| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 7, July 2024, pages 120-125
Organizing Pneumonia With Diffuse Alveolar Hemorrhage Induced by the Kampo Medicine Choreito
Seijitsu Andoa, Koji Azumaa, Shinji Futamia, Kiyosi Morib, Yumiko Hiroseb, Takuma Shirasakac, Seigo Minamia, d
aDepartment of Respiratory Medicine, NHO Osaka National Hospital, Osaka, Japan
bCentral Laboratory and Surgical Pathology, NHO Osaka National Hospital, Osaka, Japan
cAIDS Medical Center, NHO Osaka National Hospital, Osaka, Japan
dCorresponding Author: Seigo Minami, Department of Respiratory Medicine, NHO Osaka National Hospital, Osaka 540-0006, Japan
Manuscript submitted April 9, 2024, accepted June 5, 2024, published online June 19, 2024
Short title: Choreito-Induced Organizing Pneumonia
doi: https://doi.org/10.14740/jmc4222
| Abstract | ▴Top |
Kampo medicine, a traditional Japanese herbal medicine, is covered by the Japanese National Health Insurance and prescribed for various purposes. While relatively safe with few adverse effects, it may potentially cause severe adverse effects, such as lung injury. Herein, we describe the case of a 61-year-old Japanese woman with choreito-induced lung injury that manifested as organizing pneumonia (OP) with diffuse alveolar hemorrhage (DAH). She was referred to our department due to multiple abnormal opacities detected on annual chest radiography. Chest computed tomography (CT) revealed multiple nodules in bilateral lungs. Bloody bronchoalveolar lavage fluid was obtained from the left lingular lobe, appearing nearly normal, while a transbronchial lung biopsy from a subpleural nodule in the left lower lobe was pathologically consistent with OP. The drug lymphocyte stimulation test result was positive for choreito, which the patient had regularly consumed for 6 - 7 months to treat hematuria. Consequently, a diagnosis of choreito-induced OP and DAH was made. Owing to the discontinuation of choreito alone and without the introduction of systemic steroid therapy, the multiple nodules shrank and eventually disappeared on follow-up chest CT. Regardless of the type of crude drug used in Kampo medicine, clinicians must always be careful for potential lung injury, which may present as OP with DAH.
Keywords: Choreito; Kampo medicine; Traditional Japanese herbal medicine; Drug-induced lung injury; Interstitial pneumonia; Organizing pneumonia; Diffuse alveolar hemorrhage; Bronchoalveolar lavage; Transbronchial lung biopsy; Scutellariae Radix
| Introduction | ▴Top |
Kampo medicine, a traditional Japanese herbal remedy, is derived from traditional Chinese medicine adopted in ancient Japan during the sixth century [1]. Some Kampo medicines have been covered under the Japanese National Health Insurance since 1967, and can currently be prescribed in clinical practice or obtained over-the-counter at local pharmacies for a wide range of purposes, ranging from self-care management to clinical control of various phases of diseases.
According to two internet-based surveys conducted by the Japan Kampo Medicines Manufacturers Association (JKMMA) in 2011 (n = 627) [2] and by a private venture company (M3 Inc.) in 2022 (n = 951) [3], 89% and 75.6% of Japanese physicians, respectively, reported prescribing Kampo medicines to their patients in daily medical practice. Additionally, another internet survey by JKMMA in 2020 (n = 2,060) revealed that approximately 70% of Japanese individuals aged ≥ 25 years had consumed Kampo medicine, with over 50% obtaining it through pharmacies, drugstores, the internet, or online platforms [4].
Choreito, a traditional Kampo medicine, is frequently used to treat urological diseases, such as cystitis, lower urinary tract symptoms, and an overactive bladder. Choreito effectively alleviates lower urinary tract symptoms and complaints of decreased urine volume, dysuria, and oral dryness in female patients [5]. Choreito comprises the following five aqueous extracts: aluminum silicate hydrate with silicon dioxide, Alisma rhizome (rhizome of Alisma orientale Juzepczuk), Polyporus umbellatus sclerotium, Poria sclerotium (dried sclerotium of Wolfiporia cocos Ryvarden et Gilbertson), and donkey glue.
