| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 1, January 2024, pages 1-6
Focal Hepatocellular Carcinoma in Pancreas
Yasir Ahmeda, e, Usama Sakhawatb, Fahad Malikb, Saadia Haleemac, Daniel Chind, Ali Marhabab
aDepartment of Internal Medicine, United Health Services Hospitals, Binghamton Primary Care, Binghamton, NY 13903, USA
bDepartment of Gastroenterology, United Health Services Hospitals, Binghamton/Johnson City, NY, USA
cDepartment of Pathology, Marshal University, Huntington, WV 25701, USA
dDepartment of Internal Medicine, Arnot Health System, Elmira, NY, USA
eCorresponding Author: Yasir Ahmed, Department of Internal Medicine, United Health Services Hospitals, Binghamton Primary Care, Binghamton, NY 13903, USA
Manuscript submitted December 5, 2023, accepted January 4, 2024, published online January 10, 2024
Short title: Focal Hepatocellular Carcinoma in Pancreas
doi: https://doi.org/10.14740/jmc4181
| Abstract | ▴Top |
A 67-year-old man was found to have a pancreatic head mass on abdominal ultrasound. He had compensated liver cirrhosis due to hepatitis C. The fine-needle aspiration (FNA) biopsy of the mass reported an adenocarcinoma of the pancreas, while the subsequent histopathology report of the supraclavicular lymph node showed features of hepatocellular carcinoma (HCC). A second read and additional stains on the FNA specimen confirmed a hepatoid (hepatocellular) carcinoma of the pancreas. He received atezolizumab and bevacizumab and had a good response. Tumors with features of HCC outside of the liver rarely occur and even more rarely in pancreas, with less than 50 cases reported so far. Pure HCC-like morphology is the most common histological form among four subtypes and has a relatively better prognosis. Surgical resection is considered the treatment of choice if amenable and variable outcomes are reported with different chemotherapies. Challenges exist in the diagnosis and the management of this rare and intriguing entity, and the potential misdiagnosis can have grave consequences as the management is completely different for a pancreatic adenocarcinoma and hepatoid carcinoma. We report a case with a challenging diagnosis of metastatic pancreatic hepatoid carcinoma which was treated as unresectable HCC with immunotherapy and the patient had a good response.
Keywords: Hepatoid carcinoma; Pancreatic hepatoid carcinoma; Hepatocellular carcinoma; Pancreatic carcinoma
| Introduction | ▴Top |
Hepatoid carcinoma (HC) is a rare tumor with features morphologically and immunohistochemically like focal hepatocellular carcinoma (HCC) [1]. It was first described in the stomach in 1985 [2] and pancreatic hepatoid carcinoma (PHC) was described in 1999 [3]. Primary sites such as the stomach, esophagus, lungs, gall bladder, urinary bladder, colon, ampulla of Vater, uterus, fallopian tubes, adrenal glands, ovaries, thymus, biliary tract, and other sites have been reported with HC [4, 5], with stomach being the most common location [5]. It is a very rare occurrence in the pancreas.
To the best of our knowledge, less than 50 cases of PHC have been reported so far in the literature. A review of literature shows that the diagnosis of PHC is mainly achieved through morphological analysis and immunohistochemical staining of the tissue specimens, since the clinical manifestations and laboratory tests are non-specific and it does not have unique radiological characteristics to set it apart from other pathologies [4]. Even the special staining required for its diagnosis might not be available at every institution. All these factors make the diagnosis difficult. It is even more challenging in cases with metastasis to the liver because a primary cancer of the liver is the closest differential. We report our experience of HC in pancreas which will add valuable information to the current literature on the diagnosis and treatment as well outcome of this rare entity.
| Case Report | ▴Top |
Investigations
A 67-year-old male with compensated cirrhosis due to hepatitis C, diabetes mellitus type II, hypertension, hypothyroidism, hyperlipidemia, and gastroesophageal reflux disease (GERD) presented with poor appetite and weight loss, progressively worsening over 3 months. He was lost to follow-up prior to that for approximately 2 years.
