| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 1, January 2024, pages 31-36
Early Detection and Diagnostic Approach Through Automated Hematological Analysis for Plasma Cell Leukemia
Joaquin Jereza, c, Francisca Sanchezb, Francisco Floresb, Lissette Guajardob, Jose Luis Brionesa, b, Carolina Selmanb
aDepartment of Haematology, Fundacion Arturo Lopez Perez, Providencia, Chile
bDepartment of Diagnostics Units, Fundacion Arturo Lopez Perez, Providencia, Chile
cCorresponding Author: Joaquin Jerez, Department of Hematology, Fundacion Arturo Lopez Perez, Providencia, Chile
Manuscript submitted January 5, 2024, accepted January 16, 2024, published online January 28, 2024
Short title: Automated Hematological Analysis for PCL
doi: https://doi.org/10.14740/jmc4188
| Abstract | ▴Top |
Plasma cell leukemia (PCL) is a clinically aggressive variant of multiple myeloma, characterized by a high burden of circulating plasma cells, necessitating swift and accurate diagnosis due to its poor prognosis. The conventional diagnostic criteria, including the recent recommendation by the International Myeloma Working Group (IMWG) of > 5% circulating plasma cells as positive, have evolved over time. In this context, we present a detailed case report that underscores the pivotal role of the ADVIA 2120 automated hematology counter in detecting plasma cells through cytogram analysis, along with the significance of routine peripheral blood smear analysis and the utility of a large unstained cells (LUCs) threshold of > 4.5% as an indicator for PCL. The case involves a 64-year-old patient with relapsed multiple myeloma and stable paraprotein levels who experienced sudden renal impairment. In this case report, we highlight how ADVIA analysis and cytochemistry assisted in the diagnosis, and further explore ADVIA’s utility in this challenging leukemia.
Keywords: Plasma cell leukemia; ADVIA 2120; Circulating plasma cells
| Introduction | ▴Top |
In 1974, Dr. Robert Kyle described 12 patients with clinically aggressive multiple myeloma presenting with over 20% plasma cells and more than 2,000 cells/mm3 in peripheral blood. He termed this entity “plasma cell leukemia” [1]. From a clinical standpoint, this entity is characterized by an increased incidence of hepatosplenomegaly, more pronounced renal insufficiency, and elevated levels of lactate dehydrogenase (LDH). In addition to reporting on these de novo cases (now referred to as primary plasma cell leukemia), he also documented five cases of multiple myeloma that progressed or relapsed into plasma cell leukemia (secondary plasma cell leukemia) [1]. It is noteworthy that subsequently reported case series, encompassing a limited number of patients, demonstrated inadequate responses to conventional chemotherapy regimens (e.g., melphalan) and a high mortality rate associated with this entity, with median survival times of less than 1 year [2-5]. Consequently, early identification assumes paramount significance.
However, the definition of plasma cell leukemia has evolved over time from Dr. Kyle’s initial description employing the aforementioned criteria [6], to the year 2013, when the International Myeloma Group (IMWG) task force proposed the initial criteria to be overly restrictive [7]. A suggestion was put forth to revise the lower cutoffs. Subsequent studies indicate that the detection of circulating plasma cells via cytology is more common than reported, and intriguingly, the prognosis for patients with over 5% of these cells appears as grim as that for the original 20% cutoff [8-10]. Consequently, in 2021, the IMWG proposed lowering the diagnostic criterion to consider those with over 5% circulating plasma cells as positive [11]. Thus, a systematic analysis of peripheral blood smears from all multiple myeloma patients is recommended to identify this entity, analyzing at least 100 - 200 nucleated cells. Systematic examination of peripheral blood smears reveals that over 20% of multiple myeloma patients exhibit circulating plasma cells [9]. Further investigation employing supplementary parameters from the automated hematological analysis system ADVIA 2120, specifically the count of large unstained cells (LUCs) as a surrogate marker for plasma cells, holds potential as an initial cautionary signal in patients with monoclonal gammopathy, prompting a comprehensive scrutiny of the smear, thereby streamlining the identification of this condition.