Although Kampo medicine is relatively safe with few adverse effects, it has been known since the 1990s that it may potentially cause severe adverse effects such as lung injury (interstitial pneumonia). The first report of lung injury caused by Kampo medicine, published in 1989, was that of a 71-year-old Japanese woman diagnosed with Shosaikoto-induced lung injury based on bronchoscopic biopsy, the drug lymphocyte stimulation test (DLST), and challenge test [6]. Similar reports were published in the 1990s. Among the 4,232 adverse event data reports on the Ministry of Health, Labor, and Welfare (MHLW) website from July 2003 to March 2018, lung injury (n = 1,177; 27.8%) was the second most frequent adverse event associated with Kampo medicine after liver injury (n = 1,193; 28.2%), followed by pseudoaldosteronism (n = 889; 21.0%) [7]. In contrast, the incidence rate of Kampo medicine-induced lung injury was 0.08% (n = 3) according to a 10-year retrospective study at the Department of Japanese Oriental Medicine, Toyama University Hospital (n = 3,590; 2008 - 2017). In this study, Scutellariae Radix (SR) (Ogon in Japanese, Scutellaria root in English) was included in the suggested causal Kampo medicines in all three cases [8].
Herein, we describe a female Japanese patient with choreito-induced lung injury who presented with organizing pneumonia (OP) and diffuse alveolar hemorrhage (DAH). To the best of our knowledge, this is the first reported case of choreito-induced lung injury presenting with the combined manifestation of OP and DAH.
| Case Report | ▴Top |
Investigations
A 61-year-old Japanese woman was referred to our department because of multiple abnormal opacities on annual chest radiography. She did not complain of any respiratory symptoms. Her vital signs revealed a body temperature of 35.7 °C, blood pressure of 150/90 mm Hg, pulse rate of 97 beats/min, and percutaneous oxygen saturation of 99% on room air. Her breath sounds were within the normal range. She had no significant medical history except for hematuria and nephritis at the age of 59 years. She regularly consumed choreito (7.5 g/day) for 6 - 7 months for her hematuria; it was prescribed by a urology clinic in her neighborhood. She also consumed Seihaito 2 - 3 times per 2 months. She did not consume any other medications or health foods. She was a current smoker with a smoking history of one pack of cigarettes per day for 31 years and drank two cans of beer (700 mL/day) five times a week. Her body mass index was 29.2 kg/m2. Chest computed tomography (CT) showed multiple nodules, mainly in the bilateral lower lobes (Fig. 1a, b), and a very small peri-bronchial ground-glass opacity in the left lingular lobe (Fig. 1c).
