| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 11, November 2024, pages 311-318
Pulmonary Kaposi Sarcoma in the Era of Antiretroviral Therapy: A Case Series
Michael Alexander Pelidisa, Lefika Bathobakaea, c, Arielle Aikena, Katrina Villegasa, Malina Mohtadia, Abraam Rezkallaa, Nargis Mateena, Hussein Mhannab, Medhat Ismailb, Patrick Michaela
aInternal Medicine, St. Joseph’s University Medical Center, Paterson, NJ, USA
bPulmonary & Critical Care Medicine, St. Joseph’s University Medical Center, Paterson, NJ, USA
cCorresponding Author: Lefika Bathobakae, Internal Medicine, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
Manuscript submitted May 16, 2024, accepted September 4, 2024, published online October 10, 2024
Short title: Pulmonary Kaposi Sarcoma in the Era of ART
doi: https://doi.org/10.14740/jmc4251
| Abstract | ▴Top |
Kaposi sarcoma (KS) is an angioproliferative neoplasm that affects the skin and lymph nodes. Human herpesvirus-8 (HHV-8) triggers KS by infecting the endothelium and inducing angiogenesis through the production of vascular endothelial growth factors and cytokines. KS is characterized by purplish or hyperpigmented plaques involving the skin and mucous membranes, and visceral involvement is very rare. Pulmonary KS (PKS) is an exceedingly rare visceral manifestation of KS and has a poor prognosis. PKS often presents with cough, hemoptysis, pleuritic chest pain, fever, and weight loss. In this case series, we share our experience in diagnosing and treating two patients with PKS. We also provide a concise review of the epidemiology, signs and symptoms, diagnosis, and management of this rare condition.
Keywords: Kaposi sarcoma; Pulmonary Kaposi sarcoma; Metastatic Kaposi sarcoma; HHV-8; AIDS; AIDS-defining illness; Antiretroviral therapy
| Introduction | ▴Top |
Kaposi sarcoma (KS) is a malignant angioproliferative mesenchymal tumor caused by human herpesvirus-8 (HHV-8) [1, 2]. HHV-8 targets spindle cells in the endothelium and causes angiogenesis through the production of vascular endothelial growth factor (VEGF) molecules, pro-inflammatory cytokines, and growth factors [2]. KS is recognized by violaceous or hyperpigmented plaques involving the skin, mucous membranes, gastrointestinal tract, and lungs [2-4]. The four types of KS include classic, endemic, iatrogenic, and epidemic or acquired immune deficiency syndrome (AIDS)-related KS [2, 5]. Epidemic KS is an AIDS-defining illness with a higher burden seen in homosexual men [2, 4]. First trialed in 1996 at the peak of the AIDS pandemic, antiretroviral therapy (ART) has reduced the incidence and burden of KS in human immunodeficiency virus (HIV)-infected patients [6, 7]. Sporadic cases continue to be reported in patients defaulting on therapy or engaging in risky behaviors.
In the era of ART, pulmonary KS (PKS) is exceedingly rare, and patients often present with fever, dyspnea, cough, wheezing, hemoptysis, or chest pain [1, 4]. PKS manifests as reticular opacities and unilateral pleural effusions on chest radiographs; however, bronchoscopy with biopsy is essential for a definitive diagnosis and excluding non-tuberculous mycobacterial infections or non-cystic fibrosis bronchiectasis [4]. PKS is treated with ART coupled with systemic chemotherapy. The National Comprehensive Cancer Network (NCCN) guidelines recommend pegylated liposomal doxorubicin and paclitaxel as first-line agents in patients seropositive for HIV [2, 8]. Herein, we describe a case series of two male patients with a history of AIDS who presented to our emergency room with cough and dyspnea. Both patients were found to have PKS and were discharged on ART after medical optimization. One patient started chemotherapy, while the other patient was lost to follow-up.
| Case Reports | ▴Top |
Case 1
A 33-year-old male with a history of tobacco and amphetamine use presented to the emergency department (ED) complaining of shortness of breath for 1 month. Dyspnea progressively worsened, and was associated with paroxysmal nocturnal dyspnea and bilateral leg swelling. The patient also reported a 30 pounds weight loss and a productive cough over the same duration, but denied night sweats, hemoptysis, chest pain, palpitations, nausea, vomiting, fever, chills, or recent international travel. The patient denied a family history of heart failure, sudden cardiac death, ischemic heart disease, or malignant arrhythmia. The patient participated in anoreceptive sexual intercourse with male partners but reported inconsistent use of barrier contraception.