In this case report, we present the utility of ADVIA analysis used to assist the diagnosis of plasma cell leukemia.
| Case Report | ▴Top |
Investigations
A 64-year-old patient with a history of type 2 diabetes mellitus and dyslipidemia, currently on atorvastatin and metformin, is a moderate smoker, consuming 10 cigarettes per day, and lacks significant family history.
The patient presented with back pain. General laboratory tests revealed hemoglobin levels of 12 g/dL (normal range: 12 - 15 g/dL), leukocytes at 7.3 × 103/µL (normal range: 3.5 - 10.5 × 103/µL), platelets at 237 × 103 mm3 (normal range: 150 - 450 × 103/mm3), creatinine at 1.28 mg/dL (normal range: 0.5 - 1 mg/dL), and calcium at 9.7 mg/dL (normal range: 8.5 - 10 mg/dL). A whole-body computed tomography scan showed multiple lytic lesions in the axial skeleton and rib arches. Furthermore, serum protein electrophoresis exhibited a monoclonal peak of 7.9 g/dL, while immunofixation indicated the presence of a monoclonal immunoglobulin G (IgG) lambda component. Serum free light chain assay displayed a lambda level of 196 mg/L (normal range: 5.7 - 26.3 mg/L) and a kappa level of 10 mg/L (normal range: 3.3 - 19.4 mg/L). Bone marrow analysis disclosed 53% plasma cells, with flow cytometry highlighting 99% clonal plasma cells. Cytogenetic analysis was not available. Beta-2 microglobulin measured 6.5 mg/dL (normal range: 0.6 - 2.37 mg/dL), and International Staging System (ISS) staging yielded a score of 3.
The patient underwent induction therapy with cyclophosphamide/bortezomib/dexamethasone for six cycles, leading to a very good partial response (paraprotein reduced to 0.4 g/dL on electrophoresis). Subsequent positron emission tomography/computed tomography (PET/CT) imaging showed a complete metabolic response. A subsequent autologous hematopoietic stem cell transplant was performed using melphalan 200 mg/m2, without deepening the response, maintaining a very good partial response with a paraprotein level of 0.3 mg/dL on electrophoresis. Finally, maintenance therapy was initiated with lenalidomide.
Diagnosis
After 2 years of maintenance treatment with lenalidomide and a consistently stable paraprotein level in the range of 0.3 - 0.5 g/dL, the patient began to experience generalized fatigue. Follow-up examinations revealed pancytopenia and acute renal impairment, with creatinine levels elevated to 11 g/dL, necessitating hospitalization. Laboratory analyses demonstrated a creatinine level of 11.1 mg/dL, blood urea nitrogen of 96 mg/dL (normal range: 7 - 17 mg/dL), calcium of 9.6 mg/dL, and LDH of 336 U/L (normal range: 120 - 246 U/L). Serum protein electrophoresis revealed an increasing monoclonal peak, while serum free light chain assay indicated lambda at 2,010 mg/L and kappa at 33.7 mg/L, consistent with a clinically aggressive relapse of multiple myeloma.
An automated hematological count was performed using the ADVIA 2120 system, revealing a hemoglobin level of 8.6 g/dL, leukocytes at 2.940 × 103/µL, and platelets at 13 × 103/mm3. The cytogram generated by the automated count was analyzed, revealing an 8.6% population of cells with loosely dispersed chromatin in the Baso channel window (Fig. 1a), suggestive of blasts. The Perox channel window exhibited a 15.6% population of LUC (Fig. 1b). Additionally, the histogram of nucleated red blood cells showed a peak of unstained events in parallel with lymphocytes (Fig. 2a). Taken together, these findings indicated the possible presence of large cells with lax chromatin, lacking myeloperoxidase (MPO) staining, and appearing distinct from large granular lymphocytes or natural killer cells. Given these characteristics and within the clinical context, these cells were highly suggestive of pathological plasma cells or plasmablasts. With this suspicion in mind, peripheral blood smears were meticulously examined, confirming an 8% population of circulating plasma cells out of a total of 200 analyzed nucleated cells (Fig. 2b), thereby confirming the diagnosis of secondary plasma cell leukemia. The study was complemented by flow cytometry, which revealed a 6% population of plasma cells expressing both CD38 and CD138 markers and demonstrating light chain lambda restriction, all of which were clonal. The blood tests conducted at the time of diagnosis were scrutinized, revealing no presence of LUCs in ADVIA, nor were plasma cells identified in peripheral blood smears.
