose every 6 h) and cefotaxime (50 mg/kg/dose every 6 h) were started. On the following day, antibiotic coverage was changed to vancomycin and cefepime (50 mg/kg/dose every 6 h). MRI was ordered and a beside ultrasound of the sacrum and spine was obtained. The tunneled site failed to show a drainable fluid collection, but spine imaging noted a segmental epidural fluid collection most consistent with a hematoma. Subsequent MRI of the spine demonstrated the dorsal epidural hematoma from C7 to the sacrum and epidural/intradural enhancement. The patient had no neurological deficits but continued to have fevers despite antibiotic treatment. Neurosurgical consultation recommended against lumbar puncture given the risk of sampling epidural fluid or seeding infection to the intradural space. On POD 10, repeat MRI of the spine demonstrated a complex epidural fluid collection from C5 to the sacrum which was concerning for an infectious process or abscess (Fig. 2). MRI of the brain was negative.
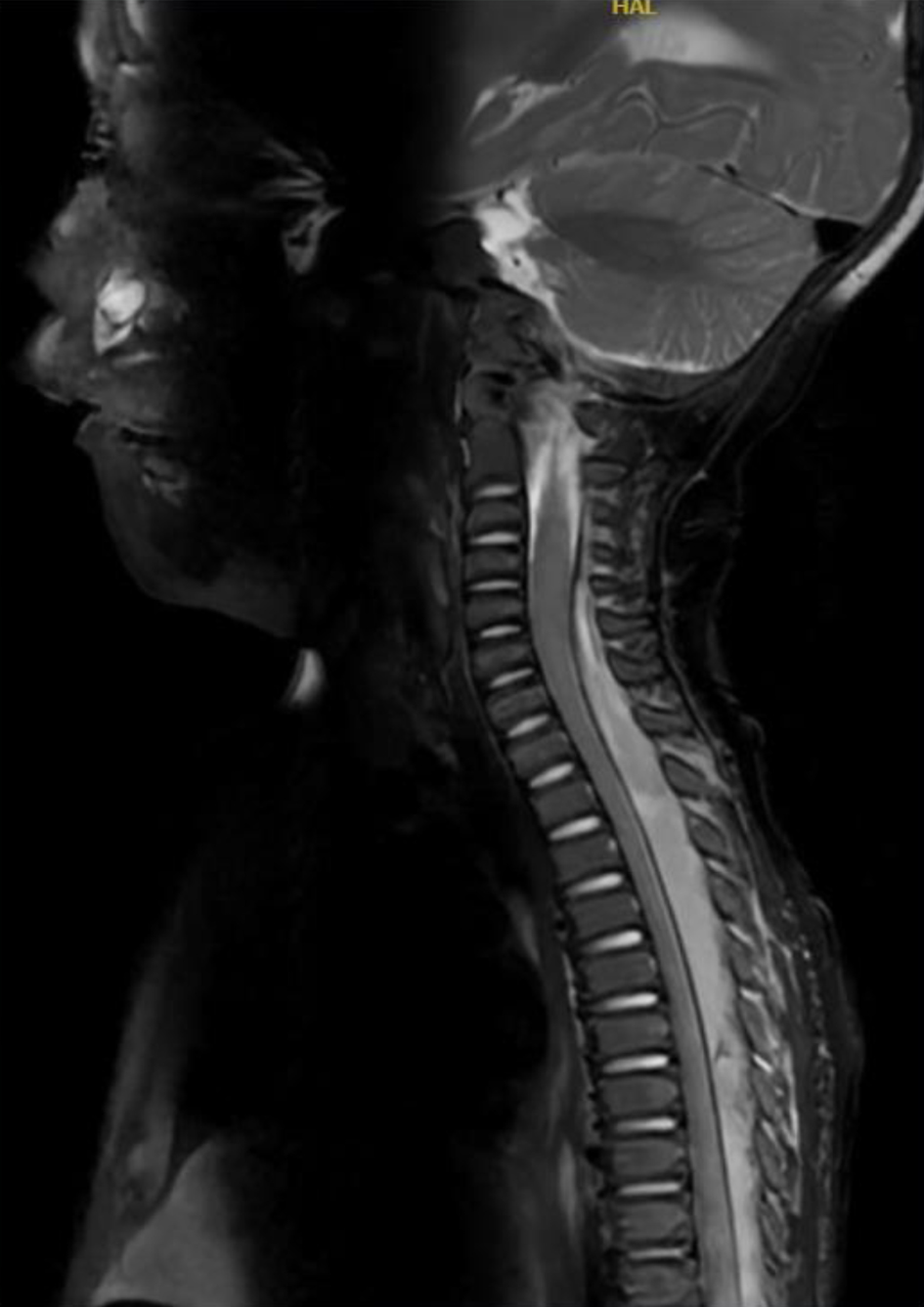 Click for large image | Figure 2. Magnetic resonance imaging of the spine obtained on postoperative day 10 demonstrating a complex epidural fluid collection with abscess formation from C5 to the sacrum (abscess). |
Treatment
The patient was taken to the operating room where a left T5-T6 hemilaminectomy was performed with evacuation of an epidural abscess and hematoma. Irrigation with antibiotic-impregnated fluids was performed superiorly (within the upper thoracic cervical epidural space) and inferiorly (within the lower thoracic and lumbar/sacral epidural space).
