| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 8, August 2024, pages 159-166
A Case With Bilateral Hippocampal Infarction Resembling Transient Global Amnesia
Tetsuya Akaishia, c , Mami Asarib, Sumireko Sekiguchib, Tomoko Muroyab, Makoto Hasebeb
aDepartment of Education and Support for Regional Medicine, Tohoku University, Sendai, Japan
bDepartment of Neurology, Izumi Hospital, Sendai, Japan
cCorresponding Author: Tetsuya Akaishi, Department of Education and Support for Regional Medicine, Tohoku University, Aoba-ku, Sendai, 980-8574 Miyagi, Japan
Manuscript submitted May 4, 2024, accepted June 11, 2024, published online July 5, 2024
Short title: Bilateral Hippocampal Infarction Resembling TGA
doi: https://doi.org/10.14740/jmc4240
| Abstract | ▴Top |
Transient global amnesia (TGA) is a benign and transient condition with a sudden short-term amnesia. One of the conditions resembling TGA is hippocampal infarction, which requires relapse prevention treatments. In this report, we present a case with bilateral hippocampal infarction in whom distinguishing these two conditions was difficult for up to 1 week from the onset. A 60-year-old female visited our hospital with sudden onset retrograde and anterograde amnesia. Thin-slice magnetic resonance imaging (MRI) with 2-mm thickness revealed hyperintense signals on diffusion-weighted imaging (DWI) with signal loss on apparent diffusion coefficient (ADC) on both sides of the hippocampus. MRI with 5-mm thickness on day 7 revealed persistent restricted diffusion on both sides, one of which was still with decreased ADC values. Based on this finding, the diagnosis of bilateral hippocampal infarction was reached, and the relapse-preventive antiplatelet was continued. This case implied the potential difficulty of distinguishing cases with TGA and those with hippocampal infarction based on MRI findings within the first several days after onset. Thin-slice brain MRI, careful search of potential cardiovascular risks, and follow-up MRI ≥ 7 days after onset will be helpful to reach a correct diagnosis in cases with sudden amnesia.
Keywords: Apparent diffusion coefficient; Diffusion-weighted imaging; Hippocampal infarction; MRI; Transient global amnesia
| Introduction | ▴Top |
Transient global amnesia (TGA) is a benign transient amnesia with sudden onset, typically involving middle-aged individuals and usually requiring no relapse prevention [1-3]. Conditions that should be discriminated from this benign condition include hippocampal infarction, which may require antiplatelets to prevent future relapses [4]. Currently, the diagnosis of TGA largely depends on clinical findings, such as the diagnostic criteria by Hodges et al [2]. The exact diagnostic criteria to discriminate TGA from hippocampal infarction based on magnetic resonance imaging (MRI) findings remains uncertain [5, 6]. In ischemic neurological conditions with cellular damages based on compromised blood flow, including hippocampal infarction, signal loss on apparent diffusion coefficient (ADC) is an important MRI finding in addition to the diffusion-weighted imaging (DWI)-high signals to estimate perfusion status [7]. However, signal loss on ADC can also be seen in cases of TGA [8-10]. A promising MRI finding to discriminate hippocampal infarction from TGA is the involvement of posterior cerebral artery (PCA) territories other than hippocampal lateral portion corresponding to the CA1 region [11]. Meanwhile, when such extra-hippocampal lesions are absent, discriminating these two conditions are often difficult. Moreover, the detection of hippocampal lesions on MRI with 5-mm-thick slices is often difficult, as the size of hippocampal lesions are often smaller than 5 mm [12]. Consequently, some cases with sudden amnesia must be followed up for more than 1 week to reach a correct diagnosis. In this report, we present a patient initially suspected of TGA based on brain MRI findings on hospital admission, but later diagnosed with bilateral hippocampal infarction based on MRI findings 1 week later.
| Case Report | ▴Top |
Investigations
A 60-year-old female with the past medical histories of hypertension and dyslipidemia, treated with pitavastatin, visited our hospital at 6:00 pm with sudden onset amnesia started from 1:00 pm on the same day, which was noticed by her colleague at the workplace. Her colleagues reported that she was normally working before the lunch break, but she suddenly started to repeat that “what am I doing here?” and “how did I get to this place today?” at approximately 1:00 pm. She was also reported to have forgotten whether she had finished lunch. She had no previous history of alcohol or cocaine use disorder. The day was in the midsummer season and the highest air temperature of the day before the onset was higher than 35 °C in the area, when she worked outside all day to guide visitors from other areas (day -1). Upon the initial neurological examination on arrival at hospital, her consciousness level was almost clear, and she showed no neurological deficits. Her memorization ability appeared to have already returned to normal on admission at 6:00 pm, but the memory from approximately 8:00 am of the same day to 5:00 pm was kept lost, suggesting the presence of both retrograde and anterograde amnesia. She also temporarily noticed tingling sensations in the periphery of four limbs before arriving at the hospital. Vital signs on arrival were normal. The blood test suggested the presence of mild dehydration that may predispose the risk of ischemic stroke, with the blood urea nitrogen (BUN) level of 10.8 mg/dL and the serum creatinine level of 0.50 mg/dL, yielding a BUN/creatinine ratio greater than 20 [13, 14]. Serum N-terminal pro-brain natriuretic peptide (NT-proBNP) level was slightly elevated with 157 pg/mL.