Diagnosis
A liver ultrasound (US) was done and showed a 70 mm pancreatic head mass, liver cirrhosis and gallbladder sludge. To further delineate the mass, a computed tomography (CT) scan of the abdomen and pelvis with intravenous contrast was done and showed a 101 mm pancreatic head mass consistent with a neoplastic disease, intra- and extrahepatic biliary dilatation was seen and there was a 41.8 mm mixed attenuation mass in lateral right hepatic lobe, suggesting metastatic neoplastic disease (Fig. 1). CA 19-9 levels were 24 U/mL (normal: < 35 U/mL). Alfa-fetoprotein (AFP) levels were markedly elevated at 411 ng/mL (normal: < 8.4 ng/mL). An endoscopic US with fine-needle aspiration (FNA) of the mass was done with a pre-procedure diagnosis of solid pancreatic neoplasm (likely adenocarcinoma) with metastasis. Color Doppler imaging was done prior to needle puncture to confirm lack of vascular structure in the path of the needle. Four passes were made with a 22-gauge needle using a trans-duodenal approach and a stylet was used. The US showed a common biliary duct (CBD) dilated at 7 mm and pancreatic duct at 2 mm. There was an oval mass in the head of pancreas which measured 100 × 80 mm in diameter (Fig. 2). Sonographic evidence also suggested invasion into portal vein. The FNA specimen was initially reported as exocrine pancreatic adenocarcinoma. Oncology team was taken on board. Fluorodeoxyglucose positron emission tomography (PET) scan was done to delineate metastatic disease, which showed a 32.3 mm hypermetabolic left clavicular mass, suggesting confluent lymphadenopathy, cirrhotic liver and hepatic metastatic neoplastic disease was not definitely visualized. There was moderate intrahepatic/extrahepatic biliary dilation. A 79.7 mm hypermetabolic mass was again visualized in the head of the pancreas. An excisional supraclavicular lymph node biopsy was performed and it had features consistent with metastatic hepatoid (hepatocellular) carcinoma.
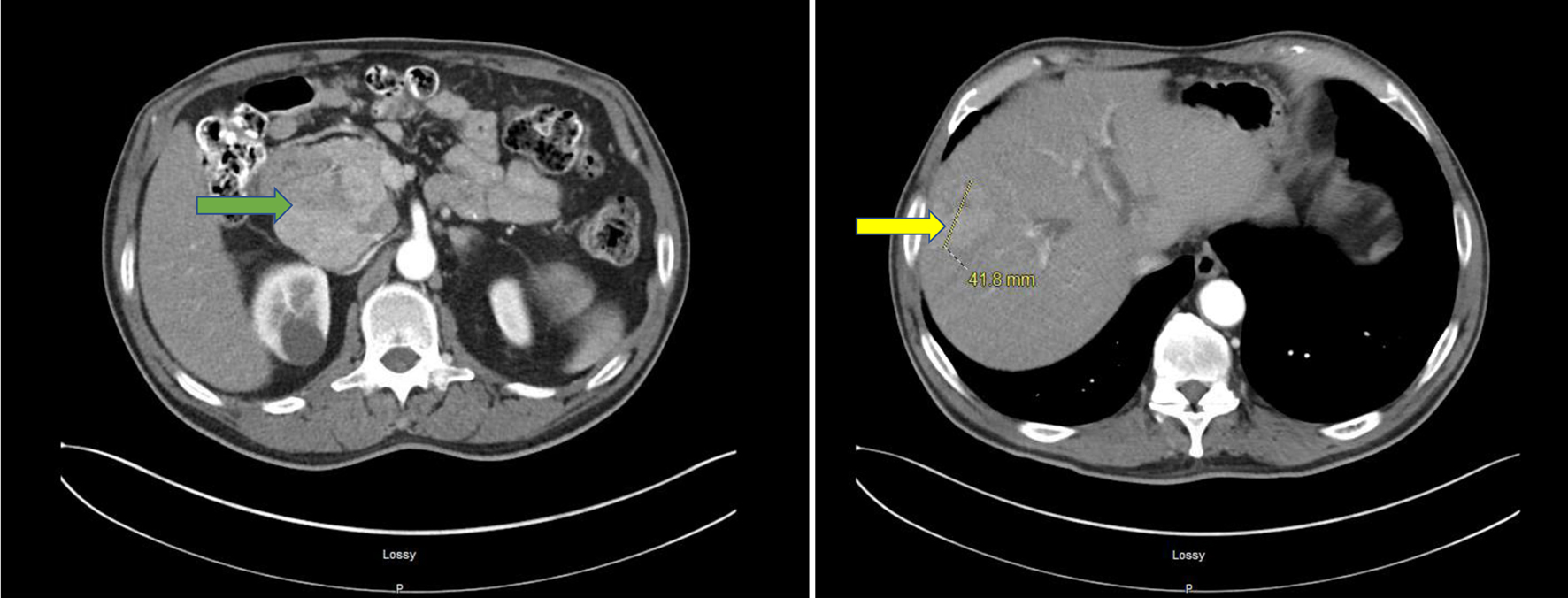 Click for large image | Figure 1. CT of abdomen and pelvis with IV contrast. A 101 × 69 mm mixed attenuation mass involving the head of pancreas (green arrow) is shown. Pancreatic duct dilation is not seen. Fatty infiltration of the liver with 41.8 × 63 mm mixed attenuation mass in the right hepatic lobe (yellow arrow) is shown. Right renal parenchymal cyst is shown. CT: computed tomography. |
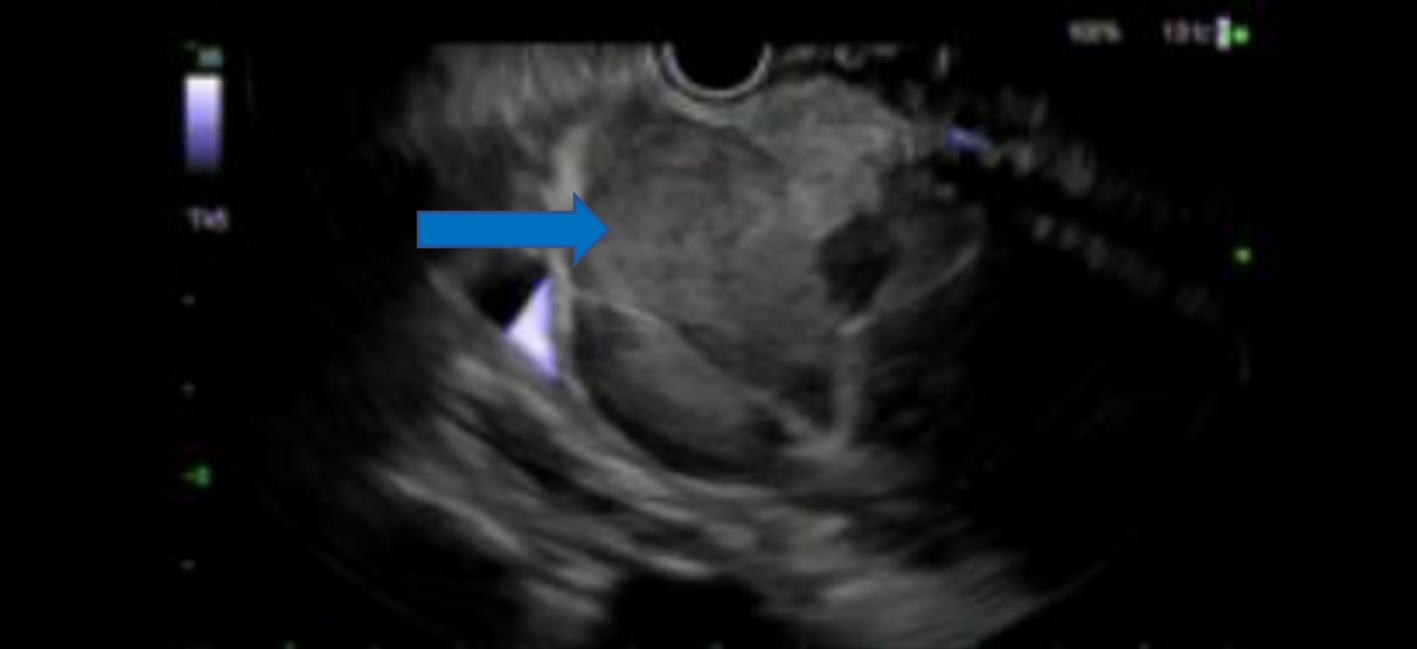 Click for large image | Figure 2. An oval mass measuring 100 × 80 mm in the head of pancreas seen on endoscopic ultrasound (blue arrow). |