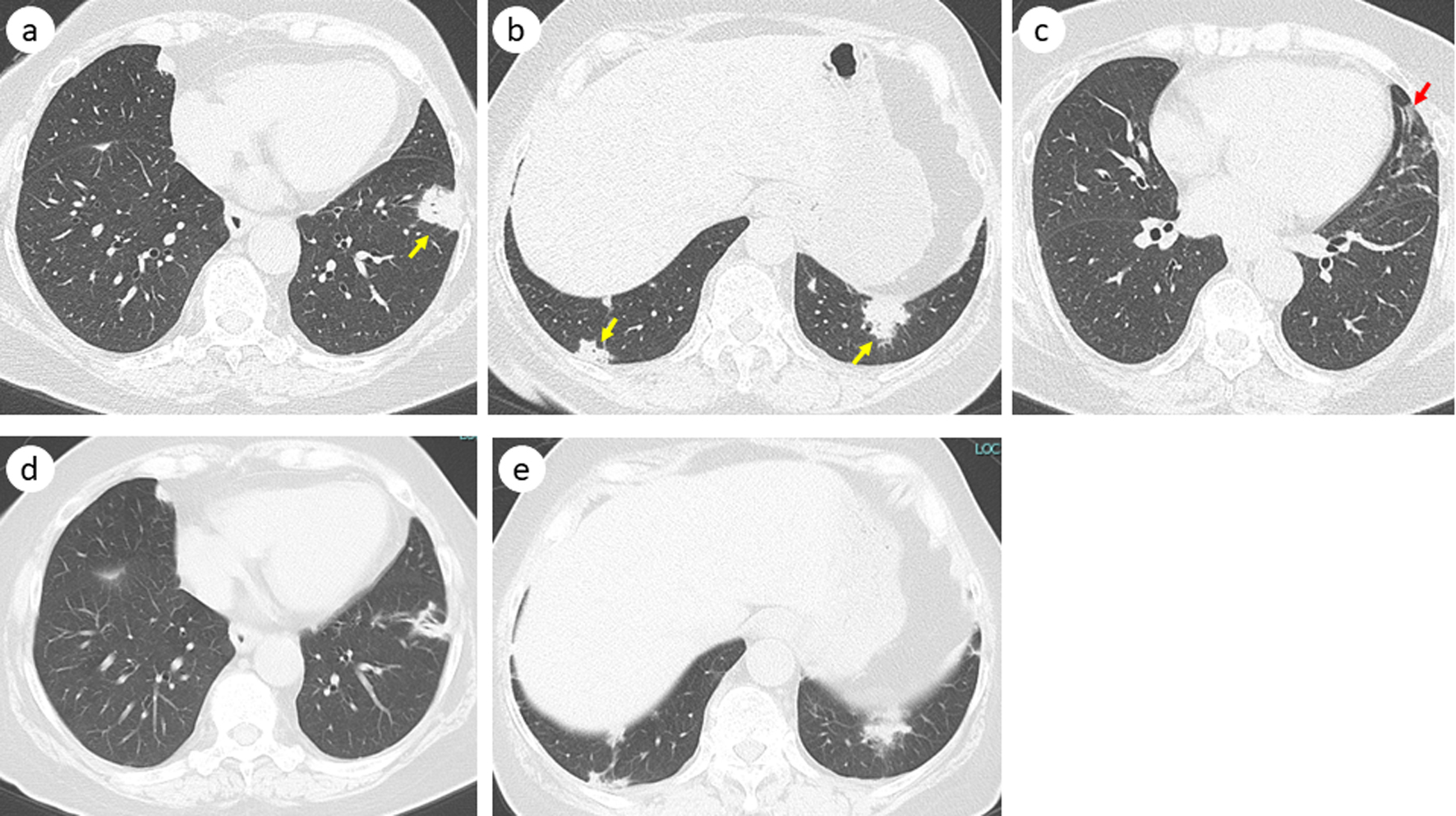 Click for large image | Figure 1. Chest computed tomography before bronchoscopy showing multiple nodules (yellow arrow) (a, b) and a slight ground-glass opacity in the left lingular lobe (red arrow) (c). Five months after discontinuation of choreito, these nodules shrank or vanished (d, e). |
Diagnosis
Except for a slightly elevated C-reactive protein (CRP) level (0.33 mg/dL) and positive antinuclear antibody (ANA) test result (1:80, speckled pattern; 1:40, nucleolar pattern), the results of laboratory tests using peripheral venous blood were negative for proteinase 3 anti-neutrophil cytoplasmic antibody (ANCA), myeloperoxidase ANCA, T-SPOT tuberculosis test, anti-Mycobacterium avium complex antibody test, Aspergillus antigen, Cryptococcus neoformans antigen, β-D-glucan, angiotensin converting enzyme, carcinoembryonic antigen, squamous cell carcinoma associated antigen, pro-gastrin releasing peptide, and soluble interleukin-2 receptor. The absolute and differential counts of leukocytes (6,500/µL, 64.4% of neutrophil, 25.2% of lymphocytes, and 2.0% of eosinophils), aspartate aminotransferase (16 IU/L), and alanine aminotransferase (11 IU/L) were within the normal ranges. We did not examine Krebs von den Lungen-6 or surfactant proteins A and D. CRP, ANA, or other blood laboratory tests were not performed. Urinalysis results were normal, except for slight hematuria. Her spirometry results were within the normal range with a vital capacity of 2.38 L (96.4% of expected) and a forced expiratory volume in the first second of 1.91 L (94.1% of expected).
Neither bleeding nor bloody sputum was visually observed in the oral cavity, larynx, or bronchial tract during bronchoscopy. Following our standard procedure, bronchoalveolar lavage fluid (BALF) was obtained from the left B5 segment (fluid recovery rate, 56%), although no abnormal shadows were observed in this lobe. However, the BALF unexpectedly became serially and increasingly hemorrhagic (Fig. 2). The total cell count in the BALF was 3.72 × 105/mL, and the differential cell count showed 0.5% lymphocytes, 1.5% neutrophils, 97.5% macrophages, and 0.5% eosinophils with a CD4/CD8 ratio of 1. Bacterial and mycobacterial cultures of the BALF were negative, and cytological examination did not detect any malignant cells. Hemosiderin-laden macrophages (HLMs) were not observed in the BALF. We subsequently performed a transbronchial lung biopsy of the subpleural nodule on the left S8 (Fig. 1a). The biopsy specimens demonstrated alveolitis and intraluminal polypoid structures with mild infiltration of chronic inflammatory cells, such as small lymphocytes and plasma cells (Fig. 3), which were pathologically consistent with OP. Thereafter, the DLST was positive for choreito with a stimulation index (SI) of 337% but negative for Seihaito with an SI of 140%. Based on these results, choreito-induced OP and DAH was diagnosed.