Diagnosis and treatment
In the day of the onset, multiple old ischemic changes were confirmed in both hemispheres, but no DWI-high signals were confirmed with 5-mm-thick MRI imaging (Fig. 1), obtained using a Siemens MAGNETOM Symphony 1.5 Tesla MRI system. The magnetic resonance (MR) angiography revealed no abnormalities, such as reversible cerebral vasoconstriction syndrome, with patent PCAs on both sides [15, 16]. An additionally performed 2-mm thin-slice MRI (day 1) revealed bilateral hyperintense DWI signals with signal losses on ADC in the hippocampal lateral portions corresponding to CA1 areas on both side (Fig. 2), indicating the presence of cellular damages and the potential diagnosis of bilateral hippocampal infarction. At this point, both TGA and bilateral hippocampal infarction were possible, and intravenous argatroban for 7 days (60 mg/day for 2 days, followed by 20 mg/day for 5 days) was decided to be temporarily started.
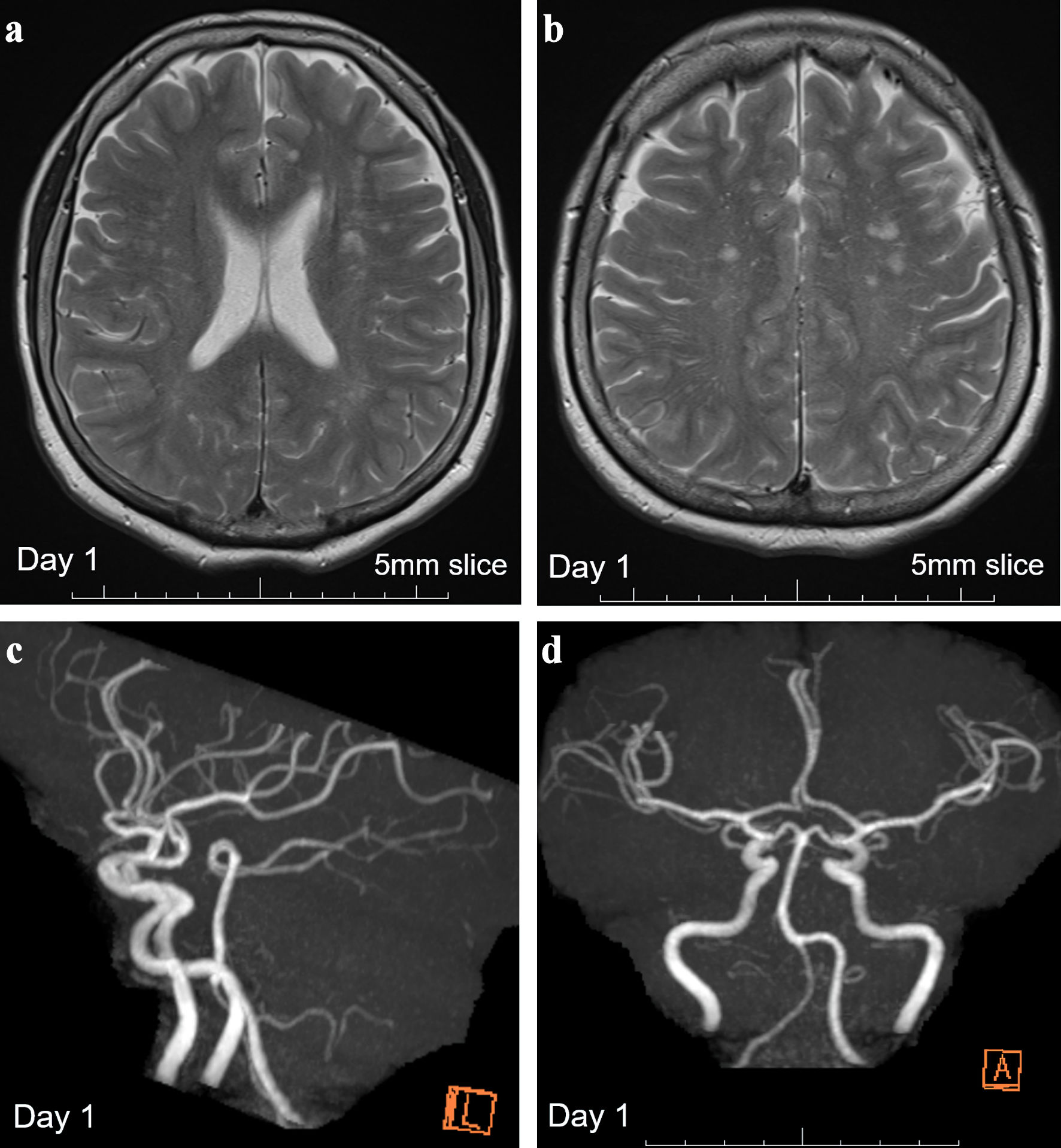 Click for large image | Figure 1. Brain MRI showing multiple old ischemic changes and MR angiography on day 1. (a, b) T2-weighted images with 5-mm-thick brain MRI on day 1 revealed multiple old ischemic changes in both hemispheres. (c, d) Normal MR angiography findings with a patent posterior circulation system. MRI: magnetic resonance imaging; MR: magnetic resonance. |
