| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 8, August 2024, pages 171-179
Peripartum Cardiomyopathy: A Case Report of Mortality From a Rare and Potentially Fatal Condition
Diana Marcela Perea Rojasa, d , Christian David Seni Hernandeza, Indiana Luz Rojas Torresa, Marianela Olivares Olmosb, Camila Maria Garcia Jaravab, Dario Jose Gaivao Arciniegasb, Sebastian Andre Seni Hernandezb, Luis Miguel Corrales Calderonc, Luis Enrique Perea Vasquezc, Silvia Salva Camanoc
aFaculty of Health Sciences, Universidad Simon Bolivar, Barranquilla, Colombia
bFaculty of Health Sciences, Universidad Del Norte, Barranquilla, Colombia
cLa Misericordia Clinica Internacional, Barranquilla, Colombia
dCorresponding Author: Diana Marcela Perea Rojas, Faculty of Health Sciences, Universidad Simon Bolivar, Barranquilla, Colombia
Manuscript submitted April 13, 2024, accepted June 14, 2024, published online July 18, 2024
Short title: Peripartum Cardiomyopathy Mortality
doi: https://doi.org/10.14740/jmc4228
| Abstract | ▴Top |
Peripartum cardiomyopathy (PPCM) poses a significant challenge in maternal health, characterized by heart failure with reduced ejection fraction during late pregnancy or early postpartum. Despite advances in understanding PPCM, it remains life-threatening with substantial maternal morbidity and mortality. This article reviews the epidemiology, etiology, diagnostic challenges, management strategies, and outcomes associated with PPCM. A case report of a 29-year-old woman with PPCM is presented, emphasizing the importance of early recognition and tailored management. The patient’s presentation was marked by atypical symptoms, including dysuria, lumbar pain, persistent fever, and oral intake intolerance. Despite aggressive medical intervention, the patient experienced a tragic outcome, succumbing to cardiopulmonary arrest within 48 h of admission. This case underscores the challenges in diagnosing and managing PPCM, particularly when presenting with nonspecific symptoms and emphasizes the urgent need for improved diagnostic criteria and therapeutic interventions to mitigate adverse outcomes in affected individuals.
Keywords: Peripartum cardiomyopathy; Pregnancy; Heart failure; Mortality; Maternal health
| Introduction | ▴Top |
Peripartum cardiomyopathy (PPCM) represents a significant challenge in maternal health, manifesting as heart failure with reduced ejection fraction during late pregnancy or in the early postpartum period. Despite advances in understanding PPCM, it remains a life-threatening condition associated with substantial maternal morbidity and mortality due to its timing and individual characteristics [1]. It was first described in the late 1800s, but it is only in recent decades that it has gained recognition as a distinct cardiac condition.
The incidence of PPCM varies globally, ranging from one in 968 to one in 4,000 live births. In the USA, it affects approximately one in 2,230 births, with an increasing trend noted over time. Asian countries exhibit varying rates, with Japan reporting a lower incidence than other parts of Asia. Additionally, countries like Haiti and Nigeria report cases from one in 300 and one in 100 live births, respectively [2]. This condition also carries significant morbidity and mortality, with rates ranging from 0% to 40% globally. African regions have higher prevalence and worse outcomes, with mortality rates reaching 47.4% in Nigeria. Also, African and African American women face elevated risk compared to Caucasian populations, highlighting socioeconomic and health care access disparities [3].
PPCM is characterized by left ventricular systolic dysfunction, often with a reduced ejection fraction below 45%, occurring in previously healthy young women without identifiable cardiac causes [4]. The etiology of PPCM is complex and multifactorial, involving factors such as oxidative stress, hormonal influences, inflammation, and genetic predisposition. While the precise mechanisms underlying PPCM remain elusive, recent research has implicated antiangiogenic 16-kDa prolactin (PRL) as a key trigger, leading to cardiomyocyte damage via microRNA-mediated pathways [2].
PPCM is likely multifactorial in origin with several implicated risk factors, these include advanced maternal age, African origin, preeclampsia, use of tocolytics, twin pregnancy, high parity, obesity, poor socioeconomic status, malnutrition, customary birth practices, and selenium deficiency [4]. The mean age of PPCM onset is typically around 30 to 31 years, with incidence rising with age, especially among women over 40. Additionally, PPCM disproportionately affects women of African descent, particularly African Americans, who are significantly more likely to develop PPCM compared to White or Hispanic women; factors like high parity rates, socioeconomic status, and hypertension may contribute to this racial disparity. Furthermore, higher gravidity, parity, and multigestational pregnancies increase the likelihood of PPCM. Hypertensive disorders of pregnancy, including gestational hypertension and preeclampsia, are strongly linked too, consequently, managing hypertensive disorders during pregnancy is crucial in reducing the risk of PPCM [5].
Diagnostic challenges persist in PPCM, necessitating a comprehensive evaluation incorporating clinical suspicion, advanced imaging modalities such as echocardiography, and the exploration of potential biomarkers to aid in early detection and differentiation from physiological changes of pregnancy [1].
Management strategies for PPCM are currently based on general heart failure protocols [5], with bromocriptine emerging as a specific treatment by inhibiting PRL secretion from the pituitary gland [1, 4]. However, the response to treatment varies among patients, highlighting the need for further research into alternative pathophysiological pathways and novel therapeutic targets [5].