The patient developed signs and symptoms of obstructive jaundice including pale colored stools and dark urine. Laboratory workup was significant for elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) at 207 and 349 U/L, respectively (normal: 10 - 50 and 7 - 52 U/L), elevated alkaline phosphatase 298 U/L (normal: 34 - 104 U/L), and increased total bilirubin 5.9 mg/dL (normal: 0.3 - 1.0 mg/dL) with direct bilirubin levels at 4.5 mg/dL (normal: 0.0 - 0.4 mg/dL). Patient was scheduled for a magnetic resonance imaging (MRI) of the abdomen and pelvis and a plan for a liver biopsy after that. MRI showed moderate central biliary ductal dilation secondary to mass effect from the pancreatic head mass, T1 hypointense lesions predominantly in the right hepatic lobe, consistent with metastasis and intra-and extrahepatic duct dilations. Pancreatic head mass measured at 61 × 77 × 117 mm, obstructing distal CBD, and encasing the common hepatic artery and abutting the portal vein. The appearance of the pancreatic head mass was thought to be atypical for primary pancreatic cancer. Unfortunately, a liver biopsy was not done as the patient was admitted to the hospital. He underwent an endoscopic retrograde cholangiopancreatography (ERCP) procedure showing the main bile duct contained a single segmental stenosis of 20 mm in length, the common hepatic duct and left and right hepatic ducts, and all intrahepatic branches were severely dilated, secondary to a stricture likely from malignancy. A temporary plastic stent was successfully placed. The patient’s symptoms improved significantly after the procedure; hence he was discharged home.
A consideration was given to initial diagnosis of adenocarcinoma of the pancreas in light of all the clinical and radiological information, and excisional lymph node histopathology. Additional immunohistochemical staining and evaluation of the pancreatic FNA sample confirmed a hepatoid (hepatocellular) carcinoma of the pancreas. The neoplastic cells were strongly positive for Cam 5.2, Hep Par-1, arginase-1, glypican-3, villin, beta-catenin and SMAD-4 (Figs. 3-5). This also correlated with the morphologic and immunostaining pattern of malignant cells in the lymph node. Because of the clinical and radiological presentation and histomorphology features, the metastatic deposit in the lymph node was deemed to be of pancreatic origin, likely a PHC. The smaller tumors in the liver were likely metastatic deposits from the PHC and less likely a separate neoplastic disease.