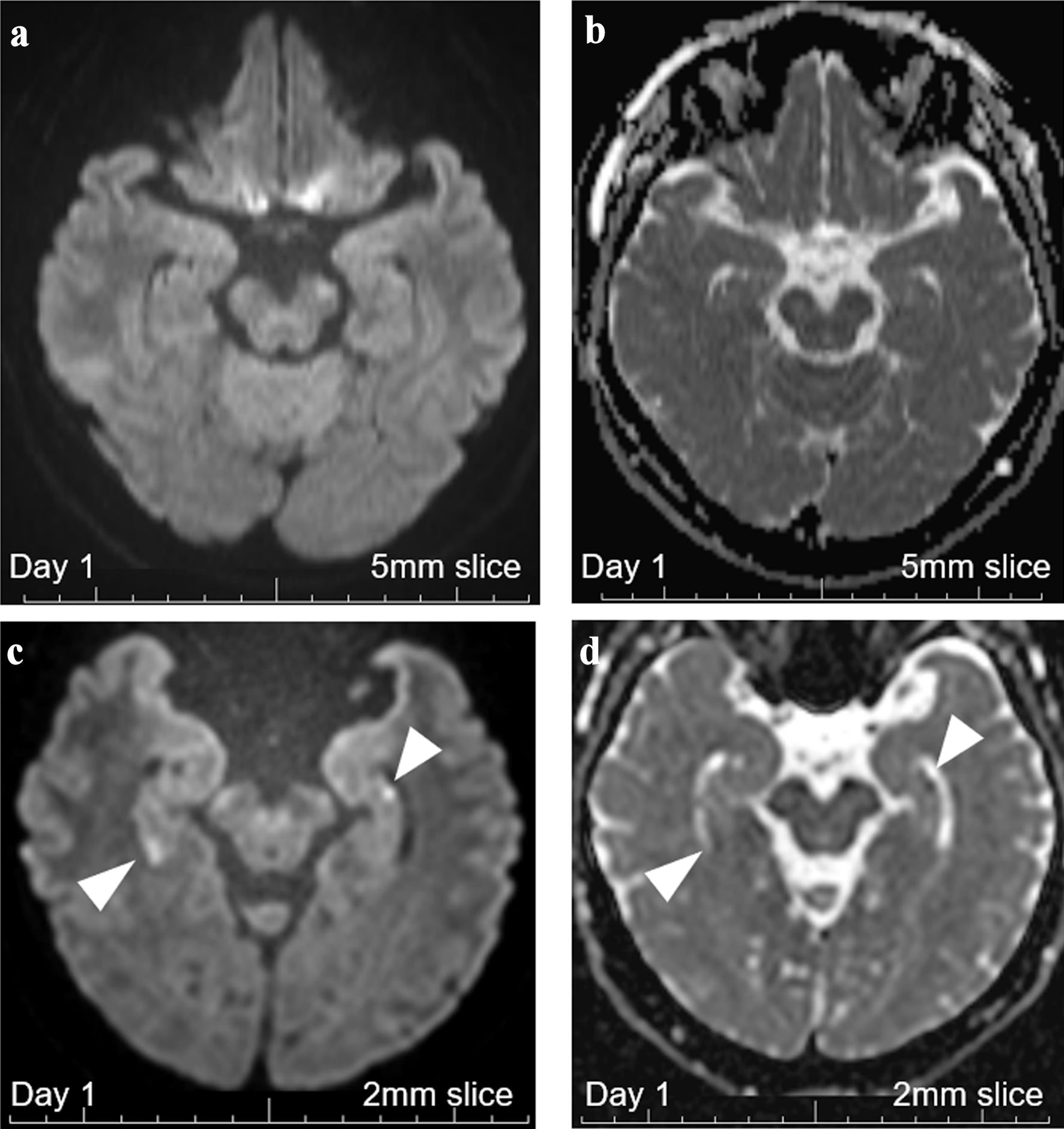 Click for large image | Figure 2. Brain MRI with 5-mm thickness and 2-mm thin-slice on day 1. (a) DWI with 5-mm-thick brain MRI on day 1 revealed no abnormalities. (b) ADC with 5-mm-thick brain MRI on day 1 revealed no abnormalities. (c) DWI with 2-mm thin-slice brain MRI on day 1 revealed two hippocampal signal high lesions each on both sides (white arrowheads). (d) ADC with 2-mm thin-slice brain MRI on day 1 revealed signal losses for the two DWI-high hippocampal lesions (white arrowheads). MRI: magnetic resonance imaging; ADC: apparent diffusion coefficient; DWI: diffusion-weighted imaging. |
On day 2, her lost memories of the previous day between 8:00 am and 5:00 pm were kept lost, but she fully remembered the episodes after arriving at the hospital. She had a reduction in appetite and could not eat solid food at breakfast. A 2-mm thin-slice MRI was performed again. This time, the signal loss on ADC became more conspicuous (Fig. 3). Additional extra-hippocampal lesions were still absent. Carotid ultrasonography and cardiovascular MRI, covering the aortic arch level, identified no apparent thromboembolic risks like unstable plaques. Even at this stage, both possibilities of TGA and bilateral hippocampal infarction still remained.