Follow-up and outcomes
Bacterial cultures of the epidural abscess revealed pan-sensitive Pseudomonas aeruginosa. Postoperative antibiotics were continued with vancomycin and cefepime with the addition of metronidazole. Intraoperative cultures grew Pseudomonas aeruginosa. The patient completed a 6-week course of intravenous cefepime to treat the epidural abscess. She continued to progress with no deficits noted on her neurological examination. There were no other postoperative concerns.
| Discussion | ▴Top |
The incidence of an epidural abscess following use of an indwelling epidural catheter in pediatric-aged patients remains extremely low. However, catheter-related infections including abscess formation can result in increased hospital length of stay, increased healthcare costs, the need for surgical intervention, and even patient mortality. Given these concerns, strict adherence to aseptic technique with catheter placement and its subsequent use is mandatory to limit the occurrence of this adverse effect. Additionally, early identification and intervention is needed whenever a catheter-related infection is suspected.
The first reports of caudal epidural anesthesia in children were published in 1933 followed by its use in neonates in 1950 [9, 10]. Widespread clinical use of caudal epidural anesthesia expanded in the 1970s and 1980s as regional anesthesia was used instead of general anesthesia to avoid the potential for apnea following general anesthesia with halothane as well as a means to control postoperative pain in infants and children. As regional anesthesia techniques progressed in neonates and infants, there was increased use of continuous epidural anesthesia in place of a single shot caudal epidural block to provide more prolonged postoperative analgesia following major surgical procedures. Options for such techniques included direct placement of the catheter at the level of surgery or threading the catheter from the caudal epidural space to the desired level [11, 12]. Early experience with the combination of general anesthesia and caudal epidural anesthesia for major abdominal surgery in neonates and infants was reported by Bosenberg et al in 1988 [11]. To demonstrate the feasibility of threading a catheter from the caudal space to the thoracic level, the authors performed a three-phase study. The first phase demonstrated passage of a catheter from the caudal to thoracic level in cadaveric specimens. The second phase demonstrated the feasibility of the technique in piglets. Phase three involved a cohort of 20 neonates and infants undergoing biliary tract surgery. In 19 of 20 cases, the catheter tip was placed within one vertebrae of the desired thoracic dermatome (T8). Subsequent authors reported similar successful experiences with passage of the catheter from the caudal to thoracic levels in neonates and infants. Given the potential to avoid complications related to direct needle/catheter placement at the level of surgery, as was chosen in our patient, the routine clinical practice in neonates continues to rely on the threading of an epidural catheter from the caudal space to the desired dermatome [13, 14].
As with any procedure, adverse effects may occur with caudal epidural anesthesia in neonates and infants. With regional anesthesia, adverse effects may be related to placement of the needle and catheter, the medications that are subsequently infused, or physiological effects of the medications (sympathectomy resulting in hypotension or bradycardia). To date, there are no reports of infectious complications following single shot caudal epidural anesthesia [5, 7]. However, placement of an indwelling catheter may increase the potential for an infectious complication. Following the introduction of this practice, various investigators have attempted to evaluate the potential for bacterial colonization of epidural catheters and clinically significant infections [15-17].