 Click for large image | Figure 2. Macroscopic findings of bronchoalveolar lavage fluid from the left B5. The numbers are the order of the samples obtained. |
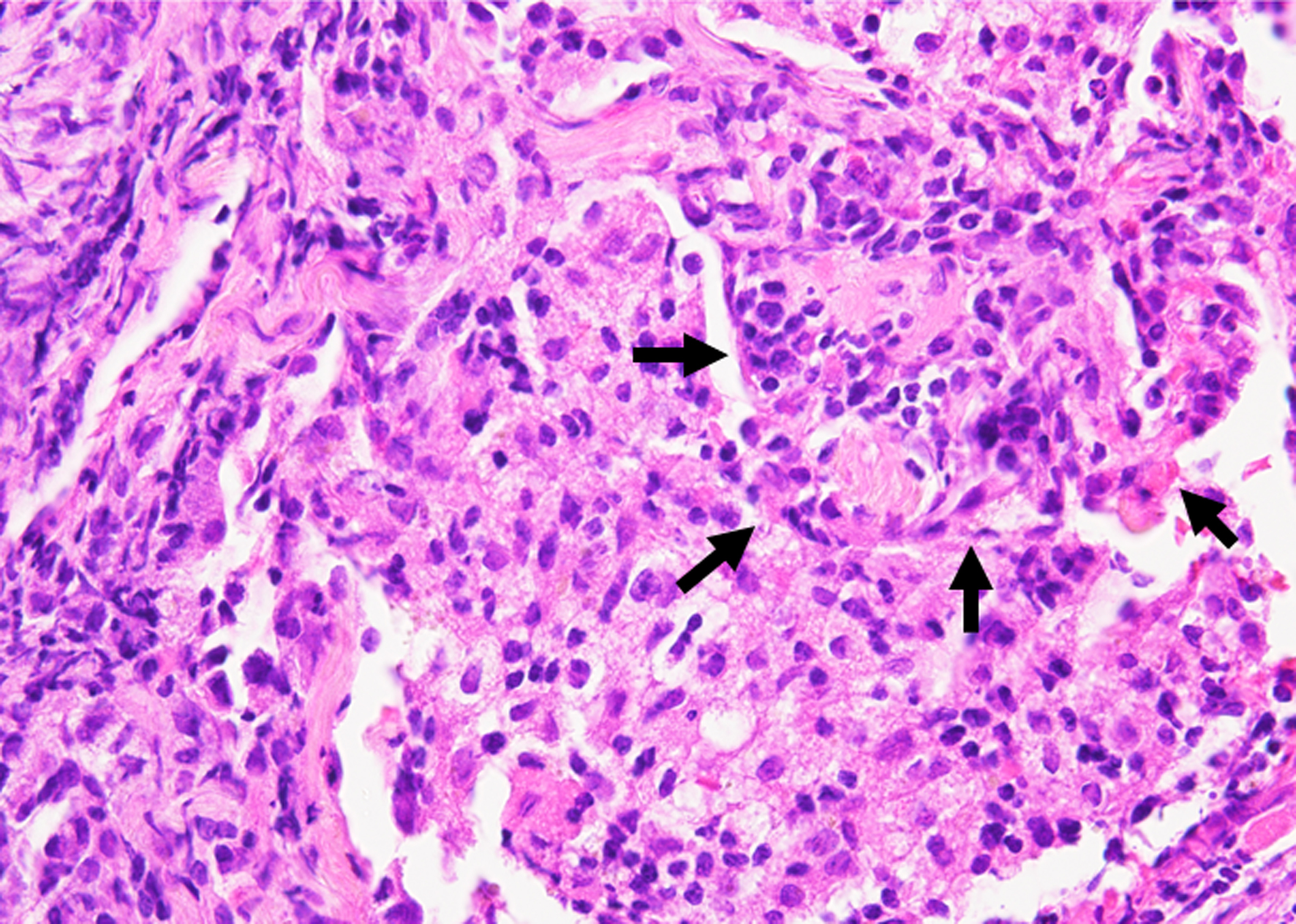 Click for large image | Figure 3. Histology of bronchoscopic specimen from the tumor in the left B8a (hematoxylin and eosin staining, magnification × 400). The black arrows indicate an intraluminal polypoid structure with mild infiltration of chronic inflammatory cells. |
Treatment
The patient discontinued taking choreito immediately before bronchoscopy at her own discretion. Despite the presence of DAH, we did not introduce systemic steroid therapy, partially because she did not have any respiratory symptoms and maintained normal respiratory conditions and partially because she refused steroid and immunosuppressive therapy. The patient’s cigarette and alcohol consumption were transiently reduced but could not be discontinued; these habits then returned to nearly the same levels at the first visit.