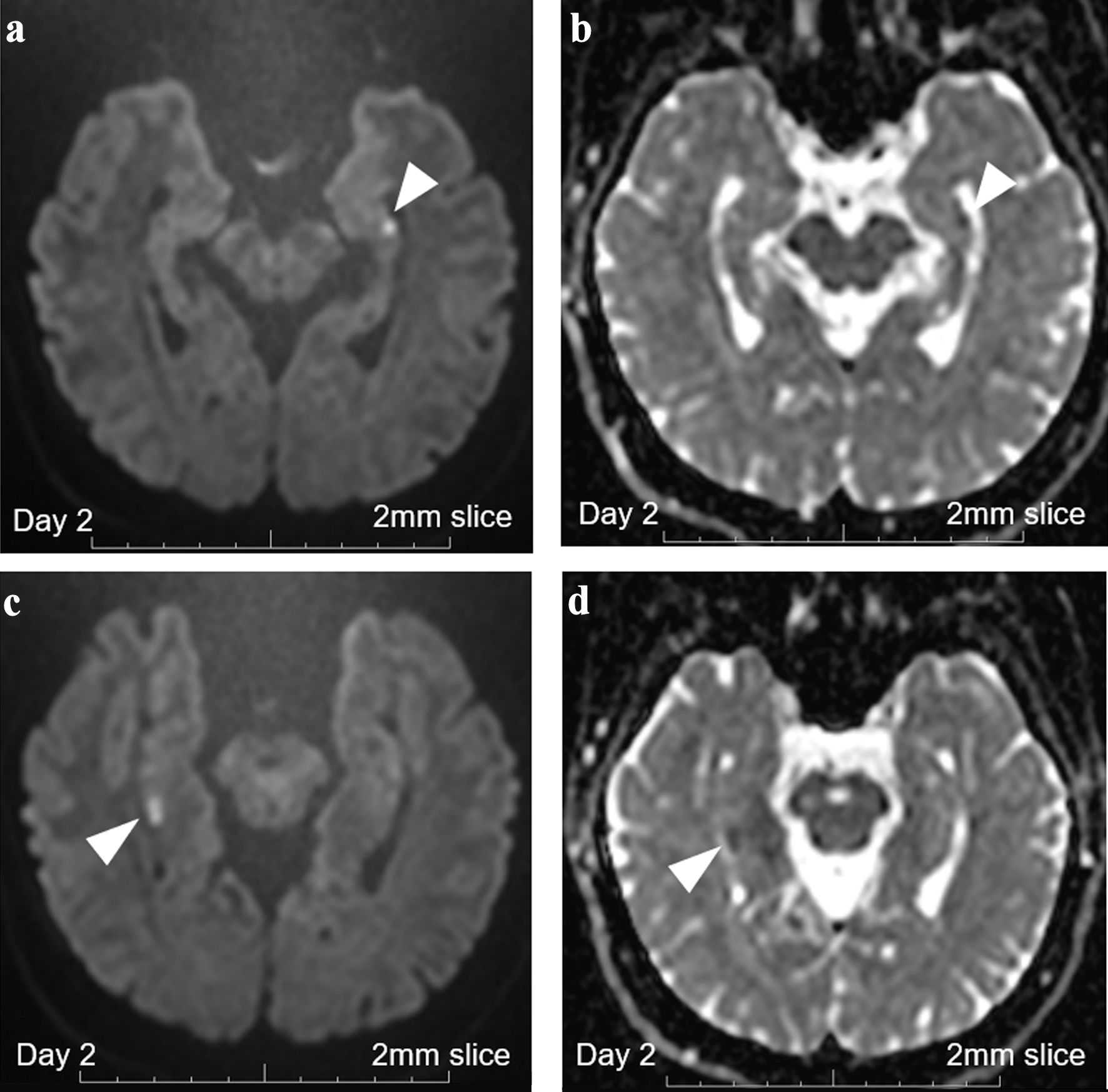 Click for large image | Figure 3. Brain MRI with 2-mm thin-slice on day 2. (a) DWI with 2-mm thin-slice brain MRI on day 2 revealed persistent punctate hyperintense DWI signals in the left hippocampus (white arrowhead). (b) ADC with 2-mm thin-slice brain MRI on day 2 revealed persistent punctate signal loss in the left hippocampus (white arrowhead). (c) DWI with 2-mm thin-slice brain MRI on day 2 revealed persistent high signals in the right hippocampus (white arrowhead). (d) ADC with 2-mm thin-slice brain MRI on day 2 revealed persistent signal loss in the right hippocampus (white arrowhead). MRI: magnetic resonance imaging; ADC: apparent diffusion coefficient; DWI: diffusion-weighted imaging. |
On day 7, a 5-mm-thick MRI revealed persistent hyperintense DWI signals on both hippocampi, one of which was still with a signal loss on ADC (Fig. 4). These findings of persistent MRI abnormalities even after 3 - 6 days after the onset indicated the diagnosis of bilateral hippocampal infarction. Based on these findings, antiplatelet therapy was decided to be continued, and dual antiplatelet therapy (DAPT) with aspirin 100 mg/day and clopidogrel 75 mg/day was started. Other additional diagnostic examinations including the 24-h Holter monitoring and echocardiogram revealed no suspectable thrombotic risks. The Wechsler Memory Scale-Revised test on day 12 revealed an impaired verbal short-term memory (Table 1). The patient did not present with nausea or vomiting during the hospitalization. She left the hospital on day 15, and DAPT was switched to single antiplatelet therapy by aspirin 100 mg/day. Her lost memory between 8:00 am and 5:00 pm on the day of onset was not recovered at last. She has had no relapses with a single oral antiplatelet as of 6 months from the amnestic event.