In this case report, we present a 29-year-old woman of mixed ethnicity who presented with dysuria, lumbar pain, persistent fever and oral intake intolerance, ultimately diagnosed with PPCM. We discuss the clinical presentation, diagnostic workup, management approach, and outcomes of this case in the context of existing literature and current understanding of PPCM. This case highlights the importance of early recognition and tailored management in improving outcomes for patients with PPCM.
| Case Report | ▴Top |
The case involves a 29-year-old woman at 22.4 weeks of gestation as determined by ultrasound, a non-smoker of mixed ethnicity housewife from an urban area, with a history of three pregnancies and two cesarean sections, both with high risk due to extreme prematurity. She also had a previous hospitalization for an Escherichia coli (E. coli) urinary tract infection treated with antibiotic therapy, followed by discharge.
She was admitted to the emergency unit with a 15-day history of dysuria, lumbar pain, persistent fever and oral intake intolerance. On physical examination, she presents hypotension, tachycardia, tachypnea, fever, no neurological signs, tolerance to ambient oxygen, and fetal tachycardia on Doppler, likely secondary to maternal fever (Table 1). As an important note, the patient reported receiving treatment 4 days ago for the urinary tract infection with sultamicillin at another healthcare center. However, due to experiencing an allergic reaction to this medication, she discontinued it.
 Click to view | Table 1. Vital Signs Record |
Given these findings, sepsis with a probable urinary focus was considered. Hydration replacement was initiated with 0.9% saline solution at 120 mL/h via infusion pump, and broad-spectrum antibiotic therapy with intravenous piperacillin-tazobactam 4.5 g every 6 h after obtaining a urine culture. The patient was transferred to the intensive care unit for multidisciplinary management.
In the intensive care unit, prophylactic thrombosis therapy was initiated with subcutaneous enoxaparin 60 mg every 24 h, progesterone 200 µg intravaginally every 24 h due to history of premature delivery and short cervix, and nasal cannula oxygen therapy at 3 L/min. Additionally, due to experiencing vomiting, she was given antiemetic therapy, and the previously initiated analgesic and empirical antibiotic therapy was continued.
The patient subsequently continued to present blood pressure readings with a tendency towards hypotension, tachycardia and tachypnea. Due to these findings, in order to maintain vital organ function, restore circulating blood volume and improve organ perfusion, fluid resuscitation was initiated with a 500 mL bolus of 0.9% saline solution. Additionally, laboratory results showed respiratory alkalosis in arterial blood gases, neutrophilia in the complete blood count (Table 2), consequently, a 15 mL bicarbonate ampule was administered.
 Click to view | Table 2. Arterial Gases Record |
Following this, due to the patient’s persistent tachycardia, hypotension, tachypnea, and a history of recurrent urinary tract infections, she was considered at high risk for Pseudomonas and multidrug-resistant bacteria producing beta-lactamases. Therefore, the spectrum of antibiotics was broadened to carbapenems, and intravenous meropenem 2 g every 8 h for 48 h was initiated, followed by 1 g every 8 h thereafter.
The following day, she reported dyspnea on moderate exertion with accessory muscles with signs of hemodynamic instability such as somnolence and precordial pain. An electrocardiogram showed ST segment elevation in the inferior leads, with troponin T levels of 610.3 pg/mL, and pro-B-type natriuretic peptide (proBNP) levels of 8,397 pg/mL, prompting an urgent repeat chest X-ray that indicated acute heart failure (Fig. 1).
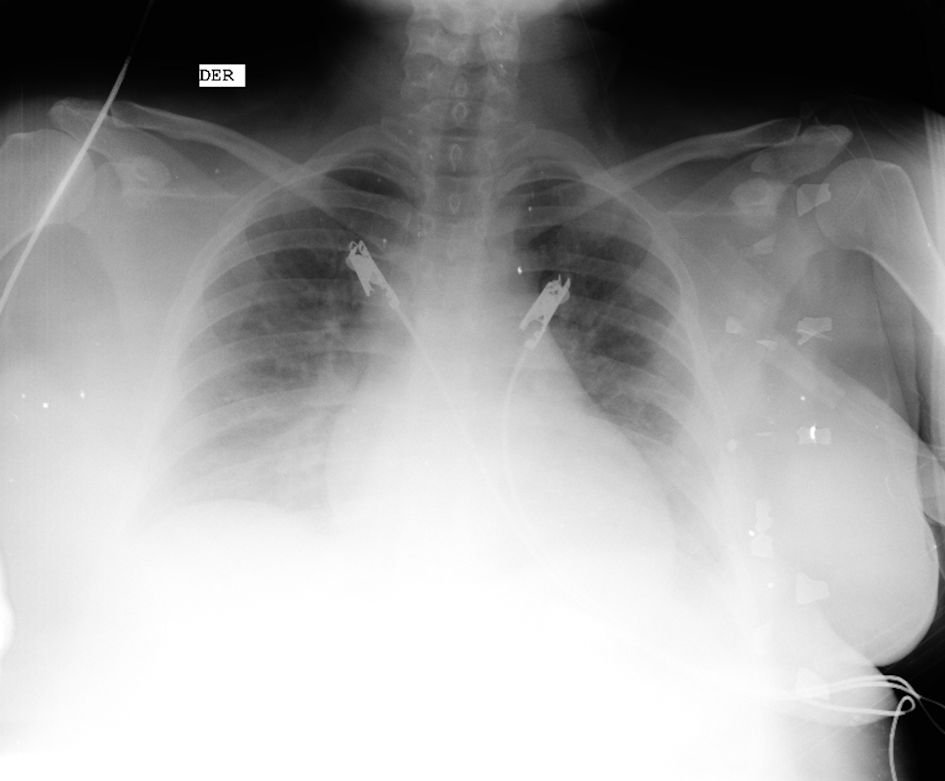 Click for large image | Figure 1. Chest X-ray. Figure shows enlarged cardiac silhouette, bilateral pulmonary vascular congestion and diffuse interstitial edema consistent with pulmonary congestion (source: patient’s medical chart, 2023). |
Additionally, a transthoracic echocardiogram (Fig. 2 and Table 3) showed a mild concentric ventricular hypertrophy and global akinesia with a left ventricular ejection fraction (LVEF) of 23%, depressed right ventricular systolic function, and mild pericardial effusion, leading to a diagnosis of heart failure with depressed ejection fraction (Stevenson class C) and acute respiratory failure secondary to cardiomyopathy. Consequently, treatment was initiated with a single dose of the inotropic agent dobutamine, administered as two intravenous ampules, and loop diuretic furosemide 40 mg bolus followed by 20 mg every 8 h.
