| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 7, July 2024, pages 126-129
An Atypical Presentation of Metastatic Renal Cell Carcinoma
Selia Chowdhurya, b, Samiul Haquea, Harshavardhan Sanekommua, Brandon Nightingalea, Shazli Razia, Mohammad A. Hossaina
aDepartment of Medicine, Jersey Shore University Medical Center, Neptune City, NJ, USA
bCorresponding Author: Selia Chowdhury, Department of Medicine, Jersey Shore University Medical Center, Neptune City, NJ 07753, USA
Manuscript submitted April 12, 2024, accepted May 22, 2024, published online June 19, 2024
Short title: An Atypical Case of Metastatic RCC
doi: https://doi.org/10.14740/jmc4225
| Abstract | ▴Top |
Renal cell carcinoma (RCC) is notorious for spreading to various organs, however, its occurrence in the gastrointestinal (GI) tract is uncommon and poses diagnostic challenges due to vague symptoms. Here, we present the case of a 64-year-old man experiencing recurrent RCC metastasis in the GI tract. He presented with multiple episodes of hematochezia and was found to have masses in the colon, liver, and peritoneum, with histopathology confirming RCC. The patient underwent systemic chemotherapy and palliative radiation therapy, leading to symptom relief. This case emphasizes the rarity of RCC metastasizing to the GI tract and the importance of timely recognition and frequent surveillance during the remission phase to detect recurrence.
Keywords: RCC; Metastasis; Colon
| Introduction | ▴Top |
Renal cell carcinoma (RCC) is the primary tumor originating in the cortex of the kidney and constitutes approximately 85% of all renal malignant tumors [1]. Ranking as the third most common urological cancer, RCC represents 3% of all malignancies in adults in the world, following prostate and bladder cancer [2]. The classic initial triad of symptoms, which includes hematuria, flank pain, and a palpable mass, is reported in only up to 17% of patients [1]. Most RCC cases are asymptomatic, and the widespread use of imaging in the diagnosis of urological disorders in routine medical practice often leads to incidental discovery of RCC cases [1]. RCC carries the highest mortality rate (40%) among patients with urinary tract tumors [3]. Surgical resection stands as the primary curative approach for individuals with localized disease. Despite appropriate oncologic resection, almost 40% of patients may develop metastases post-nephrectomy [3]. Notably, there is no specific time limit for metastatic activity, with late metastatic disease identified in 10% of patients after 5 years [3].
Histologically, RCC is the seventh most common cancer in the Western nations. Most common histologic subtypes include clear cell, papillary and chromophobe [3]. RCC has the potential to metastasize to various distant organs after many years. The most common sites for metastases include the lungs (75%), lymph nodes (36%), bone (20%), and liver (18%) [4]. Metastasis is seen in 33% of patients with RCC, and it occurs mainly via hematogenous route [1]. Diagnostic tools such as ultrasound, magnetic resonance imaging, arteriography, and positron emission tomography/computed tomography (PET/CT) are valuable for disease diagnosis, staging, and management. However, contrast-enhanced thin-slice CT exhibits higher sensitivity in evaluating local recurrence and metastatic disease [3, 4].
In this case report, we present a patient who underwent sigmoid colonic mass resection for colonic metastasis of RCC and presented with recurrence of colon metastasis 3 years post-resection. Metastasis to the gastrointestinal tract, specifically the colon, is exceedingly rare, with fewer than 15 cases of solitary colon metastases reported in the global literature to date [3].
| Case Report | ▴Top |
This is a 64-year-old male with a past medical history of metastatic clear cell RCC. He was found to have a left renal mass incidentally in magnetic resonance imaging (MRI) and diagnosed with RCC at the of 58. He underwent a robotic-assisted left radical nephrectomy. Subsequent surveillance with PET/CT, MRI, and colonoscopy in 2 years revealed a mass in the sigmoid colon, for which he underwent surgical resection of the sigmoid colon serosal mass, peritoneal mass, and abdominal wall soft tissue mass with ileosigmoid bypass surgery. Biopsy of the sigmoid colon mass confirmed metastatic RCC with peritoneal metastasis (positive for PAX 8 and negative for CK 20, CDX 2, CK7 (Fig. 1). The patient commenced pembrolizumab along with low-dose axitinib after the surgery. He had poor tolerance to axitinib and was switched to cabozantinib, which he was on for 3 years, then he was started on nivolumab. He also received palliative radiation therapy.