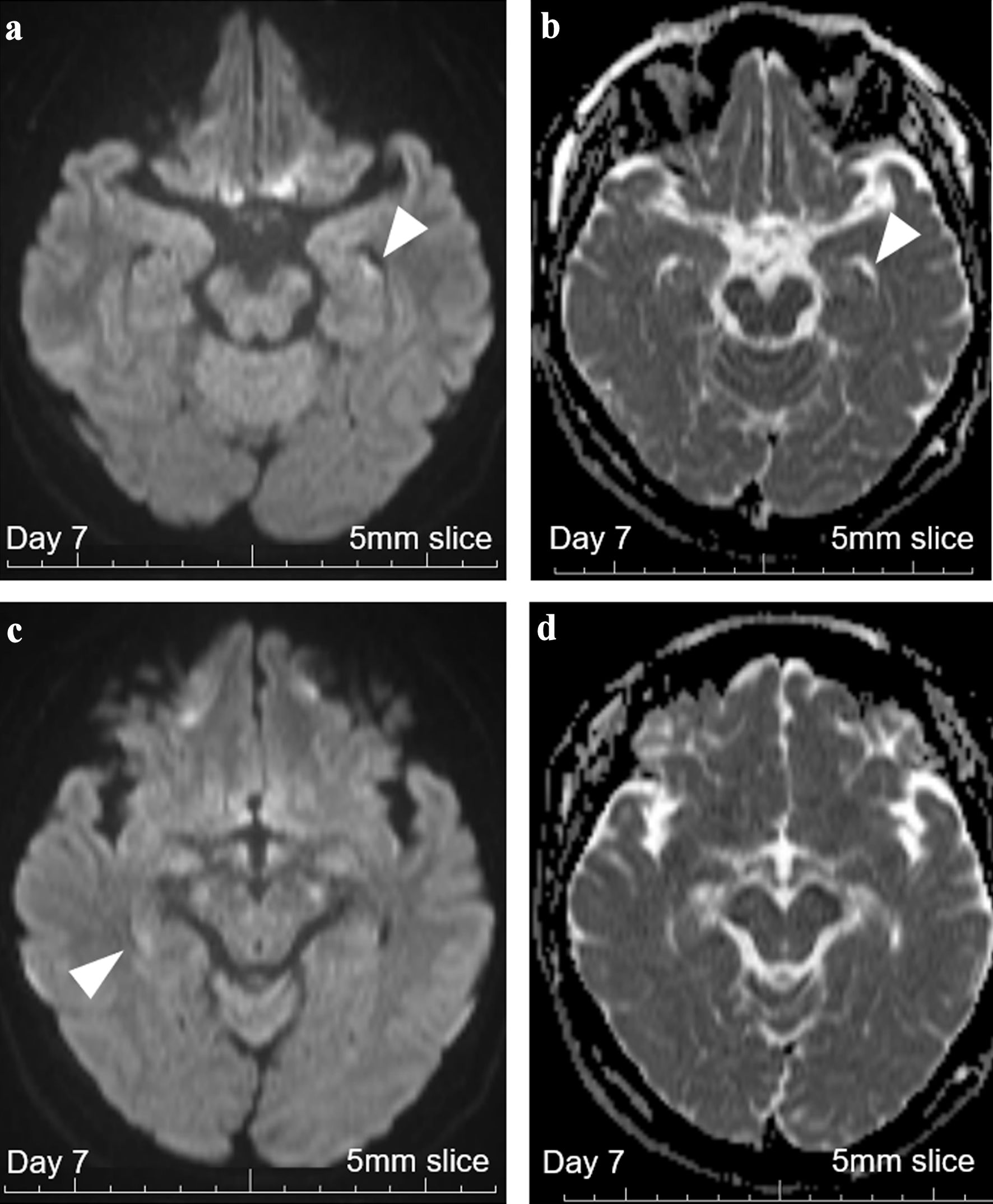 Click for large image | Figure 4. Brain MRI with 5-mm thickness on day 7. (a) DWI with 5-mm-thick brain MRI on day 7 revealed persistent high signals in the left hippocampus (white arrowhead), increasing the probability of hippocampal infarction. (b) ADC with 5-mm-thick brain MRI on day 7 revealed persistent signal loss in the left hippocampus (white arrowhead). (c) DWI with 5-mm-thick brain MRI on day 7 revealed persistent vague high signals in the right hippocampus (white arrowhead). (d) ADC with 5-mm-thick brain MRI on day 7 revealed no apparent signal loss, suggesting that cellular damages were milder on this side. MRI: magnetic resonance imaging; ADC: apparent diffusion coefficient; DWI: diffusion-weighted imaging. |
 Click to view | Table 1. Results of the Wechsler Memory Scale-Revised Test (WMS-R) on Day 12 |
| Discussion | ▴Top |
In this case report, a middle-aged woman with sudden amnesia was reported, for whom making the correct diagnosis based on clinical and MRI findings in the first several days was difficult. Her clinical symptom and neuroimaging findings in the first week from the onset were compatible with those in TGA. Because the possible diagnosis of bilateral hippocampal infarction could not be ruled out, antiplatelet therapy was started. Based on the persistent diffusion restrictions with decreased ADC values on day 7, we made the final diagnosis of bilateral hippocampal infarction, and antiplatelet therapy was decided to be continued. Persistent deficits of verbal memory on day 12 further supported the diagnosis of hippocampal infarction rather than TGA [17-21]. This case report emphasizes the importance of careful medical history taking and diagnostic approaches for the individuals with sudden transient amnesia.
As the same in cases with hippocampal infarction, hyperintense DWI signals can be found in patients with TGA, especially when a thin-slice MRI is performed. Furthermore, utilizing a 7 Tesla MRI can realize a higher detection rate of DWI-high lesions in TGA, reportedly with the detection rate of 80-90% [22]. The detection rate of hyperintense DWI signals on MRI in TGA is thought to be the highest after 12 - 48 h from the onset [23]. Meanwhile, it is unestablished whether a signal loss on ADC is also an indicative finding of TGA. Many previous reports utilized the findings of signal loss on ADC in making the diagnosis of TGA [8, 10, 24, 25]. Therefore, signal loss on ADC cannot be a rationale to rule out TGA. Additional MRI abnormalities outside the hippocampal CA1 region in the PCA territories would indicate the diagnosis of hippocampal infarction.