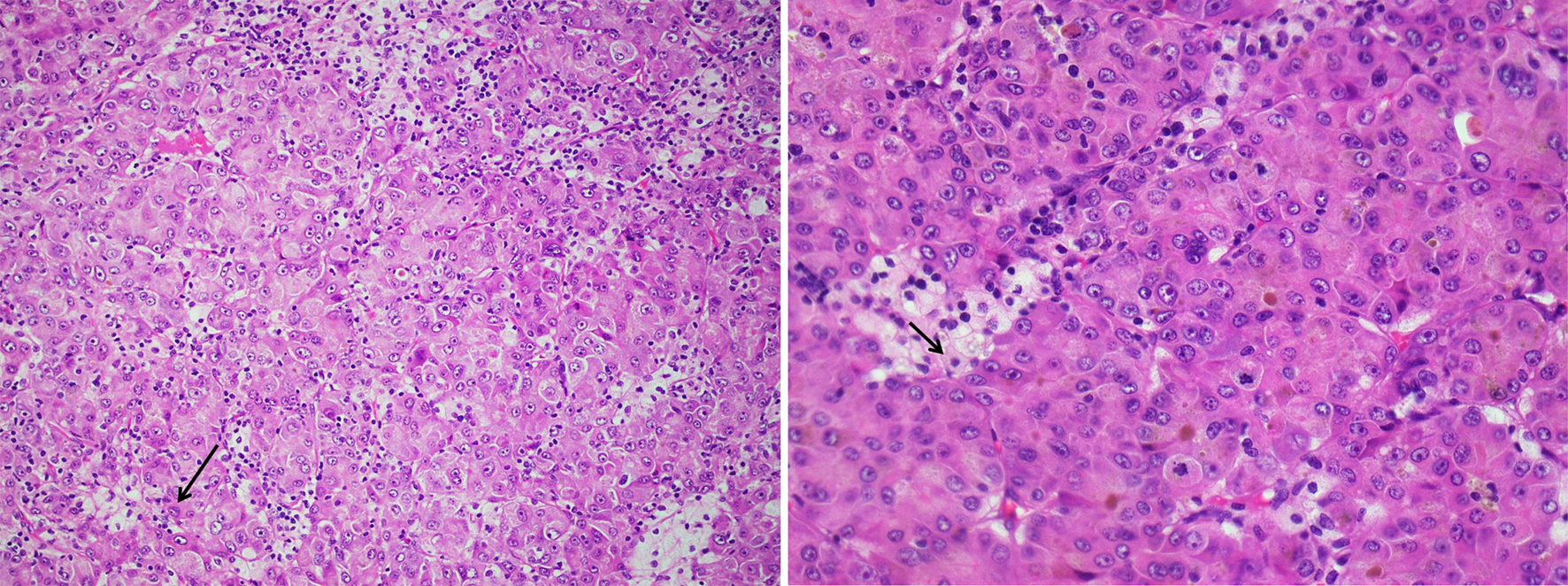 Click for large image | Figure 3. Hematoxylin and eosin stain of lymph node specimen showing small and large nests of neoplastic cells with large amount of cytoplasm, prominent nucleoli and intranuclear inclusions (thin black arrows). Few areas of tumor necrosis are seen. |
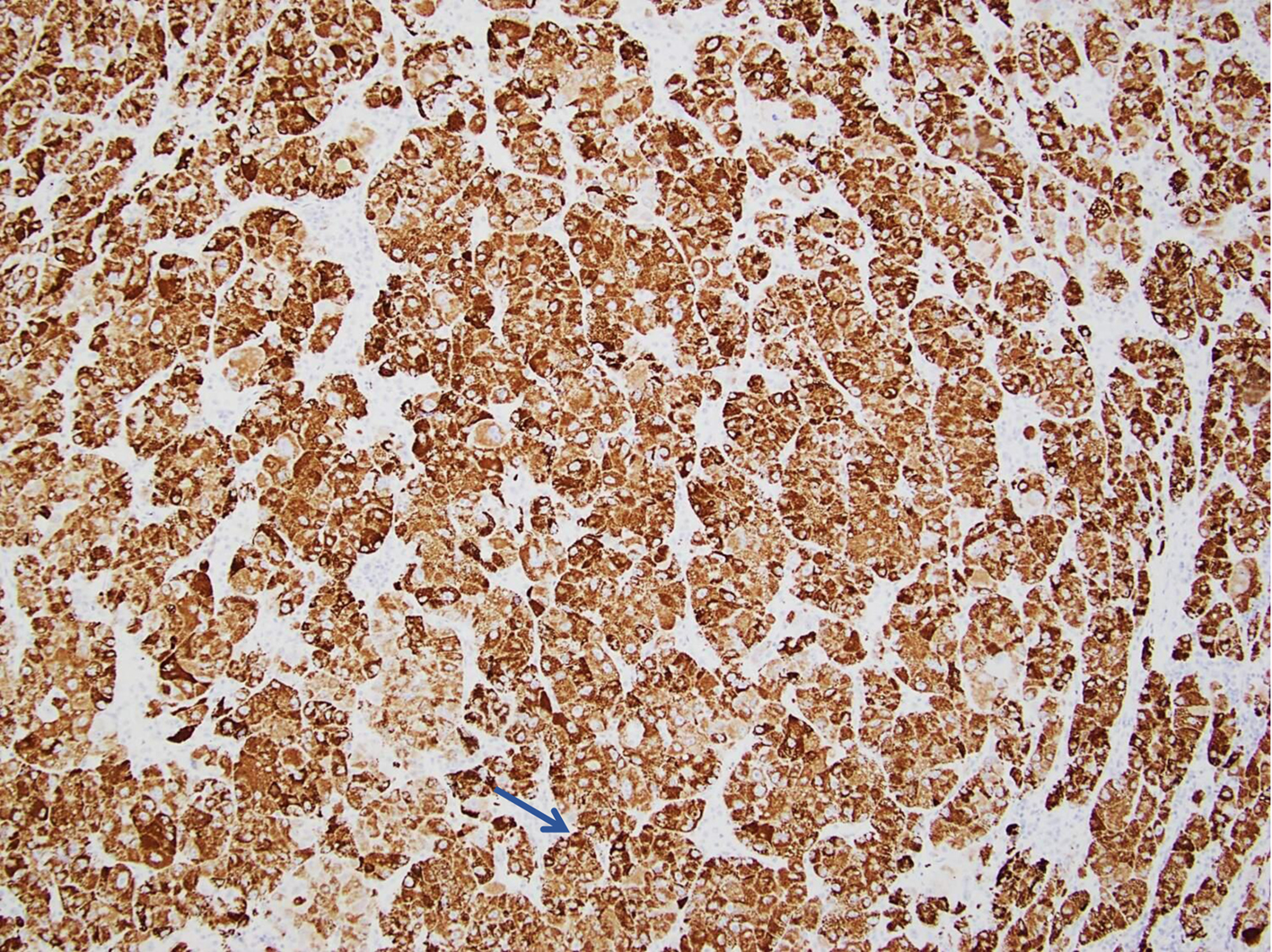 Click for large image | Figure 4. Pancreatic FNA specimen showing tumor cells staining positive for Hep Par-1 on immunohistochemical stain (thin blue arrow). FNA: fine-needle aspiration. |
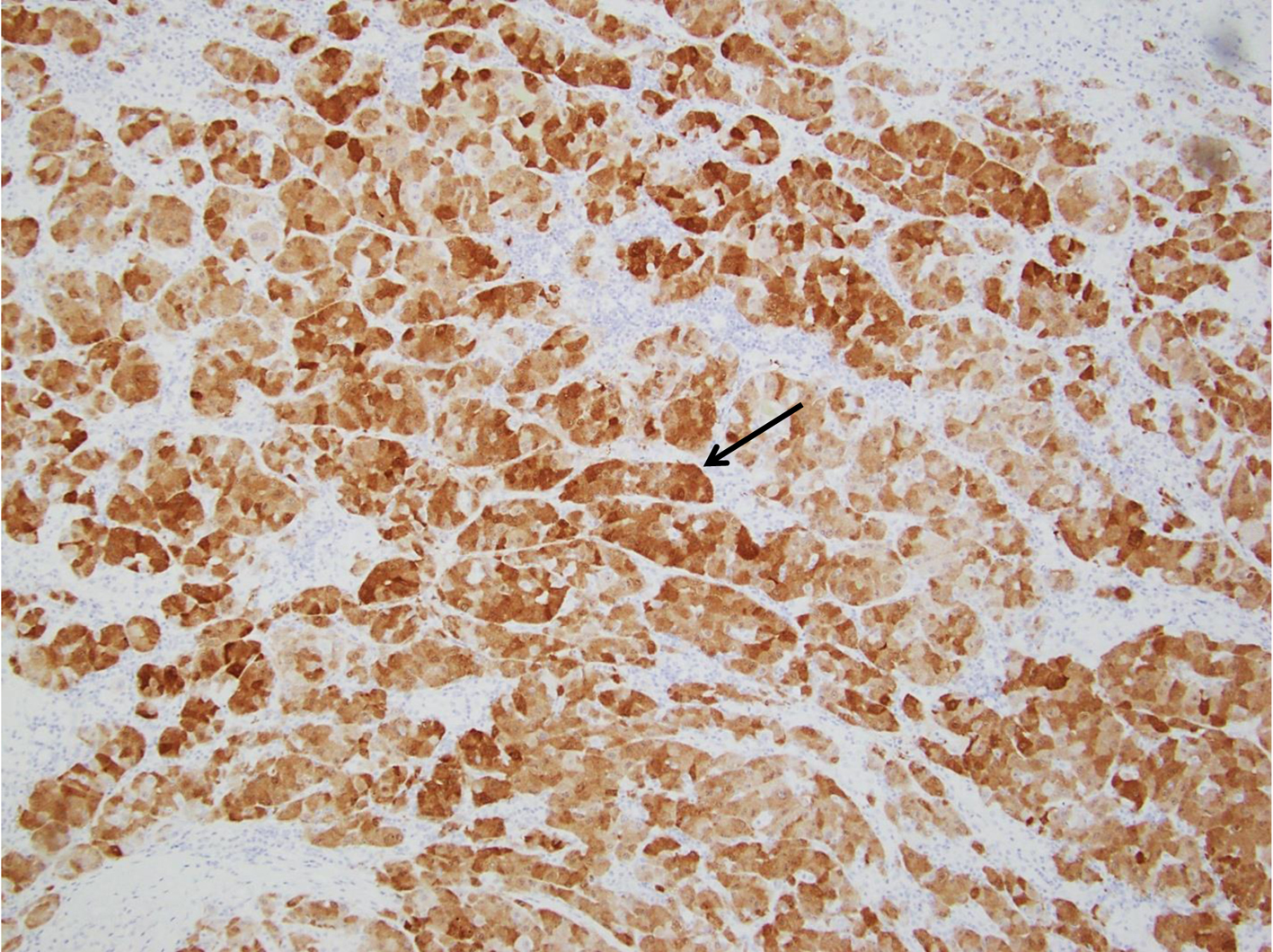 Click for large image | Figure 5. Pancreatic FNA specimen showing tumor cells staining positive for arignase-1 on immunohistochemical stain (thin black arrow). FNA: fine-needle aspiration. |
Treatment
The patient was started on atezolizumab and bevacizumab therapy, and regularly followed up with the oncologist to monitor response to therapy.
Follow-up and outcomes
Imaging studies including PET scans showed stable disease (or even minimal improvement) for up to 14 months of continued treatment with atezolizumab and bevacizumab and the patient tolerated the therapy relatively well. The patient’s disease progressed, based on the increased AFP levels, i.e., approaching 1,000 U/mL (normal: < 35 U/mL), and the increase in the size of liver lesions on CT scan of the abdomen and pelvis. Therapy with atezolizumab and bevacizumab was stopped and he was started on lenvatinib. He developed infection with COVID-19 followed by an episode of sinusitis 2 weeks later. Lenvatinib was ultimately discontinued (about 2 months later) due to symptoms like worsening shortness of breath and abdominal pain, and