McNeely et al prospectively cultured the distal tip of epidural catheters following their removal in a cohort of 91 pediatric patients (45 caudal and 46 lumbar epidural catheters) [15]. Cultures were positive in nine of the 45 (20%) caudal epidural catheter tips compared with two of the 46 (4%) lumbar epidural catheter tips. Staphylococcus epidermidis was the predominant skin and catheter tip organism isolated in both groups; however, four of nine caudal epidural catheter tips grew gram-negative bacteria. No patient developed a clinical epidural infection during the study period. Kost-Byerly et al prospectively studied the incidence of bacterial colonization of caudal and lumbar epidural catheters, as well as the incidence of serious systemic and local infections, in 210 (caudal epidural in 170 and lumbar epidural in 40) children after short-term epidural analgesia (3 days or fewer) [16]. All catheters were percutaneously placed using standard aseptic technique without subcutaneous tunnelling. Following removal, the subcutaneous portion of the catheter was cultured. There were no serious systemic infections in any patient. Culture revealed that 35% (73 of 210) of the catheters were colonized. Gram-positive colonization was similar in caudal (25%; 43 of 170) and lumbar (23%; 9 of 40) catheters. Gram-negative organisms were cultured from 16% of the caudal catheters (27 of 170) and 3% of the lumbar catheters (1 of 40). When using caudal epidural catheters, colonization was less likely in patients more than 3 years of age. These studies demonstrated that although catheter colonization occurs, systemic infections did not with short-term use. The incidence of epidural catheter tip colonization was increased with the caudal route of insertion. Additionally, gram-negative bacteria were cultured more commonly with caudal insertion.
Despite relatively widespread use to provide postoperative analgesia, clinically significant infectious complications (cellulitis, exit tract infections, and epidural abscess) are relatively uncommon even with the prolonged use of indwelling epidural catheters in infants and children (Table 2) [5, 7, 17-25]. Our review of the literature identified seven observational studies and four case reports with either superficial (cellulitis) or deep (abscess) infections. These reports include a total of 162 superficial infections, seven epidural catheter-related abscesses, and various other infectious-related complications including one patient with meningeal signs, one case of epidural inflammation without abscess, two paravertebral muscular infections and one suspected catheter-related fever without signs of infection. Despite the occurrence of superficial infections, we found only seven previous reports of a catheter-associated epidural abscess from four prospective observational studies and two case reports [7, 18, 20, 21, 23, 25]. The patients ranged in age from 2 months to 13 years. The placement site of the epidural catheters included three lumbar, three thoracic, and one sacral (caudal). Symptoms started while the catheters were still in place in three patients and after removal in four patients. The most common clinical signs were erythema and tenderness. Although MRI was the most common diagnostic imaging modality used for diagnosis of an epidural abscess, CT imaging and ultrasonography have also been used [20, 21, 23]. In the case reports that noted the organisms responsible, Staphylococcus aureus was cultured in three and Pseudomonas aeruginosa (as reported in our case) in one. Of note, while all patients received broad spectrum antibiotics, the epidural catheter-related abscesses were treated surgically with open drainage in only three of the seven patients [7, 18, 21]. The data from the pediatric population with significant numbers for prospective observational studies indicate an extremely low incidence of infectious complications from epidural anesthesia. The incidence is even lower for the development of a documented epidural abscess with only seven cases reported in the literature, an incidence that is significantly lower than that reported in the adult population [26, 27].