Follow-up and outcomes
Two months after drug discontinuation, we observed remarkable shrinkage of all nodules on CT imaging. Five months after discontinuation, we confirmed the near disappearance of the nodules on CT (Fig. 1d, e).
| Discussion | ▴Top |
This case clinically presented the following three interesting and instructive characteristics.
First, choreito, which does not contain SR, causes drug-induced lung injury. According to a literature review of 73 patients with Japanese Kampo medicine-induced lung injury (59 articles), the most frequent causative drugs were Shosaikoto (n = 19), Saireito (n = 12), Seishinrenshiin (n = 8), and Botsusyosan (n = 8) [9]. These medicines commonly contain crude SR or Glycyrrhizae Radix (known as Kanzo in Japanese and licorice in English) [8]. Among the total 1,177 event reports related to lung injury from the analysis of adverse event report data of the MHLW in Japan from July 2003 to March 2018, 818 (69.5%) events were suggested to be induced by Kampo medicines containing SR. Among the top seven suggested medicines, all contained SR: Saireito (n = 142), Bofutsushosan (n = 108), Otsujito (n = 65), Hangeshashinto (n = 62), Shosaikoto (n = 56), Seishinrenshiin (n = 50), and Saikokaryukotsuboreito (n = 46) [7]. Based on the Japanese Adverse Drug Event Report database containing 830,079 reports published between April 2004 and 2023, the adjusted reporting odds ratios (95% confidence interval) using multivariate logistic regression models for SR (daily intake) and SR (daily intake) interacted with age (≥ 60 years) were 1.47 (1.36 - 1.59) and 3.35 (3.12 - 3.60), respectively [10]. Thus, SR-containing drugs are more likely to cause lung injury. However, choreito does not contain SR, which suggests a different pathologic mechanism from that of conventional SR-induced lung injury. We were unable to identify the specific crude drug that caused lung injury, particularly because we could not obtain the five extracts from choreito. Thus, regardless of whether the prescribed Kampo medicine contains SR, we must always pay attention to the possible adverse events of lung injury.
Second, choreito-induced lung injury presents with OP, a rare manifestation. Although there has been a case of Shoseiryuto-induced lung injury, in which a reverse halo sign, relatively specific to OP, appeared on chest CT [11], our case presented with only nodular shadows without ground-glass opacities. Of the total 1,177 reported adverse events related to lung injury, the most frequent were interstitial lung disease (n = 852), lung injury (n = 133), pneumonia (n = 114), eosinophilic pneumonia (n = 22), and dyspnea (n = 17). In this study, OP was reported in only six cases [7]. Considering the various sizes of scattered, multiple, and bilateral nodules in our patient, we suggested a cryptococcal infection at her first visit. Without a detailed interview regarding her drug history, a differential diagnosis of drug-induced lung injury could not be made initially.