weakness in the setting of recent COVID-19 infection, being on targeted chemotherapy with lenvatinib and increased overall burden of the disease. Therapy with tremelimumab and durvalumab was initiated but the patient was hospitalized for a fever of unknown origin a week later. The patient was discharged to hospice care and passed away two and a half weeks later.
| Discussion | ▴Top |
The patient’s presentation in combination with imaging and initial pancreatic biopsy report, all pointed towards a metastatic adenocarcinoma of the pancreas. The supraclavicular biopsy specimen had features of a hepatoid (hepatocellular) carcinoma. Additional staining of the pancreatic FNA specimen along with an abdominal MRI findings of multiple liver lesions suggested a diagnosis of PHC with metastasis to liver and left supraclavicular lymph node.
The theories proposed to explain pathogenesis of PHC are: 1) pancreas has ectopic liver tissue where an HC originates [6-8]; 2) the pancreatic cells transdifferentiate into hepatocytes [8, 9]; and 3) liver and pancreas are both derived from the foregut endoderm and there may be activation of the genes controlling hepatic differentiation of pancreatic cells during carcinogenesis, which are normally suppressed [3, 10]. The most common location for PHC is pancreatic tail [1].
In a recent literature review of 41 cases, four histological subtypes were noticed: pure HCC-like morphology, with neuroendocrine differentiation, and with acinar or glandular differentiation [4]. Different terms have been used for the subtype with pure HCC such as hepatoid carcinoma, hepatic adenocarcinoma, hepatoid variant of pancreatic cancer, primary HCC of the pancreas, a pancreatic tumor with hepatoid differentiation or ectopic HCC, etc. [11].