In the ED, the patient experienced respiratory distress and spoke in short sentences. He was tachypneic and hypertensive but afebrile. His oxygen saturation was 85% on room air, prompting the initiation of noninvasive positive-pressure ventilation. On further examination, the patient had bilateral lung crackles, diminished breath sounds on the right lung, grade +2 peripheral pitting edema, and scattered purplish lesions in the abdomen and flanks. There was no evidence of jugular vein distension, bruits, murmurs, wheezing, stridor, or stertor. An arterial blood gas analysis showed chronic primary respiratory acidosis with appropriately compensated metabolic alkalosis. Electrocardiogram (ECG) showed normal sinus rhythm with no acute ischemic changes or segment prolongation. A chest X-ray demonstrated bilateral infiltrates and a large right-sided pleural effusion, causing the collapse of the right lung (Fig. 1). The patient was administered 40 mg of furosemide intravenously (IV), and eventually started on nitroglycerine infusion to reduce the fluid overload.
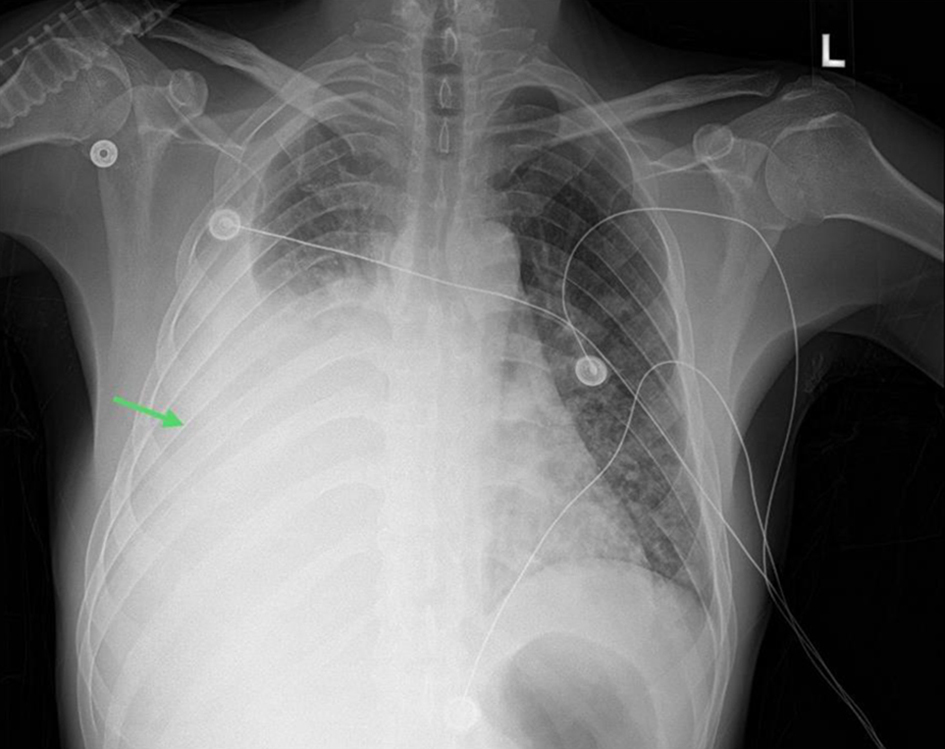 Click for large image | Figure 1. Frontal view chest X-ray showing large right-sided pleural effusion with adjacent atelectasis (green arrow). |
A battery of tests was ordered due to concerns for acute-onset heart failure versus pulmonary embolism, and malignancy. Triage blood test results revealed leukopenia, anemia, hyponatremia, and elevated D-dimer levels (Table 1). B-type natriuretic peptide (BNP) test, troponin level, viral respiratory panel, pneumonia panel, procalcitonin, lactic acid, urinalysis, sexually transmitted disease panel, and urine drug screening tests were unremarkable. Computed tomography (CT) angiography of the chest showed a large right-sided pleural effusion, bilateral lung infiltrates, and para-aortic lymph nodes in the upper abdomen and ruled out pulmonary embolism (Fig. 2). On re-evaluation, the patient was noted to be borderline hypotensive, and the nitroglycerine drip was paused. A right-sided chest tube insertion drained 1 L of sanguineous fluid, resulting in some clinical improvement. The patient was admitted to a medical ward with airborne precautions for continued care. Pleural fluid analysis revealed a lymphocytic predominance of exudative fluid, with negative cultures and cytology (Table 2).