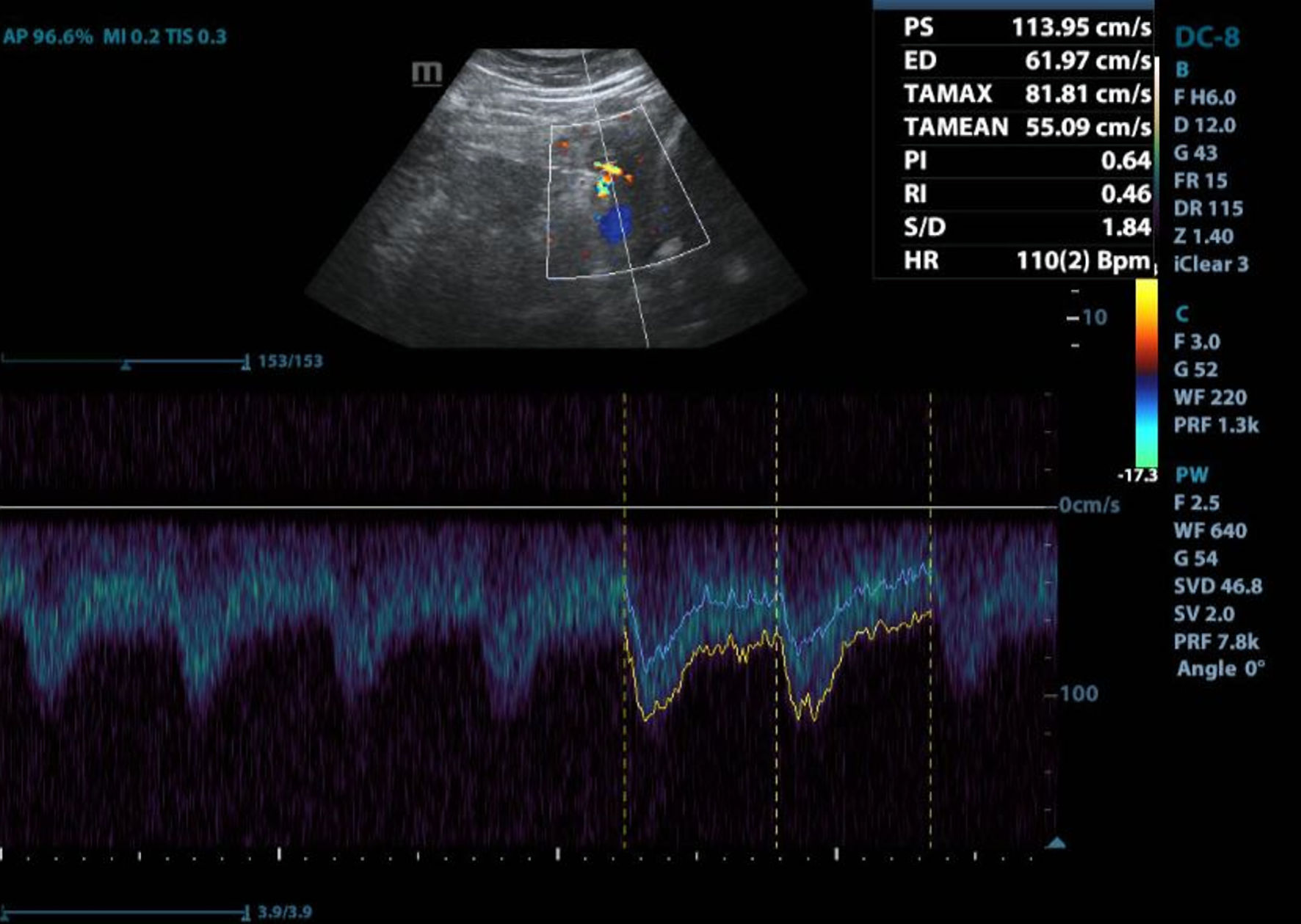 Click for large image | Figure 2. Transthoracic echocardiogram. Transthoracic echocardiogram shows left ventricular ejection fraction of 23%, right ventricle with moderately depressed systolic function, mild pericardial effusion and indirect signs with intermediate probability of pulmonary hypertension (Source: patient’s medical chart, 2023). |
 Click to view | Table 3. Transthoracic Echocardiogram Measurements |
Subsequently, the patient developed acute respiratory failure requiring 50% Venturi mask, which progressed to non-rebreather mask at 15 L/min. However, she continued to exhibit poor respiratory mechanics and hemodynamic instability necessitating vasopressor support with norepinephrine. Finally, 28 h after admission, the patient experienced cardiorespiratory arrest with ventricular fibrillation, cardiopulmonary resuscitation with chest compressions, and inotropic support was initiated without recovery of spontaneous circulation for more than 30 min; therefore, an emergency cesarean section was performed due to the state of cardiopulmonary arrest. An extremely premature without heartbeat was delivered, and resuscitation efforts were initiated for 10 min without recovery of vital signs. Figure 3 summarizes the timeline of the patient.