HC has a histological appearance of proliferating large tumor cells, with a trabecular pattern of large polygonal cells seen with abundant eosinophilic cytoplasm, central nuclei, and nucleoli [12]. A majority of PHC cases have elevated serum AFP levels, hence it can be used as a marker to determine the success of surgery or response to chemotherapy [4]. Certain primary pancreatic cancers can have elevation in AFP levels, including ductal carcinoma, neuroendocrine, germ cell tumors and undifferentiated pancreatic adenocarcinoma [13, 14]. AFP, Hep Par-1, glypican-3, arginase-1, anti-albumin, anti-α-1-antitrypisine, anti-low molecular weight cytokeratin, anti-epithelial membrane antigen antibodies and CD10 are the common immunohistochemical characteristics between PHC and HCC. Focal canalicular patterns in PHC on polyclonal carcinoembryonic antigen (CEA) stains have also been reported [15-17]. Hep Par-1 is thought to be the most sensitive among these markers [18]. Metastatic HCC from the liver and HC from another site are the closest differentials [4]. Two specific hepatocyte transporters i.e., bile salt export pump (BSEP) and multidrug resistant protein 3 (MDR3) which are only expressed by HC cells, have been suggested to differentiate [14], but they are not available everywhere. Acinar cell carcinoma is similar to HC and often shows positivity for AFP and Hep Par-1 markers on immunohistochemical staining. Seventy-five percent of HCs are positive for arginase-1 while acinar cell carcinoma is always negative for it, hence arginase-1 can be used to distinguish between the two [19].