However, in the absence of such additional extra-hippocampal lesions, like the present case, making the diagnosis based on the MRI findings is difficult. Because the CA1 region is susceptible to ischemia [12], ischemic lesions restricted to hippocampi may be possible upon dehydration and other hemodynamic changes with increased blood viscosity [26]. The number, size, or bilaterality of the hyperintense DWI signals in CA1 regions would not be determinant information for distinguishing TGA and hippocampal infarction. As the MRI abnormalities in TGA may persist up to 5 - 6 days from onset [23, 27], careful follow-up of the subsequent clinical course and MRI abnormalities for 1 week or longer are needed for making a correct diagnosis. If the MRI abnormalities persist even after 1 week from the onset, the possibility of hippocampal infarction becomes higher. If all MRI abnormalities (i.e., DWI, ADC, T2, and fluid attenuated inversion recovery (FLAIR)) vanish in the first week after the onset, the diagnosis of TGA will become more likely and the relapse-prevention antiplatelets may be unnecessary. Further studies are needed to establish MRI criteria to longitudinally distinguish the cases with punctate hippocampal infarctions and those with TGA presenting hippocampal diffusion restrictions.
Amnestic symptoms can be caused by any cerebral lesions in the areas involved in memory functions, such as cingulate gyrus and mammillary bodies comprising the Papez circuit. Previous studies reported the development of amnesia in cases with lesions in cingulate gyrus, mammillary bodies, and fornix [28-31]. Careful evaluation of these cerebral regions associated with memory functions is recommended in amnestic patients, because a small punctate lesion in any of these cerebral components including the limbic system structures may result in amnestic symptoms resembling TGA [30].
As a limitation of this case report, electroencephalogram (EEG) was not performed for this case. As an epileptic condition resembling TGA, transient epileptic amnesia (TEA) is sometimes seen typically in middle-aged and older people [32]. TEA is considered to be a rare subtype of temporal lobe epilepsy with amnesic seizure. To diagnose the condition, detection of ictal EEG abnormalities is a key finding [33]. However, the present case has not relapsed after the initial amnesic episode, and the diagnosis of TEA seems to be less likely for this case. Another limitation of this case report was that the existence of patent foramen ovale (PFO) has not been investigated. To detect the PFO, transthoracic echocardiography with bubble contrast is among the standard diagnostic approaches [34]. As the presence of PFO is one of the causes of paradoxical cerebral embolism, cases with unknown thrombotic origin are better to be investigated for the presence of PFO not to overlook thrombotic backgrounds including deep venous thrombosis [35].