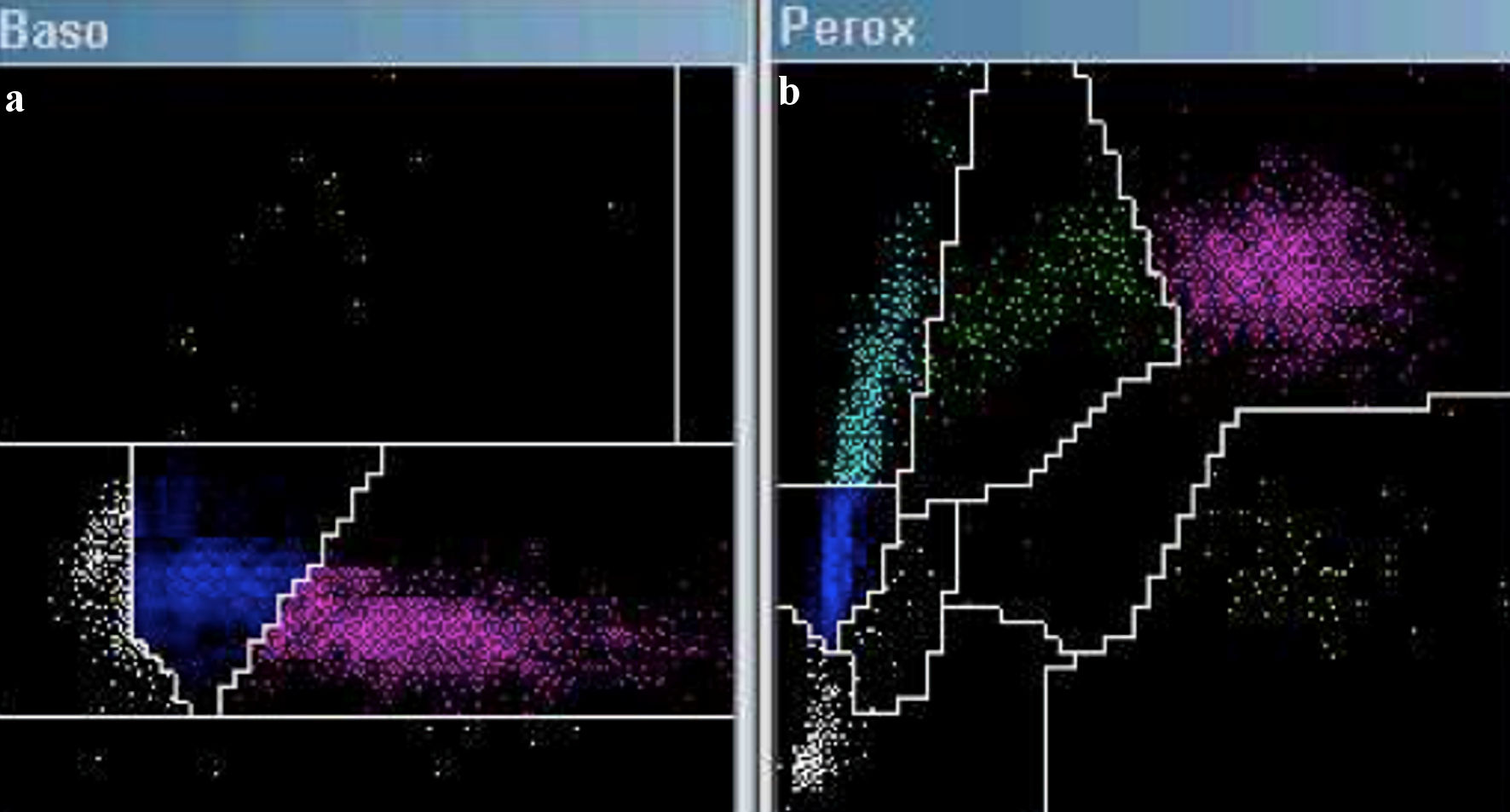 Click for large image | Figure 1. Cytogram in ADVIA 2120: Baso channel (a) and Perox channel (b). An increase in cell count is observed in the Baso channel within the blast region (highlighted in white). The Perox channel reveals an elevation of cells identified as large unstained cells (highlighted in cyan). |
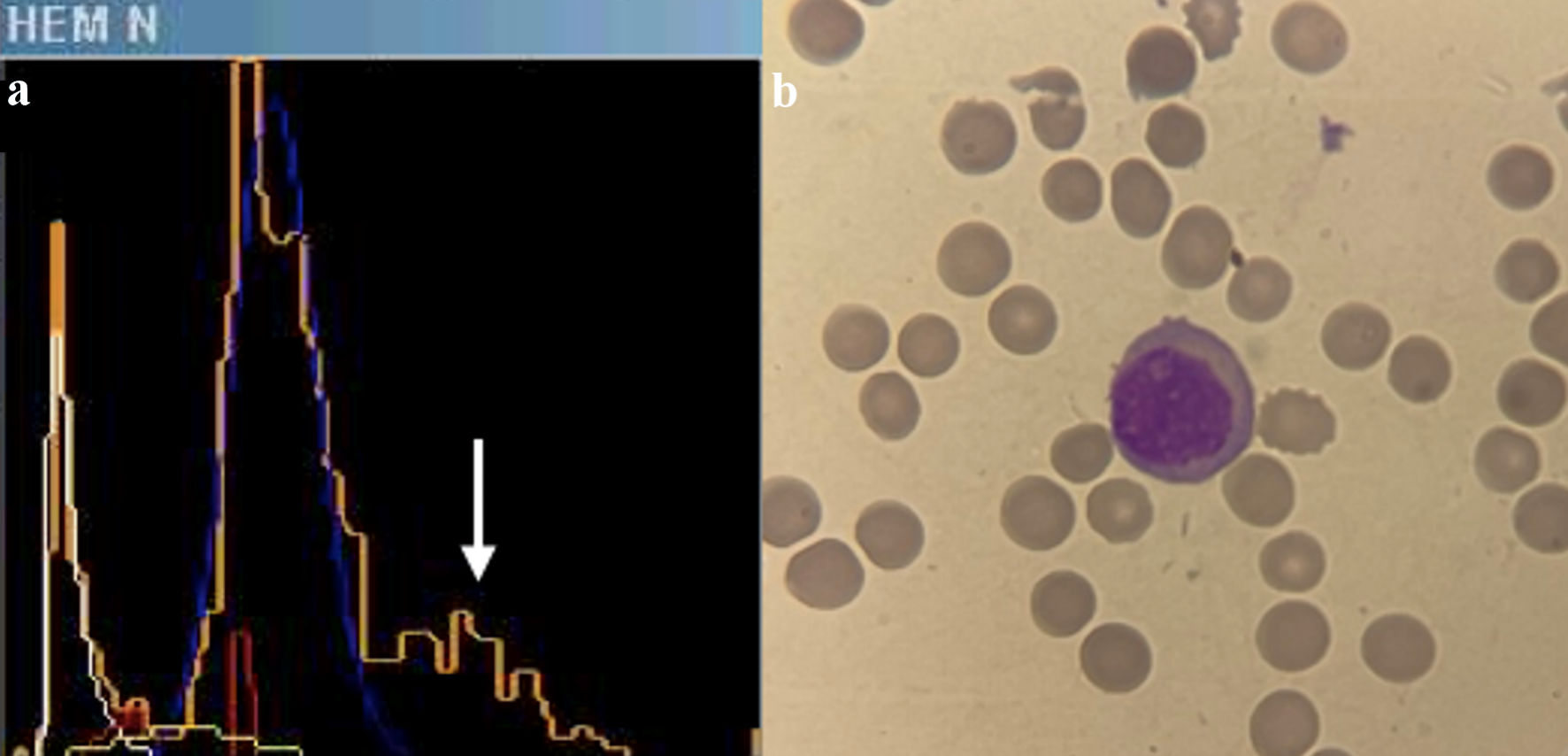 Click for large image | Figure 2. (a) Histogram of nucleated red blood cells in ADVIA 2021. A third peak is observed in orange (indicated by a white arrow), identifying non-stained cells with myeloperoxidase, which runs parallel to the lymphocyte peak in blue. (b) Plasma cell in peripheral blood film. |
Treatment
The patient was admitted to the hospital and emergency hemodialysis was initiated. High-dose dexamethasone pulses were administered; however, the patient experienced rapid deterioration of consciousness, necessitating urgent intubation and connection to invasive mechanical ventilation. To assess the situation, a computed tomography scan of the brain was performed, revealing evidence of an acute subdural hematoma with significant mass effect and signs of intracranial hypertension. In this clinical context, it was concluded that the patient was not a candidate for systemic therapy.