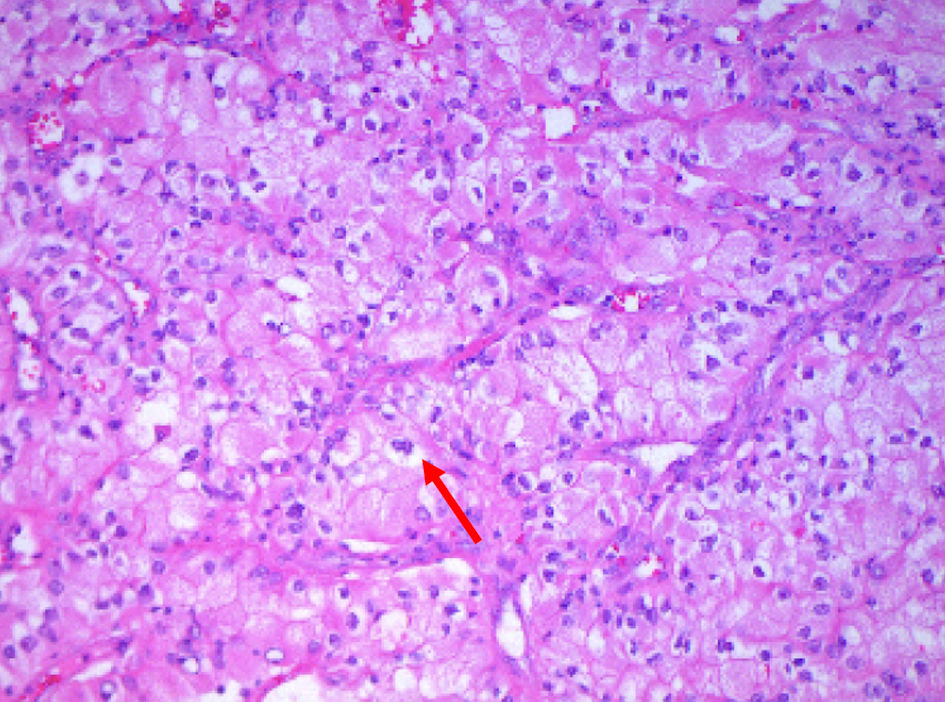 Click for large image | Figure 1. Surgical specimens from the sigmoid mass revealing neoplastic cells (arrow) with ample granular to clear cytoplasm, vesicular chromatin and scattered inflammatory cells growing in trabecular growth pattern (hematoxylin and eosin stain × 200). |
The patient presented to the emergency department with five episodes of bright red blood per rectum for 1 day. The patient endorsed dizziness and fatigue and denied various symptoms, including abdominal pain, nausea, vomiting/hematemesis, fever, chills, chest pain, shortness of breath, or headache. He was hemodynamically stable: blood pressure of 108/64 mm Hg, heart rate of 94 beats per minute, and respiratory rate of 18 breaths per minute with an oxygen saturation of 100% on room air.
On physical examination, he appeared chronically ill without any signs of acute distress. The abdomen was soft and nontender, with normoactive bowel sounds. Laboratory results showed a significant drop in hemoglobin (Hb) to 6.7 g/dL (baseline Hb ranges between 10.1 and 11.2 g/dL), with notable results listed in Table 1.