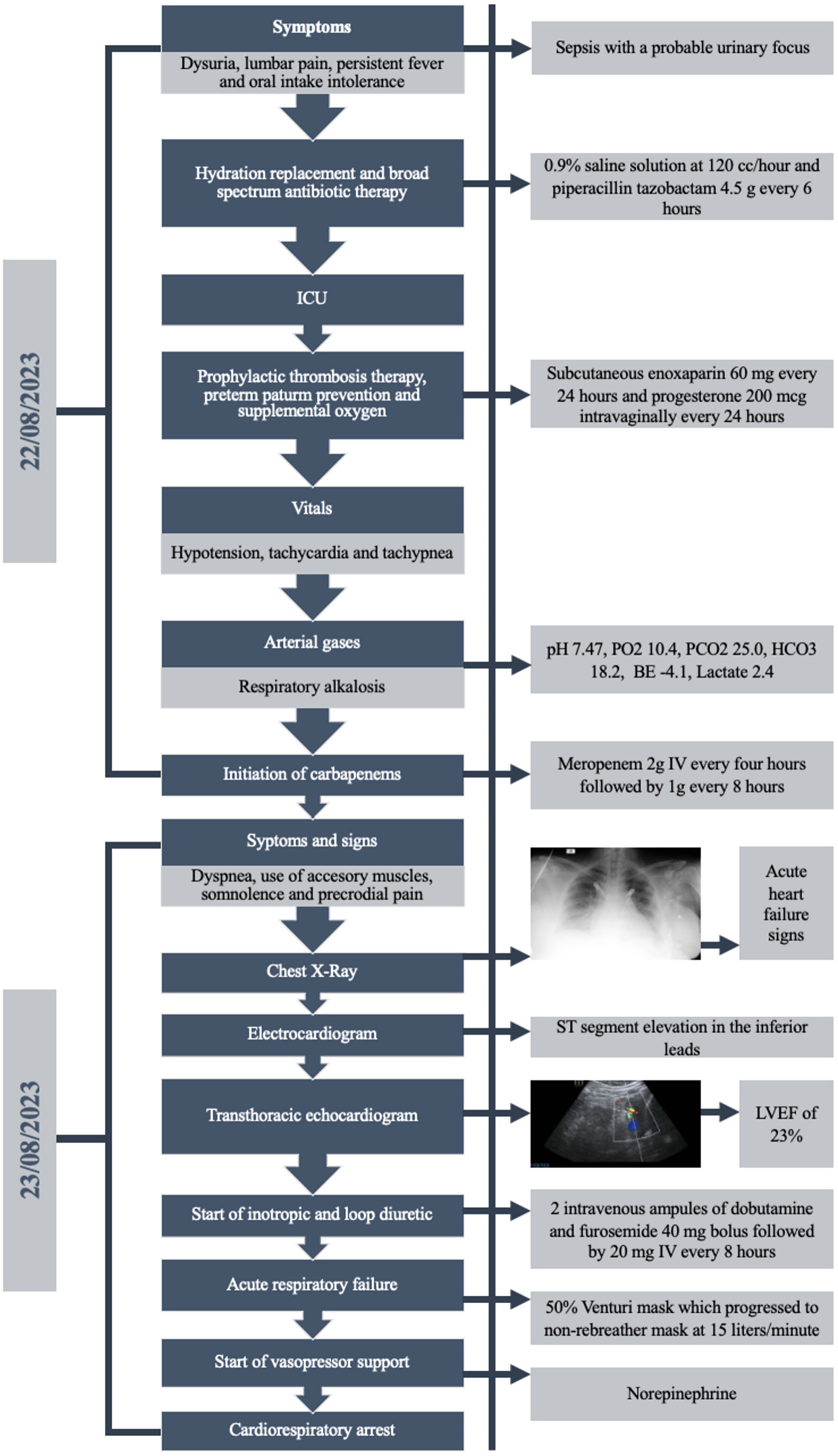 Click for large image | Figure 3. Case timeline. Figure summarizes the case presentation of the patient, exam findings, symptoms, treatments and progression to cardiorespiratory arrest (source: made by authors, 2024). ICU: intensive care unit; IV: intravenous; LVEF: left ventricular ejection fraction; PO2: partial pressure of oxygen; PCO2: partial pressure of carbon dioxide; HCO3: bicarbonate; BE: base excess. |
| Discussion | ▴Top |
PPCM is a serious condition marked by reduced left ventricular function during or after pregnancy, often leading to heart failure. It is distinct from other cardiomyopathies due to its timing and unique features [1] with a higher prevalence and significantly worse outcomes reported amongst African women; the variable incidence between and within countries could be attributed to the differences in prevalence of both genetic and non-genetic risk factors, as well as disparities in socioeconomic status and access to healthcare [3].
The patient in the case report exhibited several risk factors associated with PPCM, including a history of multiple pregnancies, previous cesarean sections, and a urinary tract infection, which align with the known risk factors outlined in the literature. These risk factors include advanced maternal age, high parity, and hypertensive disorders of pregnancy, all of which increase the likelihood of developing PPCM.
Various mechanisms are implicated in PPCM, including hemodynamic stresses of pregnancy, vasculo-hormonal factors, inflammation, immunology, genetics, autoantibodies, myocarditis, and hemodynamic stress. PPCM is considered primarily idiopathic, but several plausible etiologies have been proposed. Vasculo-hormonal factors play a role in PPCM, particularly angiogenic imbalance accentuated by preeclampsia [6]. In a recent multicenter study involving women with PPCM, researchers observed a significant link between levels of activin A and sFlt-1 and both systolic and diastolic blood pressure, particularly in those with a history of hypertensive disorders of pregnancy, like preeclampsia. These findings suggest that women with PPCM and a history of HDP may have a different vascular profile compared to those without such a history [7].