Follow-up and outcomes
The patient’s condition severely deteriorated, displaying platelet refractoriness and progressive escalation of neurological involvement. Their condition further worsened, and they passed away within a matter of a few days.
| Discussion | ▴Top |
The ADVIA 2120 automated hematology counter operates by combining flow cytometry and cytochemistry, conducting a joint analysis of nuclear density, cellular volume, and cytochemical staining in two distinct channels (Perox and Baso), in order to quantify the total and differential leukocyte count [12]. In the Perox channel, the introduction of hydrogen peroxide initiates a reaction with the endogenous MPO enzyme. This reaction, combined with the addition of the chromogenic agent 4-chloro-1-naphthol, leads to the formation of a dark brown precipitate. Subsequently, utilizing a cytometer, light scatter variables (corresponding to volume, Y-axis) and light absorption (determined by the level of positive MPO staining, X-axis) are analyzed. Through a cluster analysis system, the following cell types are identified (Fig. 3a): neutrophils (large cells and MPO-positive), monocytes (slightly smaller and partially MPO-positive), eosinophils (intense MPO staining and slightly smaller than neutrophils), lymphocytes (small cells and MPO-negative), and LUCs (large cells and MPO-negative). While LUCs typically correspond to large granular lymphocytes or reactive/atypical lymphocytes; this category also includes MPO-negative blasts (e.g., monoblasts).
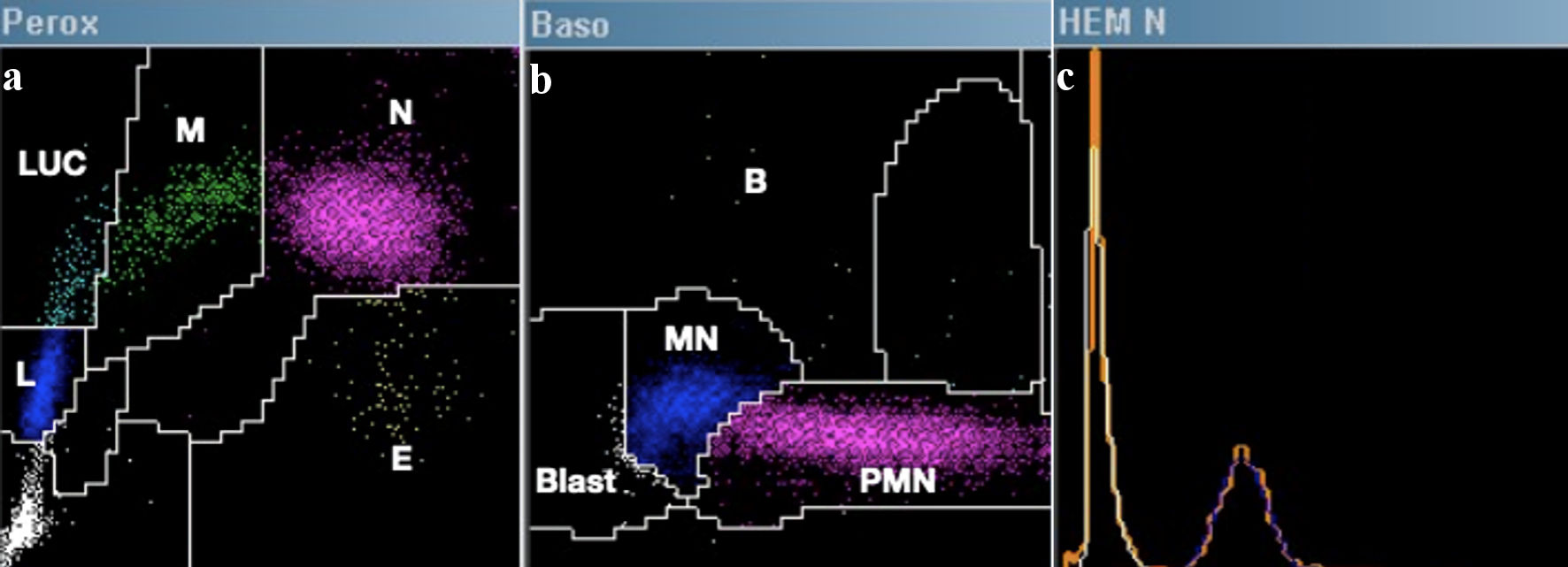 Click for large image | Figure 3. Example of a normal hematological count in ADVIA 2021. (a) Perox channel: E = eosinophils, N = neutrophils, M = monocytes, L = lymphocytes, LUCs = large unstained cells. (b) Baso channel: B = basophils, PMN = polymorphonuclear cells, MN: mononuclear cells, Blast = blasts. (c) The nucleated red blood cell enumeration histogram corresponds to the unstained area on the Y-axis of the Peroxidase channel. This visualization depicts overlaps of non-stained cells (orange), platelets (white), nucleated red blood cells if present (yellow and red), and lymphocytes (blue). |
In the Baso channel, a combination of phthalic acid and surfactants is employed to lyse the membranes of all cells except basophils, which are inherently resistant. Subsequently, utilizing a cytometer (Fig. 3b), high-angle light scatter variables (corresponding to nuclear density/lobularity, X-axis) and low-angle light scatter (corresponding to cellular volume, Y-axis) are analyzed. Since basophils remain intact, they are isolated and quickly analyzed based on their volume. Finally, nuclear analysis enables the classification of mononuclear cells (such as lymphocytes and monocytes), polymorphonuclear cells (including neutrophils and eosinophils), and blasts, identified by their lax chromatin (detected to the left on the X-axis).