 Click to view | Table 2. Published Reports Regarding the Incidence of Epidural Catheter-Related Infections in the Pediatric Population |
Prevention starts with adherence to standard sterile technique including all of the providers in the room wearing a hat and mask. The site should be cleansed with a chlorhexidine solution followed by draping with coverage of the entire patient. All those performing or assisting with the procedure should perform standard surgical hand-washing and in addition to a hat and mask, wear a sterile surgical gown and gloves. In our case, we applied our standard practice of care regarding preparation and draping, healthcare provider sterile precautions, chlorhexidine preparation, placement of a chlorhexidine impregnated disk over the exit site, tunneling of the catheter, and placement of a sterile bio-occlusive dressing [28, 29]. The only minor variation noted was the need for placement by a second provider when the initial attempts failed. Additionally, per standard practice, the site was monitored and directly inspected on a daily basis by a member of the Acute Pain Service. The local anesthetic solution (chloroprocaine + clonidine) was changed under aseptic condition. Moreover, in clinical practice, we generally limit the duration of epidural catheter use to 4 - 5 days as the potential for infectious complications may increase with the duration of catheter use [7, 30].
In an effort to decrease the risk of soiling of the entry site of the caudal epidural catheter entry site from urine and feces as well as stabilizing the catheter in place, tunnelling of the catheter has been suggested [31, 32]. In a prospective randomized trial of 50 neonates and infants, tunneling of the catheter compared to fixation at the sacral entry site resulted in longer retention of the epidural catheter with decreased clinical concern and need for catheter removal related to soiling. Others reported no bacterial colonization of removed caudal epidural catheters that were tunneled for postoperative use in a cohort of 18 pediatric patients [32].
Epidural catheter-related infections may occur through one of four potential mechanisms. The first is entry-site contamination from surrounding skin flora, which spreads along the catheter track. This may result in either a superficial or deep infection. Organisms may also be introduced during catheter insertion. Thirdly, there may be hematogenous spread from bacteremia. Lastly, contamination may occur due to the infusion of contaminated fluids or medications [33, 34]. One factor that may limit the potential for infectious complications during epidural anesthesia is that many of the local anesthetic agents have inherent antimicrobial properties against a wide spectrum of bacterial pathogens [35]. At clinically relevant concentrations, bupivacaine 0.125-0.75% and lidocaine 1-3% inhibit the growth of numerous bacteria and fungi. Different local anesthetic agents show variation in their antimicrobial capacity with bupivacaine and lidocaine inhibiting growth to a significantly greater extent than ropivacaine. These properties are attributed to the disruption of microbial cell membrane permeability, leading to leakage of cellular components and cell lysis. However, no previous studies have evaluated the bactericidal and bacteriostatic capacity of chloroprocaine.
Learning points
The diagnosis of an infectious complication of a caudal epidural catheter starts with immediate attention to any change in the clinical status of the patient including the development of temperature instability, changes in the white blood cell count, localized tenderness or erythema over the entry site, back pain, neck stiffness, or purulent discharge. The key for achieving a prompt diagnosis is a high index of suspicion with daily inspection of the insertion site and a review of the patient’s clinical status. As in our patient, this is complemented by radiological imaging including ultrasonography, CT imaging, or MRI with immediate neurosurgical consultation if an abscess is identified. If an epidural abscess is identified, immediate broad spectrum antibiotic coverage should be initiated with consideration for catheter removal and surgical consultation with drainage as needed to prevent progression with loss of neurological function.
Conclusion
We present a 4-month-old infant who developed an epidural abscess after placement of a tunneled caudal epidural catheter. Purulent discharge from the insertion site and track was sent for culture and sensitivity, MRI was urgently ordered, and a beside ultrasound of the sacrum and spine was obtained. Urgent neurosurgical and infectious disease consultation were obtained to evaluate the need for surgical intervention and provide guidance as to choice of antibiotic therapy. Although no surgical intervention was immediately deemed necessary, when repeat MRI demonstrated complex abscess formation impinging on the spinal cord, hemilaminectomy was performed with evacuation of an epidural abscess and hematoma. The patient recovered without neurological sequelae. When there is a concern for epidural catheter-related infection, the catheter should be removed immediately. Epidural catheter tip as well as any purulent discharge from the insertion site should be sent for culture and sensitivity. Urgent neurosurgical and infectious disease consultation is suggested. Given the limited incidence in the population, no specific recommendations can be given regarding the need for surgical intervention. Primary indications for surgery include acute or progressive neurological deficits, spinal cord compression on radiological imaging, ring-enhancing lesions, or disease progression on antibiotic therapy (as seen in our patient). Initial broad spectrum, triple antibiotic therapy is suggested to cover methicillin-resistant Staphylococcus species as well as gram-negative organisms including Pseudomonas. The appropriate duration of antimicrobial therapy is determined on a case-by-case basis, but generally includes therapy for 4 - 8 weeks.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from a parent for anesthetic care and use of patient data for publication purposes. The patient information was de-identified for publication.