 Click to view | Table 1. Notable Laboratory Values on Admission |
Computed tomography angiogram (CTA) of the abdomen and pelvis revealed a lobulated mass regional to the splenic flexure of the colon up to 7 cm (Fig. 2a), multiple densities in the peritoneum, and multiple enhancing lesions in the liver suggestive of central necrosis (Fig. 2b).
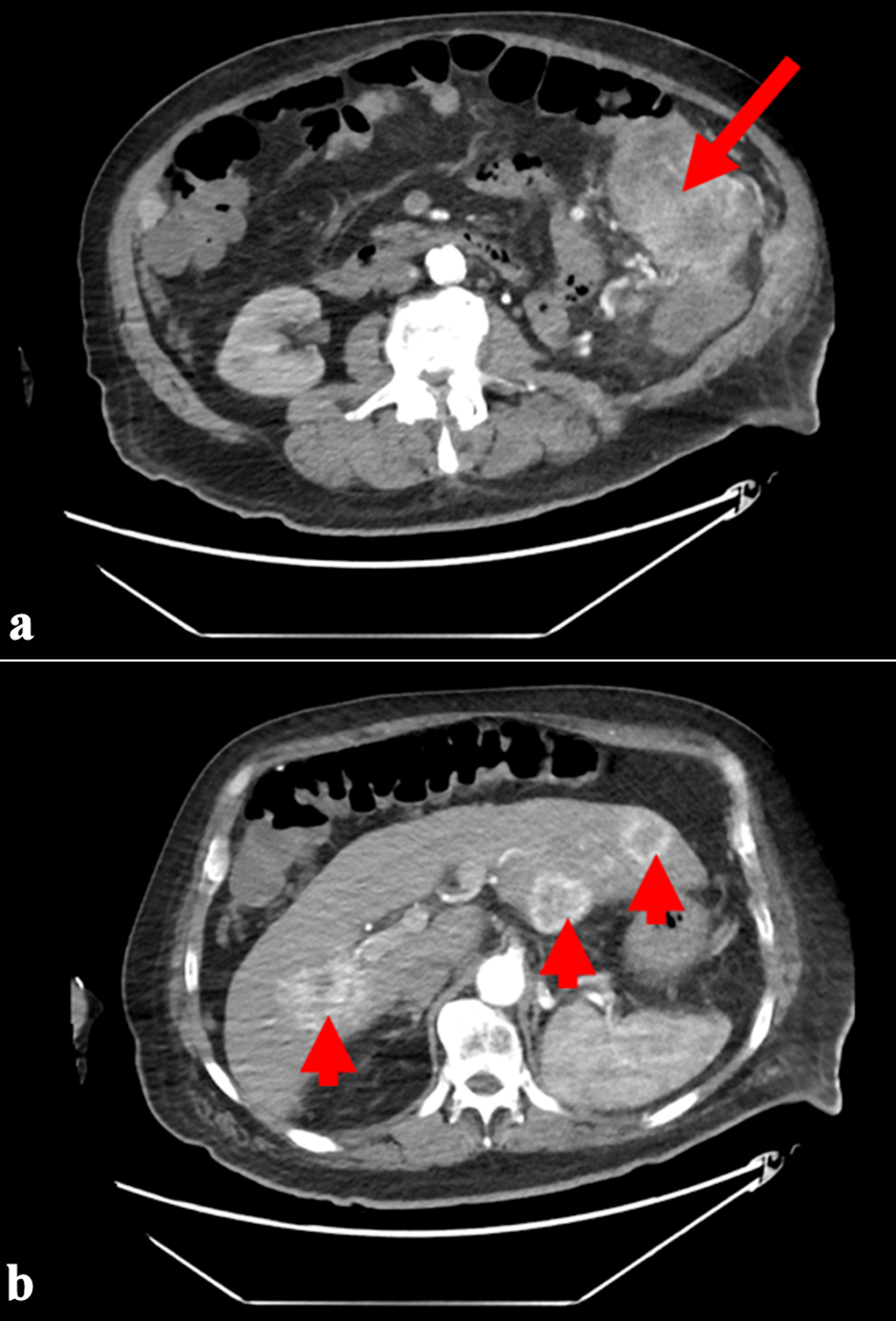 Click for large image | Figure 2. (a) CT angiography of the abdomen and pelvis showing a mass (arrow) in the splenic flexure of the colon. (b) Multiple hypodense lesions (arrowheads) in the liver suggestive of central necrosis. CT: computed tomography. |
During the patient’s hospitalization, nivolumab was temporarily halted, and careful monitoring of the complete blood count (CBC) was initiated. He was transfused with two units of packed red blood cells. Concurrently, the intravenous administration of ferric gluconate was continued. His symptoms resolved completely with the improvement of biochemical parameters, and his diet was advanced gradually as tolerated. Due to diffuse metastatic colon cancer, surgical resection was not considered from an oncologic standpoint. Interventional radiology embolization was recommended in case of persistent active bleeding. Post-discharge, the patient’s bleeding resumed, and he was further continued with nivolumab with active discussions on goals of care, subsequently went for hospice care.