There is no consensus on treatment protocol due to limited number of cases reported in the literature. Surgical resection is considered the preferred option [4, 12]. Oral multitarget tyrosine kinase inhibitor, sorafenib, resulted in a 7-month progression-free survival, in a case of metastatic PHC [20]. A 13-month progression-free survival was reported in a case of PHC with liver metastasis treated with neoadjuvant modified-FOLFIRINOX (MFOLFIRINOX) chemotherapy followed by surgical resection [21]. A 46-month survival was reported in a patient with pancreatic neuroendocrine carcinoma treated with six cycles of chemotherapy (gemcitabine intravenously) after successful surgery [22]. Next-generation nucleic acid sequencing can be used for detection of mutated genes, hence helping define the characteristics of the tumor as well as the choice of chemotherapy. It is a novel approach for rare tumors that was used in a few instances of PHC with promising results [23, 24]. We chose a combination therapy of atezolizumab and bevacizumab as this combination showed better overall and progression-free survival outcomes compared with sorafenib in patients with unresectable HCC, in the IMbrave150 trial. The hazard ratio for death with atezolizumab-bevacizumab compared to sorafenib was 0.58 (95% confidence interval (CI): 0.42 - 0.79; P < 0.001), and overall survival at 12 months was 67.2% (95% CI: 61.3 - 73.1) versus 54.6% (95% CI: 45.2 - 64.0) [25]. Tremelimumab plus durvalumab (STRIDE) combination therapy was noticed to significantly improve overall survival when compared to sorafenib in unresectable HCC, in the HIMALAYA trial [26]. Studies comparing STRIDE and atezolizumab plus bevacizumab head-to-head have not been conducted yet.
Prognosis is difficult to predict due to limited number of cases in the literature. PHC is thought to have an aggressive course. Among different subtypes of PHC, pure HCC-like morphology has a better outcome than other subtypes [4]. The 1-year and 5-year survival rates in a case series of 23 cases of HC were reported to be 71.1% and 40.4%, respectively. The median was 13 months, and the mean was 18.1 ± 21.8 months [11].
The present case had an interesting journey until the final diagnosis was made. Diagnosing rare pathologies is difficult in general, and it can be even more challenging when the entity has non-specific manifestations and lacks clearly defined diagnostic criteria. Furthermore, making the correct diagnosis is crucial in cases like metastatic PHC, as a misdiagnosis or a delay in the diagnosis can have grave consequences.
Learning points
PHC is a very rare tumor with limited number of cases reported in literature. Its diagnosis is challenging since it does not have characteristic clinical or radiological features and elevated AFP might be a clue. The diagnosis is invariably through special staining of the tissue specimen. Long-term survival is better in patients where the tumor is resected compared to chemo-/immunotherapy. Metastasis to other organs such as liver and lymph nodes is a poor prognostic indicator.
Acknowledgments
The paper was presented at an annual ACG meeting in Charlotte, North Carolina, on October 21 - 26, 2022. It was awarded the outstanding presenter award as well.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
Yasir Ahmed: wrote/drafted the manuscript, and final review and editing. Usama Sakhawat: case description for initial draft, review process. Fahad Malik: review of literature, editing. Saadia Haleema: description of histopathology slides. Daniel Chin: review of literature, editing. Ali Marhaba: supervisor, conceptualization, final review and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Zeng SX, Tan SW, Fong CTH, Liang Q, Zhao BL, Liu K, Guo JX, et al. Hepatoid carcinoma of the pancreas: A case report and review of the literature. World J Clin Cases. 2020;8(6):1116-1128.
doi pubmed pmc - Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56(4):840-848.
doi pubmed - Yano T, Ishikura H, Wada T, Kishimoto T, Kondo S, Katoh H, Yoshiki T. Hepatoid adenocarcinoma of the pancreas. Histopathology. 1999;35(1):90-92.
doi pubmed - Lei Y, Wang Y, Hao J, Liu M. Pancreatic hepatoid carcinoma: A case report and literature review. World Acad Sci J. 2021;3:49.