Author Contributions
AE provided patient care, case review and preparation of the manuscript including drafts and final version; SA and RJB provided intraoperative care of the patient and review of the final manuscript; JDT contributed to manuscript preparation, review, and editing. RJB and GH provided postoperative care of the patient and review of the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Suresh S, Schaldenbrand K, Wallis B, De Oliveira GS, Jr. Regional anaesthesia to improve pain outcomes in paediatric surgical patients: a qualitative systematic review of randomized controlled trials. Br J Anaesth. 2014;113(3):375-390.
doi pubmed - Cravero JP, Agarwal R, Berde C, Birmingham P, Cote CJ, Galinkin J, Isaac L, et al. The Society for Pediatric Anesthesia recommendations for the use of opioids in children during the perioperative period. Paediatr Anaesth. 2019;29(6):547-571.
doi pubmed pmc - Tobias JD. Acute pain management in infants and children-Part 2: intravenous opioids, intravenous nonsteroidal anti-inflammatory drugs, and managing adverse effects. Pediatr Ann. 2014;43(7):e169-e175.
doi pubmed - Martin LD, Adams TL, Duling LC, Grigg EB, Bosenberg A, Onchiri F, Jimenez N. Comparison between epidural and opioid analgesia for infants undergoing major abdominal surgery. Paediatr Anaesth. 2019;29(8):835-842.
doi pubmed - Polaner DM, Taenzer AH, Walker BJ, Bosenberg A, Krane EJ, Suresh S, Wolf C, et al. Pediatric Regional Anesthesia Network (PRAN): a multi-institutional study of the use and incidence of complications of pediatric regional anesthesia. Anesth Analg. 2012;115(6):1353-1364.
doi pubmed - Goldman LJ. Complications in regional anaesthesia. Paediatr Anaesth. 1995;5(1):3-9.
doi pubmed - Walker BJ, Long JB, Sathyamoorthy M, Birstler J, Wolf C, Bosenberg AT, Flack SH, et al. Complications in pediatric regional anesthesia: an analysis of more than 100,000 blocks from the Pediatric Regional Anesthesia Network. Anesthesiology. 2018;129(4):721-732.
doi pubmed - Brull R, McCartney CJ, Chan VW, El-Beheiry H. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg. 2007;104(4):965-974.
doi pubmed - Campbell MF. Caudal anesthesia in children. Am J Urol. 1933;30:245-249.
- Yaster M, Maxwell LG. Pediatric regional anesthesia. Anesthesiology. 1989;70(2):324-338.
doi pubmed - Tobias JD, Lowe S, O'Dell N, Holcomb GW, 3rd. Thoracic epidural anaesthesia in infants and children. Can J Anaesth. 1993;40(9):879-882.
doi pubmed - Bosenberg AT, Bland BA, Schulte-Steinberg O, Downing JW. Thoracic epidural anesthesia via caudal route in infants. Anesthesiology. 1988;69(2):265-269.
doi pubmed - Rasch DK, Webster DE, Pollard TG, Gurkowski MA. Lumbar and thoracic epidural analgesia via the caudal approach for postoperative pain relief in infants and children. Can J Anaesth. 1990;37(3):359-362.
doi pubmed - van Niekerk J, Bax-Vermeire BM, Geurts JW, Kramer PP. Epidurography in premature infants. Anaesthesia. 1990;45(9):722-725.
doi pubmed - McNeely JK, Trentadue NC, Rusy LM, Farber NE. Culture of bacteria from lumbar and caudal epidural catheters used for postoperative analgesia in children. Reg Anesth. 1997;22(5):428-431.
doi pubmed - Kost-Byerly S, Tobin JR, Greenberg RS, Billett C, Zahurak M, Yaster M. Bacterial colonization and infection rate of continuous epidural catheters in children. Anesth Analg. 1998;86(4):712-716.
doi pubmed - Pula R, Suravaram S, Thakur N, Gazula S, Gooty S, Prathyusha. Continuous epidural catheter for analgesia-risk and incidence of infection in pediatric population undergoing surgeries. Int J Health Sci Res. 2020;10(1):43-48.