Increased oxidative stress and apoptosis, triggered by hormonal factors like prolactin, also contribute to PPCM pathogenesis. Evidence suggests that a proinflammatory state could be a factor in the pathophysiology of PPCM, as supported by studies indicating elevated levels of proinflammatory cytokines such as interleukin-6, tumor necrosis factor-α, interferon-γ, C-reactive protein, and soluble death receptor sFas/Apo1 circulating in the body [8]. In this case, upon admission, the patient exhibited signs of sepsis with a probable urinary focus, and her clinical presentation included hypotension, tachycardia, tachypnea and fever, indicating systemic inflammation and hemodynamic instability, which were a critical trigger for the development of PPCM.
Genetic factors play a significant role too. It is believed that about 15-20% of PPCM cases may involve a genetic predisposition, with mutations in genes like TTN, FLNC, DSP, and BAG3, which interacts with environmental factors such as low serum selenium levels. This electrolyte is crucial for antioxidant enzyme function, and its deficiency can promote oxidative stress and myocardial injury, potentially exacerbating other contributing factors like viral infections such as myocarditis [5, 6]. Other research suggests that one prominent pathophysiologic theory implicates prolactin, which can undergo enzymatic changes, leading to vasoconstriction, inflammation, and impaired heart muscle function. Additionally, disturbances in proangiogenic and antiangiogenic factors may play roles in PPCM development, affecting endothelial repair and function [1].
Regarding the diagnosis, women with PPCM typically present in the last month of pregnancy or the first 5 months postpartum, exhibiting symptoms such as dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, lower extremity edema, fatigue, chest pain, and cough. Severe cases may present in cardiogenic shock, resembling conditions like myocardial infarction (MI), pulmonary embolism (PE), or amniotic fluid embolism, while some may have severe arrhythmias. Physical examination may reveal signs like pulmonary rales, raised jugular venous pressure, peripheral edema, tachypnea, tachycardia, and irregular peripheral pulses, along with murmurs of mitral and tricuspid regurgitation [6].
However, early signs often present atypically, complicating diagnosis for healthcare providers, while delayed medical consultation by patients exacerbates prognostic outcomes. Consequently, diagnosis typically relies on exclusion, leading to treatment delays and increased morbidity and mortality rates. Thus, differential diagnosis primarily involves distinguishing PPCM from conditions such as preeclampsia, pulmonary thromboembolism, amniotic fluid embolism, pulmonary edema due to other causes, acute MI, and acute coronary dissection, among others [9]. The symptoms exhibited by this patient, such as dysuria, lumbar pain, persistent fever, and oral intake intolerance, are atypical for PPCM. However, they suggest an underlying infectious process, likely a urinary tract infection, as the initial trigger for PPCM development in this case. The patient’s history of a urinary tract infection followed by sepsis indicates a potential link between infection-induced inflammatory response and PPCM pathogenesis. Sepsis can lead to systemic inflammation and myocardial injury, exacerbating the risk of PPCM development, particularly in susceptible individuals.
Echocardiography serves as the primary imaging technique based on criteria established by the PPCM Working Group of the European Society of Cardiology. While left ventricular dilation may not always be present, a reduced LVEF below 45% is a hallmark, with left ventricular end-diastolic diameter exceeding 60 mm and LVEF dropping below 30% indicating poorer prognosis [5]. Additional echocardiographic findings may include right ventricular dilatation and dysfunction, pulmonary hypertension, enlargement of the left atrium or biatrial, functional regurgitation of the mitral and tricuspid valves, as well as intracardiac thrombosis [10]. Additionally, since physiological ventricular hypertrophy occurs during pregnancy due to the increase in maternal blood volume, it may be found in the echocardiography as well [11].
Although echocardiography is the first imaging option, magnetic resonance imaging (MRI) offers more precise measurements of chamber volumes and ventricular function, especially detecting left ventricular thrombus. MRI, particularly with gadolinium, aids in diagnosing myocarditis, but this contrast should be avoided during pregnancy unless absolutely necessary [12].
Diagnostic evaluation also includes serum B-type natriuretic peptide (BNP) and N-terminal pro-BNP levels, which are often elevated in PPCM but show minimal increases in normal pregnancy and moderate increases in preeclampsia. Electrocardiography plays a vital role too, with over 95% of PPCM patients exhibiting abnormalities such as signs of left ventricular hypertrophy, tachycardia, nonspecific T wave changes, left atrial dilation, or conduction abnormalities [11]. On the other hand, chest X-ray aids in identifying cardiac enlargement and pulmonary edema, as well as ruling out other conditions mimicking heart failure particularly in low- and middle-income countries [4, 6].
Prompt diagnosis and comprehensive management are essential for pregnant women with acute heart failure, requiring collaboration among various medical specialists including cardiologists, intensivists, obstetric physicians, neonatologists, anesthesiologists, and cardiac surgeons. The management approach prioritizes both maternal and fetal well-being, with therapeutic strategies tailored to the severity of heart failure categorized under the “BOARD” acronym that includes bromocriptine, oral heart failure drugs, anticoagulation, relaxants and diuretics [6].
Bromocriptine is being employed as a supplementary treatment to inhibit the 16-KDa receptor implicated in the disease process, aiming to restore left ventricular function, the treatment protocol involves administering 2.5 mg every 12 h for 15 days, followed by a maintenance dose of 2.5 mg per day for 6 weeks [13, 14]. The oral heart failure drugs used are beta-blockers, hydralazine and digoxin which are considered safe during pregnancy; however, medications like angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, sacubitril-valsartan, sodium-glucose cotransporter 2 inhibitors, and mineralocorticoid receptor antagonists should be avoided during pregnancy [15].