Further analysis of the Perox and Baso channels has proven successful in various contexts [13]. D’Onofrio et al, in a publication from 2001 [14], proposed the PANDA (peroxidase and nuclear density analysis) system relating to MPO staining and nuclear density. This system describes a visual classification based on both channels, identifying seven MPO staining patterns and three nuclear density patterns. The correlations uncovered in this analysis are highly intriguing. For instance, the pattern PO/D1 (presence of MPO-negative blasts) primarily clusters acute lymphoblastic leukemias and MPO-negative myeloid leukemias (such as monoblastic, erythroid, and megakaryoblastic).
However, an entity not included in this pattern, likely due to its low incidence, is plasma cell leukemia. In this case, due to the absence of MPO expression and their size, these cells will be grouped as LUCs in the Perox channel. Depending on the compactness of their chromatin (and the percentage of plasmablasts present), these cells may exhibit lax chromatin (identified as blasts) or compact chromatin (similar to mononuclear cells). To date, only one study has evaluated plasma cell detection using the described analysis, comparing them with morphology and flow cytometry [15]. In this analysis, the identification of plasma cells as LUCs showed excellent performance, demonstrating high correlations with both morphology (r = 0.92) and flow cytometry (r = 0.89). In our particular case, the early and focused application of the ADVIA cytogram analysis facilitated the specific search for circulating plasma cells.
Additionally, ADVIA analysis poses a challenge concerning the MPO-negative blast pattern, specifically in distinguishing between MPO-negative lymphoid and myeloid lineages. To address this issue, an analysis was performed on the histogram of nucleated red blood cells [16], in which MPO-negative cellular events are arranged (Fig. 3c). In this analysis, peaks corresponding to lymphocytes and LUCs are observed. Typically, lymphoblastic leukemias present a single peak encompassing both lymphocytes and LUCs. In contrast, myeloid leukemias show a double peak, indicating that the lymphoid population originates from a common clone and forms a single peak. In our case, this analysis was conducted, revealing a third peak, suggesting that plasmablasts derive from a mature cell not corresponding to normal mature lymphocytes. Therefore, in the described differential diagnosis, the possibility of plasma cell leukemia is added.