Conclusions
A case with bilateral hippocampal infarction was presented, whose clinical symptom and MRI findings on admission were totally compatible with those with TGA. In some cases, with sudden amnesia, distinguishing these two conditions within the first several days is difficult, as DWI- and ADC-abnormalities can be found in both conditions within this time window. If the possibility of hippocampal infarction cannot be confidently ruled out in the first several days after onset, relapse-preventive antiplatelet therapy may be temporarily started unless contraindicated. A follow-up MRI after 7 days from onset will aid to determine the diagnosis and decide whether to continue the antiplatelet therapy. Persistent MRI abnormalities after this time period may indicate ischemic lesions, and relapse preventions may be required.
Learning points
Although TGA is a benign condition with no required relapse preventions, all cases with sudden onset amnesia are better to be evaluated with brain MRI on day 1, day 2, and after day 7. Only reassuring the patients that they are possibly with TGA, and no therapeutic intervention are needed, based only on the episode without follow-up MRI, may be insufficient. This is because the differential diagnosis of hippocampal infarction and other resembling conditions that require specific treatments cannot be confidently ruled out within the first several days after the onset in some cases.
In addition to the usual 5-mm- or 6-mm-thick brain MRI, a thin-slice hippocampal MRI with 2-mm or 3-mm thickness is better to be performed to correctly estimate the presence and extent of cellular damages.
If hippocampal infarction cannot be ruled out because of the presence of diffusion restriction with decreased ADC values, antiplatelet therapy may be temporarily started unless contraindicated. A follow-up MRI after day 7 after the onset will aid to determine the diagnosis and decide whether to continue the antiplatelet therapy or not.
Acknowledgments
None to declare.
Financial Disclosure
The authors did not receive specific aid from any funding agencies.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Written informed consent was obtained from the patient.
Author Contributions
All authors participated in patient care. TA drafted the manuscript. MA, SS, TM, and MH contributed to reviewing and revising the manuscript.
Data Availability
All data were obtained during the patient’s hospitalization. Any inquiries regarding the additional information should be directed to the corresponding author.
Abbreviations
ADC: apparent diffusion coefficient; DWI: diffusion-weighted imaging; PCA: posterior cerebral artery; TGA: transient global amnesia
| References | ▴Top |
- Logan W, Sherman DG. Transient global amnesia. Stroke. 1983;14(6):1005-1007.
doi pubmed - Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843.
doi pubmed pmc - Owen D, Paranandi B, Sivakumar R, Seevaratnam M. Classical diseases revisited: transient global amnesia. Postgrad Med J. 2007;83(978):236-239.
doi pubmed pmc - Marinkovic I, Lyytinen J, Valanne L, Niinikuru R, Pekkonen E. Bilateral hippocampal infarction as etiology of sudden and prolonged memory loss. Case Rep Neurol. 2012;4(3):207-211.
doi pubmed pmc - Naldi F, Baiardi S, Guarino M, Spinardi L, Cirignotta F, Stracciari A. Posterior hippocampal stroke presenting with transient global amnesia. Neurocase. 2017;23(1):22-25.
doi pubmed - Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208.
doi pubmed - Lin W, Lee JM, Lee YZ, Vo KD, Pilgram T, Hsu CY. Temporal relationship between apparent diffusion coefficient and absolute measurements of cerebral blood flow in acute stroke patients. Stroke. 2003;34(1):64-70.
doi pubmed - Wilkinson T, Geranmayeh F, Dassan P, Janssen JC. Neuroimaging in transient global amnesia. Pract Neurol. 2013;13(1):56-57.
doi pubmed - Jan K, Chuin S. A case of recurrent transient global amnesia: don't forget the hippocampal punctuate diffusion restriction. Oxf Med Case Reports. 2018;2018(6):omy025.
doi pubmed pmc - Vella S, Grech R. Highlighting the classical MRI findings in transient global amnesia. BJR Case Rep. 2020;6(2):20190111.
doi pubmed pmc - Lee HY, Kim JH, Weon YC, Lee JS, Kim SY, Youn SW, Kim SH. Diffusion-weighted imaging in transient global amnesia exposes the CA1 region of the hippocampus. Neuroradiology. 2007;49(6):481-487.
doi pubmed - Yang Y, Kim S, Kim JH. Ischemic evidence of transient global amnesia: location of the lesion in the hippocampus. J Clin Neurol. 2008;4(2):59-66.
doi pubmed pmc - Lin LC, Lee JD, Hung YC, Chang CH, Yang JT. Bun/creatinine ratio-based hydration for preventing stroke-in-evolution after acute ischemic stroke. Am J Emerg Med. 2014;32(7):709-712.
doi pubmed - Riccardi A, Chiarbonello B, Minuto P, Guiddo G, Corti L, Lerza R. Identification of the hydration state in emergency patients: correlation between caval index and BUN/creatinine ratio. Eur Rev Med Pharmacol Sci. 2013;17(13):1800-1803.