On the other hand, for managing fluid levels, dietary sodium restriction is the first-line approach, while loop diuretics are safe for patients with peripheral or lung fluid buildup. It is important to note that neonates born to mothers who used thiazide diuretics during pregnancy have been reported to experience bleeding diathesis and hyponatremia. To mitigate potential risks, beta 1-selective drugs like metoprolol are preferred due to their reduced interference with beta 2-mediated uterine relaxation and peripheral vasodilatation. In instances where PPCM coexists with conditions such as preeclampsia or gestational hypertension, the utilization of labetalol is advantageous [15, 16].
In some cases where inotropic treatment has not been effective, the use of levosimendan is considered. This is the first available agent in the class of drugs known as calcium sensitizers. It enhances myocardial contractility by sensitizing calcium-sensitive myofilaments without significantly elevating intracellular calcium levels, it also exhibits superiority over both placebo and dobutamine in managing chronic heart failure and may enhance the mechanical efficiency of the right ventricle; however, its efficacy in cardiogenic shock requires further confirmation [17].
The consideration of temporary mechanical circulatory support, such as ventricular assist device therapy or extracorporeal membrane oxygenation (ECMO), in patients with hemodynamic instability despite inotropic support, is crucial for managing severe cases of cardiovascular compromise. These advanced interventions can provide temporary support to the failing heart, allowing time for myocardial recovery [18].
Anticoagulant therapy may be considered in patients with atrial fibrillation, left ventricle thrombus, systemic embolism, bromocriptine usage, and an LVEF below 30% [19]. However, there is uncertainty regarding the need for anticoagulation and the choice of medications. Options include unfractionated and low-molecular-weight heparin during pregnancy and breastfeeding, while warfarin is safe during breastfeeding but not recommended during pregnancy [15].
In the presented case, decisions from the onset of symptoms to acute management at resuscitation were meticulously guided by a step-by-step approach aimed at swiftly addressing the patient’s evolving condition. Upon recognition of symptoms suggestive of a urinary tract infection and subsequent sepsis, urgent medical intervention was initiated to stabilize the patient’s deteriorating state. The decision to administer intravenous fluid resuscitation with 0.9% saline solution was driven by the imperative to restore intravascular volume and ensure adequate tissue perfusion in the face of hypotension and signs of poor perfusion. Simultaneously, broad-spectrum antibiotic therapy with piperacillin-tazobactam was promptly initiated to combat the suspected source of infection and prevent systemic spread. These critical decisions were rooted in the urgency of halting the progression of sepsis and mitigating its potential complications. The patient also received supportive care measures such as oxygen therapy and vasopressor support, which are integral components of PPCM management in critically ill patients. However, despite appropriate management efforts, the patient in the case report ultimately succumbed to cardiopulmonary arrest, which necessitated a rapid and coordinated response to maximize the chances of successful resuscitation. The decision to perform an emergency cesarean section was made due to the critical condition of the patient and the need to expedite delivery in the setting of cardiopulmonary arrest. This decision was guided by the principle of prioritizing maternal survival while also considering the potential benefits to the unborn child. The cesarean section allowed for the prompt delivery of the fetus, enabling the initiation of neonatal resuscitation measures and providing access to additional medical interventions if needed. Simultaneously, cardiopulmonary resuscitation efforts were initiated promptly following established protocols, including chest compressions and administration of inotropic support to restore circulation. The multidisciplinary team worked collaboratively to coordinate these efforts, ensuring that both the mother and the unborn child received the necessary medical care. Despite these interventions, the patient unfortunately did not achieve return of spontaneous circulation, underscoring the challenges and complexities associated with managing cardiopulmonary arrest in the setting of PPCM, particularly in patients with advanced disease or complications such as acute respiratory failure.
The decision not to end the pregnancy immediately upon diagnosing PPCM involved a complex consideration of multiple factors, including the gestational age of the fetus, the severity of the mother’s condition, and the risks associated with premature delivery. Ending the pregnancy prematurely may have posed significant risks to the fetus, particularly if it was not yet viable outside the womb. Additionally, the patient’s clinical status was initially stable enough to warrant a conservative approach, with close monitoring and aggressive medical management to stabilize her condition and optimize maternal and fetal outcomes.
Regarding the decision not to perform a coronary angiography when ST-elevation was diagnosed on the electrocardiogram, while ST-elevation can be indicative of acute coronary syndrome in certain contexts, in the setting of PPCM, it may not necessarily indicate coronary artery disease. Additionally, performing invasive procedures such as coronary angiography carries its own set of risks, and the benefits of doing so would need to outweigh the potential risks, especially in a critically ill patient.
Recovery typically occurs within 3 to 6 months postpartum, but in some cases, it may take up to 48 months after delivery. Good prognostic indicators include a small left ventricular size, a LVEF greater than 30-35%, absence of troponin elevation and ventricular blood clots, and non-African American ethnicity. Moreover, if the LVEF fails to surpass 50%, the likelihood of heart failure in subsequent pregnancies can notably escalate, reaching as high as 56%, with a mortality rate of 12%. Conversely, poor prognosis factors include prolonged QRS duration, delayed diagnosis, high New York Heart Association (NYHA) class, multiparity and African descent. Maternal complications may include blood clots, arrhythmias and worsening heart function, while fetal complications may involve oxygen deprivation [9, 20]. In the case report provided, mortality occurred as a result of cardiopulmonary arrest, which was precipitated by the progression of PPCM to acute heart failure and respiratory failure.