The detection of circulating plasma cells can be approached through various methods. Cytology is frequently employed, albeit with varying sensitivities, primarily due to the lack of routine screening. A Spanish study that routinely examined peripheral blood smears in a cohort of 482 patients identified circulating plasma cells in 20% of cases [9], most with counts ranging from 1% to 4%. A subgroup of adverse prognosis, comprising 3.5% of patients, exhibited > 5%. These findings were replicated by a cohort from the Mayo Clinic, revealing that the presence of > 5% circulating plasma cells is associated with a highly unfavorable prognosis [10]. Notably, this group demonstrated a graver prognosis compared to those with high-risk cytogenetic classification. It is pertinent to note that this study, which did not include routine searching for plasma cells, yielded a detection rate of only 2%, significantly differing from the Spanish cohort. Lastly, a Chinese cohort comprising 767 patients, which conducted routine blood smear analyses and defined > 2% as positive, detected circulating plasma cells in 14% of cases [8], suggesting > 2% as the prognostic threshold for overall survival.
As discernible from the aforementioned series, routine analysis inclusion is imperative. Diagnostic supplementation with other tools, such as alerts from automated hematological counting systems, can enhance detection. Particularly, in the ADVIA system, the normal average LUC count is 2%, and alerts are triggered at values > 4.5%, potentially indicating the presence of plasma cells around 2% within an appropriate context. Besides ADVIA experience, successful use of another type of automated hematological counter (Sysmex XE 5000), employing a distinct technique for leukocyte separation, has been reported [17].
Conversely, flow cytometry has heralded a paradigm shift. In one of the initial reports, analysis of 50,000 events in CD45-CD38+ cells revealed that 73% of multiple myeloma patients had circulating plasma cells, with a direct relationship between detected cell numbers and overall survival [18]. In a subsequent study, analyzing at least 150,000 events using a panel of at least six antibodies, 54% of circulating plasma cells were detected [19]. A receiver operating characteristic (ROC) analysis determined a cutoff of 400 plasma cells as a prognostic predictor, proving to be more powerful than both the ISS and cytogenetic risk assessed by fluorescence in situ hybridization (FISH) in multivariate analysis. Notably, it has been reported that up to 25% of patients with monoclonal gammopathy of undetermined significance (MGUS) and asymptomatic myeloma could exhibit circulating plasma cells through flow cytometry [20], demonstrating high discriminatory capacity between MGUS and multiple myeloma. Ultimately, a comprehensive analysis validated in a clinical trial cohort proposed > 2% as the optimal prognostic threshold for flow cytometry [21]. Furthermore, experience with next-generation flow cytometry has demonstrated that 100% of myeloma patients present circulating plasma cells, while 59% of MGUS patients also exhibit them [22].
Learning points
The detection of circulating plasma cells in patients with monoclonal gammopathy is of vital importance, as it constitutes a critical prognostic factor and a diagnostic criterion for plasma cell leukemia. Routine evaluation of peripheral blood smears in all patients with monoclonal gammopathy is essential. The use of diagnostic support tools, such as additional analysis of the ADVIA 2120 automated hematological count, can serve as an early and preliminary approach in the search for plasma cells. In this regard, it is suggested to consider an alarm for a LUC value > 4.5% in patients with monoclonal gammopathy as a direct indication to assess the possible presence of plasma cell leukemia. This suggestion should be validated in a properly designed study. In the future, the analysis of peripheral blood by flow cytometry may become a routine practice.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Joaquin Jerez authored the manuscript, while Francisca Sanchez, Lissette Guajardo, and Francisco Flores collaborated in acquiring images and managing the automated hematological analysis. Jose Luis Briones and Carolina Selman contributed to the manuscript’s revisions and provided final approval.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med. 1974;133(5):813-818.
doi pubmed - Lopez Guillermo A, Marti JM, Blade J, Nomdedeu B, Montserrat E, Rozman C. [Plasma cell leukemia. Study of 10 cases]. Sangre (Barc). 1989;34(1):28-31.