Third, DAH occurred in a nearly normal-appearing lobe, which was an unexpected finding. It is important to note that we did not detect HLMs in the BALF, which made our diagnostic evidence of DAH unstable. However, HLMs are not always found in the BALF of patients with DAH. Some retrospective studies have reported on wide ranges of proportions of HLMs in BALFs, for example, a mean of 56.4±29.1% (range 0-96%) among 47 Indian DAH patients [12], and a median of 5% (range 0-90%) among 21 patients with diffuse alveolar damage at the Mayo Clinic [13]. Among the 1,177 reports of Kampo-induced lung injury, no cases of DAH were present [7]. To the best of our knowledge, only two case reports of DAH induced by Shoseiryuto in a 78-year-old man [14] and Makyokansekito in a 64-year-old man were reported [15]. Our case was different from these two cases in the following five clinical characteristics: 1) no bilateral ground-glass opacity; 2) no persistent fever or bloody sputum; 3) relatively late onset (after 6 - 7 months) compared with 17 days after Shoseiryuto and a few hours after Makyokansekito consumption; 4) lower percentage in the BALF of lymphocytes (17.5% with Shoseiryuto and 10.5% with Makyokansekito vs. 0.5% in our case) and neutrophils (45.0% with Shoseiryuto and 6.0% with Makyokansekito vs. 1.5% in our case); and 5) bloody BALF obtained from the nearly normal-appearing lobe in our case [14, 15]. Regarding the BAL cell profiles (4), differential counts of leukocytes in the BALF varied. Among 10 patients with non-neoplastic drug-induced OP classified by CT patterns, the median percentages (ranges) in the BALF of lymphocytes, neutrophils, and eosinophils were 31.0% (3.0-66.4%), 12.0% (0-44.0%), and 21.0% (0.2-40.0%), respectively [16]. In our and Makyokansekito cases, the DLST results were positive, whereas this result was negative in the Shoseiryuto case. The discrepancy among DLST results in these three cases may suggest various mechanisms of Kampo medicine-induced DAH. Drug-induced DAH is etiologically classified into three types: 1) hypersensitivity; 2) direct toxicity; and 3) coagulation defects [17]. The cause of DAH in the present case remains unknown. The result of strongly positive DLST for choreito suggested hypersensitivity as the optimal mechanism, whereas the low percentage of lymphocytes in the BALF was not consistent with this hypothesis. In our case, DAH had already occurred in a nearly normal-appearing lung field. In an in vivo experiment, hemosiderin staining was first detected within alveolar macrophages in the BALF on day 3 after a single blood aspiration in mice [18]. Thus, HLMs in the BALF may appear in the early phase of DAH development. This phenomenon suggests a looming risk of acute deterioration due to the rapidly expanding DAH.
In conclusion, regardless of the type of crude drug used in Kampo medicine, we must always be aware of lung injury, which may present as OP with DAH.
Learning points
Choreito, a traditional Japanese Kampo medicine, may potentially cause drug-induced lung injury. It is important to note that choreito does not contain SR - the most frequent and likely causative crude drug in cases of Kampo medicine-induced lung injuries. This lung injury may present as a rare pattern of OP, which can make the diagnosis confusing to physicians without detailed interviews regarding drug history. Furthermore, this lung injury may be accompanied by the infrequent manifestation of DAH, even in a nearly normal-appearing lung field, suggesting a risk of acute deterioration. Regardless of the crude drug used in Kampo medicine, care should be taken when treating lung injury.
Acknowledgments
We thank Dr. Aturo Kawai (Director of the Kawai Clinic of Internal Medicine and Pediatrics in Higashi Sumiyoshi-ku, Osaka City, Japan) for his referral to our hospital.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The patient provided written informed consent for the submission and publication of this case report, including accompanying images and information.
Author Contributions
S. Ando and S. Minami were involved in outpatient follow-up. S. Ando, S. Minami, K. Azuma, and S. Futami performed bronchoscopy and biopsy. K. Mori and Y. Hirose made pathological diagnoses. T. Shirasaka provided advisory clinical guidance and directions. All the authors critically reviewed the report and approved the final version of the manuscript.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article.
Abbreviations
ANA: antinuclear antibody; ANCA: anti-neutrophil cytoplasmic antibody; BALF: bronchoalveolar lavage fluid; CRP: C-reactive protein; CT: computed tomography; DAH: diffuse alveolar hemorrhage; DLST: drug lymphocyte stimulation test; HLM: hemosiderin-laden macrophage; JKMMA: Japan Kampo Medicines Manufacturers Association; MHLW: Ministry of Health, Labor, and Welfare; OP: organizing pneumonia; SI: stimulation index; SR: Scutellariae Radix
| References | ▴Top |
- Fuyuno I. Japan: Will the sun set on Kampo? Nature. 2011;480(7378):S96.
doi pubmed - Japan Kampo Medicines Manufacturers Association: Actual condition survey of Kampo prescriptions [Internet]. 2011. [cited Apr 7, 2024] Available from: https://www.nikkankyo.org/serv/pdf/jittaichousa2011.pdf (in Japanese).