Patients with PPCM have been linked to thromboembolic consequences by a number of risk factors. Pregnancy-related hormonal changes make a patient more prone to thromboembolic events by causing a hypercoagulable condition that can last for several weeks after birth.
The hallmark of PPCM is left ventricular systolic dysfunction, which exacerbates blood stasis, intracardiac thrombi formation, and systemic embolism. These events can result in PE, venous thromboembolic events, cerebrovascular events, and other arterial embolic events [21].
Evidence on PPCM outcomes comes mostly from retrospective studies or small-scale prospective research, showing varied mortality rates. African American women often have poorer outcomes despite treatment, and subtle heart dysfunction may persist post-recovery [22].
When clinically required for any patient, both cardioversion and defibrillation should be performed as they are safe procedures during pregnancy. In non-emergent circumstances, fetal monitoring might be necessary to check for possible subsequent fetal arrhythmias. Due to the possibility of myocardial recovery, early implant of an implanted cardioverter defibrillator (ICD) is generally avoided, with most patients achieving a LVEF of 35% or greater within 6 months. In these situations, wearable cardioverter defibrillators (WCDs) are recommended by the European Society of Cardiology (ESC) and the American Heart Association (AHA) because they can act as a bridge to implanting an ICD in patients who do not experience myocardial recovery [13].
Due to the above, a collaborative approach involving various specialists is essential for optimal management. Also, given the association between PPCM and various chronic and obstetric conditions, enhanced surveillance during the postpartum period for high-risk patients is recommended. Strategies such as patient education, mobile health technology, community-based interventions, and telehealth could improve postpartum care [23].
Additionally, optimizing preconception risk factor management may reduce PPCM risk during pregnancy. Improvements within the acute setting of the described case could involve enhancing the timely recognition and management of PPCM through increased awareness among healthcare providers about its varied presentations and risk factors. Additionally, integrating multidisciplinary care involving obstetrics, cardiology, critical care, and neonatology could optimize the management of complex cases like PPCM, ensuring comprehensive evaluation and treatment of both maternal and fetal aspects. Incorporating advanced imaging modalities such as cardiac magnetic resonance imaging alongside echocardiography could provide more precise assessment of cardiac function, facilitating accurate diagnosis and guiding treatment decisions. These enhancements could lead to improved outcomes for both mothers and infants facing PPCM-related complications in the acute care setting [23].
Before being released from the hospital, all women with PPCM should have a conversation on contraception, with special emphasis from the obstetrician and cardiologist. In the early postpartum period, estrogen-containing contraceptives should be avoided as much as possible due to the risk of thrombosis in the presence of systolic dysfunction. Progesterone-releasing subcutaneous implants and the Mirena intrauterine device are safe and effective solutions. For those who do not wish to become pregnant in the future, tubal ligation and vasectomy are also available [13].
In terms of prevention, it is crucial to emphasize the significance of early detection and proactive measures aimed at minimizing the occurrence and severity of PPCM. This involves not only identifying at-risk individuals but also implementing strategies to mitigate potential genetic predispositions and other contributing factors. Genetic counseling and testing should be strongly considered, even in the absence of a known family history of cardiomyopathy, as certain genetic variants may predispose individuals to PPCM or related complications [24]. This is important as there are various genes implicated in disease progression and outcomes. Notably, the GNB3 gene is linked to worse prognoses in PPCM patients of African descent, while mutations in the BAG3 gene, responsible for encoding a molecular chaperone crucial for cellular stress responses, have also been identified in PPCM cases, shedding light on its role in disease pathogenesis. Additionally, sarcomeric genes such as MYBPC3, MYH6, MYH7, TNNC1, TNNT2, and TPM1, as well as X-linked genes like DMD and LAMP2, have been investigated in PPCM. Furthermore, genes such as DSP, PSEN2, VCL, HSPB7, FRMD4B, ADD2, and ZBTB17 have shown associations with PPCM or related conditions, suggesting potential shared genetic pathways [25]. Moreover, comprehensive antenatal care plays a pivotal role in early detection and management, with regular monitoring and screening helping identify any cardiac abnormalities or risk factors during pregnancy [23]. By integrating these preventive measures into clinical practice, healthcare providers can strive to reduce the incidence and impact of PPCM, ultimately improving outcomes for both mothers and their infants.
Learning points
The described case highlights the critical importance of rapid recognition and intervention in managing PPCM. Learning points include the necessity for heightened vigilance among healthcare providers regarding atypical presentations of PPCM, particularly in high-risk patients with predisposing factors such as previous cesarean sections and recurrent urinary tract infections. Additionally, the case underscores the significance of early initiation of comprehensive management strategies, including prompt administration of broad-spectrum antibiotics and aggressive fluid resuscitation, to stabilize hemodynamics and mitigate further cardiac decompensation. Decisions regarding the timing of delivery in PPCM should be individualized based on maternal and fetal factors, including the severity of cardiac dysfunction, gestational age, and overall clinical stability. While expedited delivery may be necessary in critical cases to improve maternal outcomes, it should be balanced with the risks of prematurity for the fetus. Furthermore, the case emphasizes the need for close collaboration between obstetricians and cardiologists to individualize decisions regarding the timing of delivery based on maternal and fetal considerations, balancing the urgency of maternal stabilization with the risks of prematurity. Continuous quality improvement efforts, including regular multidisciplinary team meetings and ongoing education for healthcare providers, are crucial for standardizing care practices and optimizing outcomes in cases of PPCM.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
DMP and CDS contributed to the conception and design of the case report. DMP, CDS, MOO and SAS conducted the literature research and review. ILR and LEP collected clinical data and patient information. ILR, CMG and MOO drafted the initial manuscript and prepared the case description. DJG, SAS, LMC and LEP contributed to the analysis and interpretation of the clinical data. ILR, LEP and SCS contributed to the final manuscript revisions and approval. DMP, LMC, and SCS supervised the project and provided final approval for publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
PPCM: peripartum cardiomyopathy; LVEF: left ventricular ejection fraction; MRI: magnetic resonance imaging; NYHA: New York Heart Association
| References | ▴Top |
- Kryczka KE, Demkow M, Dzielinska Z. Biomarkers in peripartum cardiomyopathy-what we know and what is still to be found. Biomolecules. 2024;14(1):103.