pubmed - Boitet R, de Gaalon S, Duflos C, Marin G, Mawet J, Burcin C, Roos C, et al. Long-term outcomes after reversible cerebral vasoconstriction syndrome. Stroke. 2020;51(2):670-673.
doi pubmed - Chandra R, Saini HS, Palmer KN, Cerejo R. The link between reversible cerebral vasoconstriction syndrome and transient global amnesia. Headache. 2023;63(1):168-172.
doi pubmed - Uttner I, Weber S, Freund W, Schmitz B, Ramspott M, Huber R. Transient global amnesia—full recovery without persistent cognitive impairment. Eur Neurol. 2007;58(3):146-151.
doi pubmed - Carota A, Lysandropoulos AP, Calabrese P. Pure left hippocampal stroke: a transient global amnesia-plus syndrome. J Neurol. 2012;259(5):989-992.
doi pubmed - Kumral E, Deveci EE, Erdogan C, Enustun C. Isolated hippocampal infarcts: Vascular and neuropsychological findings. J Neurol Sci. 2015;356(1-2):83-89.
doi pubmed - Benke T, Bodner T, Wiesen D, Karnath HO. The amnestic syndrome of posterior cerebral artery infarction. Eur J Neurol. 2022;29(10):2987-2995.
doi pubmed pmc - Jager T, Bazner H, Kliegel M, Szabo K, Hennerici MG. The transience and nature of cognitive impairments in transient global amnesia: a meta-analysis. J Clin Exp Neuropsychol. 2009;31(1):8-19.
doi pubmed - Unsgard RG, Doan TP, Nordlid KK, Kvistad KA, Goa PE, Berntsen EM. Transient global amnesia: 7 Tesla MRI reveals more hippocampal lesions with diffusion restriction compared to 1.5 and 3 Tesla MRI. Neuroradiology. 2022;64(12):2217-2226.
doi pubmed pmc - Szabo K, Hoyer C, Caplan LR, Grassl R, Griebe M, Ebert A, Platten M, et al. Diffusion-weighted MRI in transient global amnesia and its diagnostic implications. Neurology. 2020;95(2):e206-e212.
doi pubmed - Choi BS, Kim JH, Jung C, Kim SY. High-resolution diffusion-weighted imaging increases lesion detectability in patients with transient global amnesia. AJNR Am J Neuroradiol. 2012;33(9):1771-1774.
doi pubmed pmc - Menezes RB, Cavalcante ERC, Maia FM, Frota NA. Anterior portion of the cingulate gyrus: A novel location for transient global amnesia? Dement Neuropsychol. 2014;8(1):90-92.
doi pubmed pmc - Yang M, Yoo H, Kim SY, Kwon O, Nam MW, Pan KH, Kang MY. Occupational risk factors for stroke: a comprehensive review. J Stroke. 2023;25(3):327-337.
doi pubmed pmc - Ropper AH. Transient global amnesia. N Engl J Med. 2023;388(7):635-640.
doi pubmed - Tanaka Y, Miyazawa Y, Akaoka F, Yamada T. Amnesia following damage to the mammillary bodies. Neurology. 1997;48(1):160-165.
doi pubmed - Dillingham CM, Milczarek MM, Perry JC, Vann SD. Time to put the mammillothalamic pathway into context. Neurosci Biobehav Rev. 2021;121:60-74.
doi pubmed pmc - Gallardo-Tur A, Romero-Godoy J, de la Cruz Cosme C, Arboix A. Transient global amnesia associated with an acute infarction at the cingulate gyrus. Case Rep Neurol Med. 2014;2014:418180.
doi pubmed pmc - Mazzacane F, Ferrari F, Malvaso A, Mottese Y, Gastaldi M, Costa A, Pichiecchio A, et al. Acute amnestic syndrome in fornix lesions: a systematic review of reported cases with a focus on differential diagnosis. Front Neurol. 2024;15:1338291.
doi pubmed pmc - Bilo L, Meo R, Ruosi P, de Leva MF, Striano S. Transient epileptic amnesia: an emerging late-onset epileptic syndrome. Epilepsia. 2009;50(Suppl 5):58-61.
doi pubmed - Cho S, Lee WW, Kang K, Park JM, Kim BK, Kwon O, Lee JJ. Transient epileptic amnesia with preserved consciousness: a report of two cases. J Epilepsy Res. 2017;7(1):54-56.
doi pubmed pmc - Gonnah AR, Bharadwaj MS, Nassar H, Abdelaziz HK, Roberts DH. Patent foramen ovale: diagnostic evaluation and the role of device closure. Clin Med (Lond). 2022;22(5):441-448.
doi pubmed pmc - Banana Y, Rezziki A, Kallel O, Rasras H, Bazid Z, El Ouafi N, El Mahi O, et al. Multiple paradoxical embolisms revealing a patent foramen ovale in a patient with deep venous thrombosis: A case report. Ann Med Surg (Lond). 2021;66:102426.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