- M3 Inc: [m3.com recognition survey research.] 2022. [cited Apr 7, 2024] Available from: https://www.m3.com/clinical/news/1090445 (in Japanese).
- Japan Kampo Medicines Manufacturers Association: Awareness and usage experience survey of Kampo prescriptions [Internet]. 2023. [cited Apr 7, 2024] Available from: https://www.nikkankyo.org/serv/serv7.htm (in Japanese).
- Sugihara T, Kamei J, Yasunaga H, Sasabuchi Y, Fujimura T. Prescription of Choreito, a Japanese Kampo Medicine, with antimicrobials for treatment of acute cystitis: a retrospective cohort study. Antibiotics (Basel). 2022;11(12):1840.
doi pubmed pmc - Tsukiyama K, Tasaka Y, Nakajima M, Hino J, Nakahama C, Okimoto N, Yagi S, et al. [A case of pneumonitis due to sho-saiko-to]. Nihon Kyobu Shikkan Gakkai Zasshi. 1989;27(12):1556-1561.
pubmed - Shimada Y, Fujimoto M, Nogami T, Watari H. Adverse events associated with ethical Kampo formulations: analysis of the domestic adverse-event data reports of the ministry of health, labor, and welfare in Japan. Evid Based Complement Alternat Med. 2019;2019:1643804.
doi pubmed pmc - Nogami T, Fujimoto M, Shimada Y, Watari H, Kitahara H, Kimbara Y, Nakagawa H, et al. Incidence of kampo medicine - induced interstitial pneumonia: 10 year retrospective study at a university hospital kampo medicine department. Traditional & Kampo Medicine. 2019;6(1):26-31.
- Enomoto YM, Nakamura YMP, Enomoto NMP, Fujisawa TMP, Inui NMP, Suda T. Japanese herbal medicine-induced pneumonitis: A review of 73 patients. Respir Investig. 2017;55(2):138-144.
doi pubmed - Oura K, Tanaka M, Matsumoto K, Satake R, Inoue M, Yoshida Y, Wakabayashi W, et al. Analysis of drug-induced interstitial lung disease caused by herbal medicine using the Japanese Adverse Drug Event Report database. BMC Complement Med Ther. 2024;24(1):121.
doi pubmed pmc - Hata Y, Uehara H. [A case where herbal medicine sho-seiryu-to induced interstitial pneumonitis]. Nihon Kokyuki Gakkai Zasshi. 2005;43(1):23-31.
pubmed - Prasad P, Gupta A, Nath A, Hashim Z, Gupta M, Krishnani N, Khan A. Clinical characteristics of patients with diffuse alveolar hemorrhage diagnosed by cytological examination of 1000 bronchoalveolar lavage samples. Sarcoidosis Vasc Diffuse Lung Dis. 2023;40(1):e2023004.
doi pubmed pmc - Maldonado F, Parambil JG, Yi ES, Decker PA, Ryu JH. Haemosiderin-laden macrophages in the bronchoalveolar lavage fluid of patients with diffuse alveolar damage. Eur Respir J. 2009;33(6):1361-1366.
doi pubmed - Tsuchiya K, Toyoshima M, Suda T. Pneumonitis with diffuse alveolar hemorrhage induced by Sho-seiryu-to. Intern Med. 2017;56(19):2623-2626.
doi pubmed pmc - Iida Y, Takano Y, Ishiwatari Y, Yoshida A, Shimizu T, Ito R, Hattori T, et al. Diffuse alveolar hemorrhage associated with Makyo-kanseki-to administration. Intern Med. 2016;55(22):3321-3323.
doi pubmed pmc - Takatani K, Miyazaki E, Nureki S, Ando M, Ueno T, Okubo T, Takenaka R, et al. High-resolution computed tomography patterns and immunopathogenetic findings in drug-induced pneumonitis. Respir Med. 2008;102(6):892-898.
doi pubmed - Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest. 2010;137(5):1164-1171.
doi pubmed - Epstein CE, Elidemir O, Colasurdo GN, Fan LL. Time course of hemosiderin production by alveolar macrophages in a murine model. Chest. 2001;120(6):2013-2020.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