pubmed - Kosmo MA, Gale RP. Plasma cell leukemia. Semin Hematol. 1987;24(3):202-208.
pubmed - Dimopoulos MA, Palumbo A, Delasalle KB, Alexanian R. Primary plasma cell leukaemia. Br J Haematol. 1994;88(4):754-759.
doi pubmed - Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, Marcos-Gragera R, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724-3734.
doi pubmed - International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749-757.
pubmed - Fernandez de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH, Hajek R, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27(4):780-791.
doi pubmed pmc - An G, Qin X, Acharya C, Xu Y, Deng S, Shi L, Zang M, et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann Hematol. 2015;94(2):257-264.
doi pubmed - Granell M, Calvo X, Garcia-Guinon A, Escoda L, Abella E, Martinez CM, Teixido M, et al. Prognostic impact of circulating plasma cells in patients with multiple myeloma: implications for plasma cell leukemia definition. Haematologica. 2017;102(6):1099-1104.
doi pubmed pmc - Ravi P, Kumar SK, Roeker L, Gonsalves W, Buadi F, Lacy MQ, Go RS, et al. Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 2018;8(12):116.
doi pubmed pmc - Fernandez de Larrea C, Kyle R, Rosinol L, Paiva B, Engelhardt M, Usmani S, Caers J, et al. Primary plasma cell leukemia: consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. 2021;11(12):192.
doi pubmed pmc - Gibbs G. ADVIA Haematology systems: a guide to cytogram interpretation, 2nd edn. Raleigh, NC: Lulu Press, Inc.; 2014.
- Harris N, Kunicka J, Kratz A. The ADVIA 2120 hematology system: flow cytometry-based analysis of blood and body fluids in the routine hematology laboratory. Lab Hematol. 2005;11(1):47-61.
doi pubmed - D’Onofrio G. PANDA: innovative classification of hematopoietic malignancies. Bloodline Rev. 2001;1:3-6.
- Ciriello M, Calcagno L, Cattana E, Coppo M, Vasta M, Arfini C. Peripheral plasma cell analysis on ADVIA 2120I and FACSCANTO flow cytometer. International Journal of Laboratory Hematology. 2012;34:47.
- Gibbs G, Prechtl G. Automated cytochemistry expanded using novel cellular analysis. Int J Lab Hematol. 2015;37(5):e133-134.
doi pubmed - Gounari E, Tsavdaridou V, Koletsa T, Nikolaidou A, Kaiafa G, Papaioannou M, Kostopoulos I, et al. Utility of hematology analyzer and flow cytometry in timely and correct detection of circulating plasma cells: Report of three cases. Cytometry B Clin Cytom. 2016;90(6):531-537.
doi pubmed - Nowakowski GS, Witzig TE, Dingli D, Tracz MJ, Gertz MA, Lacy MQ, Lust JA, et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood. 2005;106(7):2276-2279.
doi pubmed pmc - Gonsalves WI, Rajkumar SV, Gupta V, Morice WG, Timm MM, Singh PP, Dispenzieri A, et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high-risk myeloma. Leukemia. 2014;28(10):2060-2065.
doi pubmed pmc - Sanoja-Flores L, Flores-Montero J, Perez-Andres M, Puig N, Orfao A. Detection of Circulating Tumor Plasma Cells in Monoclonal Gammopathies: Methods, Pathogenic Role, and Clinical Implications. Cancers (Basel). 2020;12(6):1499.
doi pubmed pmc - Jelinek T, Bezdekova R, Zihala D, Sevcikova T, Anilkumar Sithara A, Pospisilova L, Sevcikova S, et al. More Than 2% of Circulating Tumor Plasma Cells Defines Plasma Cell Leukemia-Like Multiple Myeloma. J Clin Oncol. 2023;41(7):1383-1392.
doi pubmed pmc - Sanoja-Flores L, Flores-Montero J, Garces JJ, Paiva B, Puig N, Garcia-Mateo A, Garcia-Sanchez O, et al. Next generation flow for minimally-invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC). Blood Cancer J. 2018;8(12):117.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