- Pietropaoli JA, Jr., Keller MS, Smail DF, Abajian JC, Kreutz JM, Vane DW. Regional anesthesia in pediatric surgery: complications and postoperative comfort level in 174 children. J Pediatr Surg. 1993;28(4):560-564.
doi pubmed - Meunier JF, Norwood P, Dartayet B, Dubousset AM, Ecoffey C. Skin abscess with lumbar epidural catheterization in infants: is it dangerous? Report of two cases. Anesth Analg. 1997;84(6):1248-1249.
doi pubmed - Larsson BA, Lundeberg S, Olsson GL. Epidural abscess in a one-year-old boy after continuous epidural analgesia. Anesth Analg. 1997;84(6):1245-1247.
doi pubmed - Ingelmo PM, Marino G, Fumagalli R. Sepsis after epidural catheterization in a child with chronic regional pain syndrome type I. Paediatr Anaesth. 2005;15(7):623-624.
doi pubmed - Emmanuel ER. Post-sacral extradural catheter abscess in a child. Br J Anaesth. 1994;73(4):548-549.
doi pubmed - Llewellyn N, Moriarty A. The national pediatric epidural audit. Paediatr Anaesth. 2007;17(6):520-533.
doi pubmed - Aram L, Krane EJ, Kozloski LJ, Yaster M. Tunneled epidural catheters for prolonged analgesia in pediatric patients. Anesth Analg. 2001;92(6):1432-1438.
doi pubmed - Sethna NF, Clendenin D, Athiraman U, Solodiuk J, Rodriguez DP, Zurakowski D. Incidence of epidural catheter-associated infections after continuous epidural analgesia in children. Anesthesiology. 2010;113(1):224-232.
doi pubmed - Wang LP, Hauerberg J, Schmidt JF. Incidence of spinal epidural abscess after epidural analgesia: a national 1-year survey. Anesthesiology. 1999;91(6):1928-1936.
doi pubmed - Christie IW, McCabe S. Major complications of epidural analgesia after surgery: results of a six-year survey. Anaesthesia. 2007;62(4):335-341.
doi pubmed - Kerwat K, Eberhart L, Kerwat M, Horth D, Wulf H, Steinfeldt T, Wiesmann T. Chlorhexidine gluconate dressings reduce bacterial colonization rates in epidural and peripheral regional catheters. Biomed Res Int. 2015;2015:149785.
doi pubmed pmc - Kinirons B, Mimoz O, Lafendi L, Naas T, Meunier J, Nordmann P. Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children: a randomized, controlled trial. Anesthesiology. 2001;94(2):239-244.
doi pubmed - Bomberg H, Bayer I, Wagenpfeil S, Kessler P, Wulf H, Standl T, Gottschalk A, et al. Prolonged catheter use and infection in regional anesthesia: a retrospective registry analysis. Anesthesiology. 2018;128(4):764-773.
doi pubmed - Vas L, Naik V, Patil B, Sanzgiri S. Tunnelling of caudal epidural catheters in infants. Paediatr Anaesth. 2000;10(2):149-154.
doi pubmed - Fujinaka W, Hinomoto N, Saeki S, Yoshida A, Uemura S. Decreased risk of catheter infection in infants and children using subcutaneous tunneling for continuous caudal anesthesia. Acta Med Okayama. 2001;55(5):283-287.
doi pubmed - Grewal S, Hocking G, Wildsmith JA. Epidural abscesses. Br J Anaesth. 2006;96(3):292-302.
doi pubmed - Pradilla G, Nagahama Y, Spivak AM, Bydon A, Rigamonti D. Spinal epidural abscess: current diagnosis and management. Curr Infect Dis Rep. 2010;12(6):484-491.
doi pubmed - Johnson SM, Saint John BE, Dine AP. Local anesthetics as antimicrobial agents: a review. Surg Infect (Larchmt). 2008;9(2):205-213.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