| Discussion | ▴Top |
RCC is a primary kidney tumor, typically observed in adulthood, particularly in the sixth and seventh decades, with a higher incidence in men (male to female: 2/1) [5]. While the majority of RCCs are sporadic, around 4% are familial and linked to syndromes like von Hippel-Lindau disease, tuberous sclerosis, hereditary papillary renal cancer, Birt-Hogg-Dube syndrome, hereditary leiomyoma, familial renal oncocytoma, and hereditary renal cancers [6]. RCC can metastasize to various locations, and the metastatic pattern may differ based on the timing of recurrence [7]. Gastrointestinal tract metastasis is infrequent, with colon involvement being rarer compared to the stomach and small bowel [8]. Here, we report a rare recurrence of colonic metastasis from primary RCC despite radical nephrectomy and colonic mass resection.
Metastasis can transpire via lymphatic or hematogenous spread, or by direct invasion. Tumor size correlates with metastatic potential, with lymph nodes and distant metastases possible even in early RCC stages [9]. While the exact mechanism of metastasis remains elusive despite resection of the primary malignancy, Paget’s “Seed and Soil” theory may shed light on the process. Recent studies propose the formation of pre metastatic niches in target organs, where the primary tumor releases factors that attract immune cells and modify the microenvironment, facilitating the colonization and growth of metastatic cells [10]. This could potentially elucidate the recurrence of colonic metastasis in our patient. While there have been several reported cases of RCC metastasizing to the colon, none has been documented to recur [3]. Most cases of colon metastases are discovered during evaluations for symptoms such as lower gastrointestinal bleeding, similar to our patient. The locations of colonic metastases vary, with the sigmoid, splenic flexure, transverse colon, and hepatic flexure being commonly affected. Solitary metastases are uncommon and are typically associated with disseminated disease [10]. A study analyzing 1,173 autopsy reports found intestinal involvement in 9% of RCC cases, but none presented as solitary metastases, which makes this case presentation a unique one [11].
The prognosis for non-surgically treated metastatic RCC is generally poor [12]. Due to the high metastasis rate, a multidisciplinary approach is essential for RCC management. Both the National Comprehensive Cancer Network and the American Urology Association recommend routine postoperative surveillance for the first 5 years. Most recurrences of RCC tend to occur within 5 years following initial curative surgery, as many as 10% develop beyond 5 years after primary surgical treatment [13]. Although a specific duration for extended follow-up is not clearly defined, a study by Stewart et al suggested a reduction in recurrences with longer follow-up of 3,651 operated patients [14]. Considering the potential for late RCC recurrence, it may be prudent to extend postoperative surveillance beyond 15 years. Additionally, colonoscopy can be a valuable diagnostic tool for patients experiencing gastrointestinal symptoms during extended RCC surveillance.