- Tomino T, Ninomiya M, Matono R, Narutomi F, Oshiro Y, Watanabe K, Taniguchi D, et al. Pure pancreatic hepatoid carcinoma: a surgical case report and literature review. Surg Case Rep. 2019;5(1):186.
doi pubmed pmc - Cardona D, Grobmyer S, Crawford JM, Liu C. Hepatocellular carcinoma arising from ectopic liver tissue in the pancreas. Virchows Arch. 2007;450(2):225-229.
doi pubmed - Kubota K, Kita J, Rokkaku K, Iwasaki Y, Sawada T, Imura J, Fujimori T. Ectopic hepatocellular carcinoma arising from pancreas: a case report and review of the literature. World J Gastroenterol. 2007;13(31):4270-4273.
doi pubmed pmc - Shen CN, Horb ME, Slack JM, Tosh D. Transdifferentiation of pancreas to liver. Mech Dev. 2003;120(1):107-116.
doi pubmed - Rao MS, Reddy JK. Hepatic transdifferentiation in the pancreas. Semin Cell Biol. 1995;6(3):151-156.
doi pubmed - Soofi Y, Kanehira K, Abbas A, Aranez J, Bain A, Ylagan L. Pancreatic hepatoid carcinoma: a rare form of pancreatic neoplasm. Diagn Cytopathol. 2015;43(3):251-256.
doi pubmed - Kuo PC, Chen SC, Shyr YM, Kuo YJ, Lee RC, Wang SE. Hepatoid carcinoma of the pancreas. World J Surg Oncol. 2015;13:185.
doi pubmed pmc - Tomboravo C, Narindra LHRO, Ranoharison HD, et al. Hepatoid carcinoma of the pancreas: a case report. J Pancreat Cancer Treat. 2019;2(1):6-9.
- Marchegiani G, Gareer H, Parisi A, Capelli P, Bassi C, Salvia R. Pancreatic hepatoid carcinoma: a review of the literature. Dig Surg. 2013;30(4-6):425-433.
doi pubmed - Fujikura K, Yamasaki T, Otani K, Kanzawa M, Fukumoto T, Ku Y, Hirose T, et al. BSEP and MDR3: useful immunohistochemical markers to discriminate hepatocellular carcinomas from intrahepatic cholangiocarcinomas and hepatoid carcinomas. Am J Surg Pathol. 2016;40(5):689-696.
doi pubmed - Vanoli A, Argenti F, Vinci A, La Rosa S, Viglio A, Riboni R, Necchi V, et al. Hepatoid carcinoma of the pancreas with lymphoid stroma: first description of the clinical, morphological, immunohistochemical, and molecular characteristics of an unusual pancreatic carcinoma. Virchows Arch. 2015;467(2):237-245.
doi pubmed - Kai K, Nakamura J, Ide T, Masuda M, Kitahara K, Miyoshi A, Noshiro H, et al. Hepatoid carcinoma of the pancreas penetrating into the gastric cavity: a case report and literature review. Pathol Int. 2012;62(7):485-490.
doi pubmed - Pellini Ferreira B, Vasquez J, Carilli A. Metastatic hepatoid carcinoma of the pancreas: first description of treatment with capecitabine and temozolomide. Am J Med Sci. 2017;353(6):610-612.
doi pubmed - Askan G, Deshpande V, Klimstra DS, Adsay V, Sigel C, Shia J, Basturk O. Expression of markers of hepatocellular differentiation in pancreatic acinar cell neoplasms: a potential diagnostic pitfall. Am J Clin Pathol. 2016;146(2):163-169.
doi pubmed pmc - Dogeas E, Peng L, Choti MA. Hepatoid adenocarcinoma of unknown primary masquerading as a pancreatic tumor. J Gastrointest Surg. 2017;21(12):2132-2134.
doi pubmed - Petrelli F, Ghilardi M, Colombo S, Stringhi E, Barbara C, Cabiddu M, Elia S, et al. A rare case of metastatic pancreatic hepatoid carcinoma treated with sorafenib. J Gastrointest Cancer. 2012;43(1):97-102.
doi pubmed - Ma T, Bai X, Li G, Wei S, Liang T. Neoadjuvant modified-FOLFIRINOX followed by surgical resection of both the primary and metastatic tumors of a pancreatic hepatoid carcinoma with synchronous liver metastasis: A case report. Medicine (Baltimore). 2017;96(43):e8413.
doi pubmed pmc - Xin BB, Li JA, Han X, Zhao J, Ji Y, Lou WH, Xu XF. Successful treatment of a case with pancreatic neuroendocrine carcinoma with focal hepatoid differentiation: a case report and literature review. Int J Clin Exp Med. 2014;7(10):3588-3594.
pubmed pmc - Chang JM, Katariya NN, Lam-Himlin DM, Haakinson DJ, Ramanathan RK, Halfdanarson TR, Borad MJ, et al. Hepatoid carcinoma of the pancreas: case report, next-generation tumor profiling, and literature review. Case Rep Gastroenterol. 2016;10(3):605-612.
doi pubmed pmc - He J, Zhao Q, Liu Q, Li F, He L, Liu M, Yan X. Surgical resection of pancreatic hepatoid carcinoma followed by combined transarterial chemoembolization and immunotherapy: a case report. Onco Targets Ther. 2021;14:4575-4578.
doi pubmed pmc - Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.
doi pubmed - Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):Article EVIDoa2100070.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