doi pubmed pmc - Safira A, Tjahjadi AK, Adytia GJ, Waitupu A, Sutanto H. Peripartum cardiomyopathy unveiled: Etiology, diagnosis, and therapeutic insights. Curr Probl Cardiol. 2024;49(5):102474.
doi pubmed - Ejim EC, Karaye KM, Antia S, Isiguzo GC, Njoku PO. Peripartum cardiomyopathy in low- and middle-income countries. Best Pract Res Clin Obstet Gynaecol. 2024;93:102476.
doi pubmed - Bala R, Mehta S, Roy VC, Kaur G, de Marvao A. Peripartum cardiomyopathy: A review. Rev Port Cardiol. 2023;42(11):917-924.
doi pubmed - Koziol KJ, Aronow WS. Peripartum cardiomyopathy: current understanding of pathophysiology, diagnostic workup, management, and outcomes. Curr Probl Cardiol. 2023;48(8):101716.
doi pubmed - Koczo A, Marino A, Polsinelli VB, Alharethi R, Damp J, Ewald G, Givertz MM, et al. Association of activin A and postpartum blood pressure in peripartum cardiomyopathy. Pregnancy Hypertens. 2023;34:60-66.
doi pubmed pmc - Laverde-Sabogal CE, Garnica-Rosas LM, Correa-Gonzalez N. Informe de caso sobre cardiomiopatia periparto: Rara, desconocida y potencialmente fatal. Revista Colombiana de Anestesiologia. 2016;44(1):63-68.
- Iorgoveanu C, Zaghloul A, Ashwath M. Peripartum cardiomyopathy: a review. Heart Fail Rev. 2021;26(6):1287-1296.
doi pubmed pmc - Peralta-Merelo S, Leon-Alonso K, Montero-Farias D, Mendoza-Moreira R, Escobar-Segovia K. Miocardiopatia periparto, una complicacion obstetrica: a proposito de un caso clinico - Peripartum cardiomyopathy, an obstetric complication: about a clinical case. Investigatio. 2023;20:63-73.
- Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287.
doi pubmed - Rojas-Arias JL, Porras CH, Munoz-Villa M, Acuna-Osorio EM, Vargas D, Diaz-Alfonso NJ, et al. Miocardiopatia periparto. Una rara pero peligrosa complicacion obstetrica. Acta med colomb. 2019;44(2).
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, Regitz-Zagrosek V, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767-778.
doi pubmed - Carlson S, Schultz J, Ramu B, Davis MB. Peripartum cardiomyopathy: risks diagnosis and management. J Multidiscip Healthc. 2023;16:1249-1258.
doi pubmed pmc - Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M, Iung B, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165-3241.
doi pubmed - Rodriguez Ziccardi M, Siddique MS. Peripartum cardiomyopathy. In: StatPearls. Treasure Island (FL). 2024.
pubmed - Soundararajan S, Ramachandran L, Parimala A, Rajalekshmi M. Peripartum cardiomyopathy: the camouflage of symptoms in pregnancy. Journal of South Asian Federation of Obstetrics and Gynaecology. 2023;15(1).
- Espinosa R, Vidal E, Jacinto B, Vazquez JP, Vazquez J, Karchmer S. Cardiomiopatia periparto. Levosimendan en la insuficiencia cardiaca aguda. Acta Medica Grupo Angeles. 2021;19(3).
- Baker A, Caetano F, Price S, Uddin S, Trimlett R, Arachchillage D. A case of peripartum cardiomyopathy supported by VA-ECMO. Journal of the Intensive Care Society. 2019;20(2).
- Azad H, Wen T, Bello NA, Booker WA, Purisch S, D'Alton ME, Friedman AM. Peripartum cardiomyopathy delivery hospitalization and postpartum readmission trends, risk factors, and outcomes. Pregnancy Hypertens. 2023;34:116-123.
doi pubmed - Agrawal A, Jain D, Ram P, Leon JLP, Rangaswami J. Anticoagulation for intra-cardiac thrombi in peripartum cardiomyopathy: A review of the literature. Rev Cardiovasc Med. 2019;20(2):53-58.
doi pubmed - Radakrishnan A, Dokko J, Pastena P, Kalogeropoulos AP. Thromboembolism in peripartum cardiomyopathy: a systematic review. J Thorac Dis. 2024;16(1):645-660.
doi pubmed pmc - Bauersachs J, Konig T, van der Meer P, Petrie MC, Hilfiker-Kleiner D, Mbakwem A, Hamdan R, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2019;21(7):827-843.
doi pubmed - Garnier Fernandez JC, Pizarro Alvarado G, Orozco Garcia R. Generalidades sobre cardiomiopatia periparto. Revista Medica Sinergia. 2021;6(7).
- Arany Z. Peripartum cardiomyopathy. N Engl J Med. 2024;390(2):154-164.
doi pubmed - Spracklen TF, Chakafana G, Schwartz PJ, Kotta MC, Shaboodien G, Ntusi NAB, Sliwa K. Genetics of peripartum cardiomyopathy: current knowledge, future directions and clinical implications. Genes (Basel). 2021;12(1).
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