Conclusions
Metastatic RCC may present as a colonic neoplasm, especially in patients experiencing lower gastrointestinal bleeding. Recurrent metastases can arise years after curative nephrectomy for RCC, underscoring the importance of diligent, long-term follow-up for such individuals. It is essential to consider the possibility of recurrence or metastasis, particularly when patients report symptoms such as abdominal pain, anemia, or gastrointestinal bleeding. Consistent monitoring and timely evaluation of these signs can facilitate early detection and efficient management of potential complications linked to metastatic RCC.
Learning points
RCC can metastasize to the colon, though it is uncommon.
Long-term follow-up beyond 5 years is crucial for detecting late recurrences.
Gastrointestinal symptoms like bleeding or anemia in RCC patients should be investigated for potential metastasis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed patient consent has been obtained for this case report.
Author Contributions
SC contributed to conception, design, manuscript production, writing and editing. SH contributed to manuscript writing. HS contributed to manuscript writing. BN contributed to manuscript writing. SR contributed to manuscript writing. MAH contributed to conception and design of the work, and final review of manuscript. Manuscript has been read and approved by all the authors
Data Availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
| References | ▴Top |
- Saadaat R, Haidary AM, Ibrahimkhil AS, Abdul-Ghafar J. Metastatic renal cell carcinoma involving colon with unusual histologic features and diagnostic challenges: A case report. Int J Surg Case Rep. 2021;80:105627.
doi pubmed pmc - Maldazys JD, deKernion JB. Prognostic factors in metastatic renal carcinoma. J Urol. 1986;136(2):376-379.
doi pubmed - Subasi O, Aziret M, Karaman K, Ercan M. Colonic metastasis of renal cell carcinoma following curative nephrectomy: A case report and review of the literature. Int J Surg Case Rep. 2019;65:152-155.
doi pubmed pmc - Sadler GJ, Anderson MR, Moss MS, Wilson PG. Metastases from renal cell carcinoma presenting as gastrointestinal bleeding: two case reports and a review of the literature. BMC Gastroenterol. 2007;7:4.
doi pubmed pmc - Chow WH, Devesa SS, Warren JL, Fraumeni JF, Jr. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281(17):1628-1631.
doi pubmed - Brookman-May SD, May M, Shariat SF, Novara G, Zigeuner R, Cindolo L, De Cobelli O, et al. Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma—results from a comprehensive multi-centre database (CORONA/SATURN-Project). BJU Int. 2013;112(7):909-916.
doi pubmed - McNichols DW, Segura JW, DeWeerd JH. Renal cell carcinoma: long-term survival and late recurrence. J Urol. 1981;126(1):17-23.
doi pubmed - Berry AC, Nakshabendi R, Kanar O, Cai W, Persaud M. Sigmoid colonic polyp as initial presentation of metastatic papillary renal cell carcinoma. Ochsner J. 2017;17(4):417-420.
pubmed pmc - Guethmundsson E, Hellborg H, Lundstam S, Erikson S, Ljungberg B, Swedish Kidney Cancer Quality Register G. Metastatic potential in renal cell carcinomas </=7 cm: Swedish Kidney Cancer Quality Register data. Eur Urol. 2011;60(5):975-982.
doi pubmed - Akhtar M, Haider A, Rashid S, Al-Nabet AD. Paget’s “Seed and Soil” Theory of Cancer Metastasis: An Idea Whose Time has Come. Advances in Anatomic Pathology. 2019;26(1):69-74.
doi - Saitoh H. Distant metastasis of renal adenocarcinoma. Cancer. 1981;48(6):1487-1491.
doi pubmed - Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16(6):2261-2266.
doi pubmed - Kim SP, Weight CJ, Leibovich BC, Thompson RH, Costello BA, Cheville JC, Lohse CM, et al. Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology. 2011;78(5):1101-1106.
doi pubmed - Stewart SB, Thompson RH, Psutka SP, Cheville JC, Lohse CM, Boorjian SA, Leibovich BC. Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol. 2014;32(36):4059-4065.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