 Click to view | Table 1. Pertinent Laboratory Results for Cases 1 and 2 |
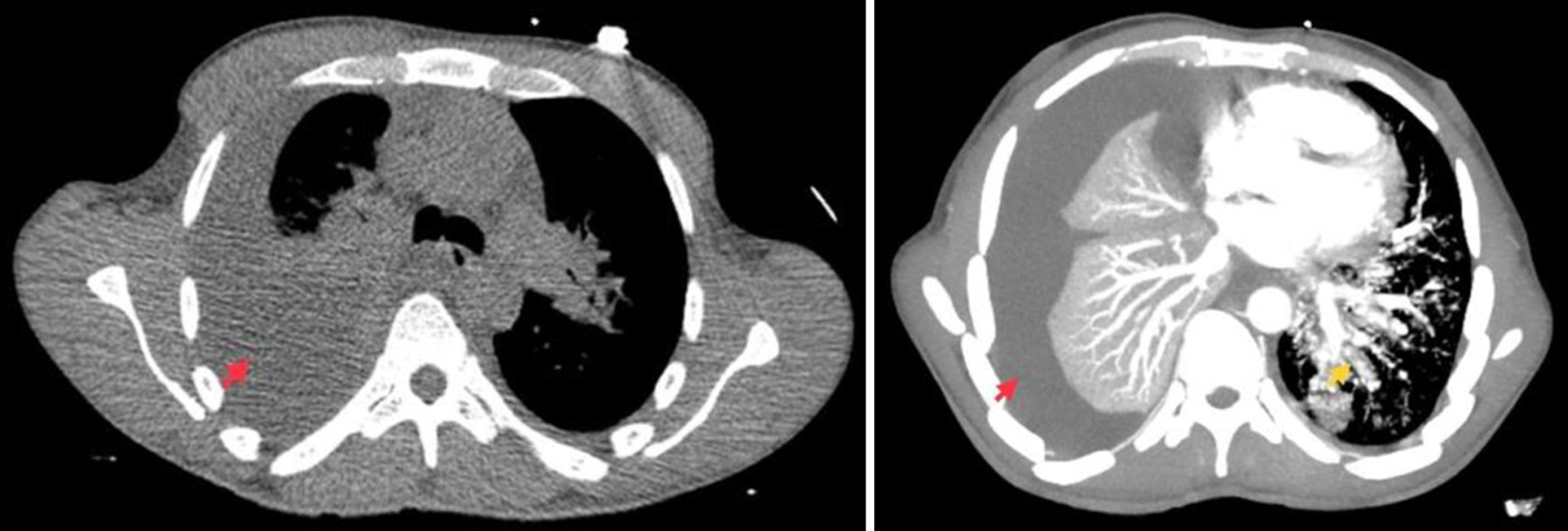 Click for large image | Figure 2. Computed tomography angiography of the chest showing a large right-sided pleural effusion (red arrows) and multifocal lung infiltrates (yellow arrow). |
 Click to view | Table 2. Results for Pleural Fluid Analysis for Cases 1 and 2 |
The fourth-generation combination test for HIV was reactive, with an RNA viral load of 134,000 copies/mL. The CD4 count was 93 cells/mm3, which confirmed a new diagnosis of HIV-AIDS. Pulmonary and infectious disease services were consulted for multidisciplinary management. Given the patient’s guarded clinical status, he was promptly started on ART with a formulary combination of bictegravir/emtricitabine/tenofovir alafenamide. He was also started on trimethoprim-sulfamethoxazole for Pneumocystis carinii pneumonia (PCP) prophylaxis. The sputum culture, viral hepatitis panel, acid-fast bacilli (AFB) culture, tuberculosis (TB) spot test, complement levels, and antinuclear antibody test results were all unremarkable (Table 1). Doppler ultrasonography of the lower extremities was negative for a deep venous thrombosis. An echocardiogram showed a normal left ventricular ejection fraction with no evidence of structural abnormalities or valvulopathy. Ceftriaxone and azithromycin were discontinued because of a negative bacterial infectious workup. Syphilis serology returned positive for latent syphilis, and the patient was treated with three doses of penicillin G administered at 7-day intervals.
Due to concerns for KS with pulmonary involvement, flexible bronchoscopy was performed, which revealed candidiasis in the hypopharynx. Multiple non-obstructing submucosal erythematous patches were noted throughout the tracheobronchial tree, most prominently in the right lower lobe (RLL) (Fig. 3). An endobronchial biopsy of a lesion in the bronchus intermedius and three transbronchial biopsies of a lesion in the RLL were obtained. Bronchial washes of the RLL were negative for malignant cells; however, histopathology of transbronchial and endobronchial biopsies showed interstitial spindle cell proliferation and positive staining for HHV-8, consistent with PKS. Furthermore, a punch biopsy of an abdominal skin lesion also stained positive for HHV-8, supporting the diagnosis of KS. Staining for AFB and fungi yielded negative results and adenosine deaminase level on pleural effusion was within normal ranges. The patient was counseled on his diagnoses and discharged on HIV-AIDS medications with plans for outpatient follow-up with the oncology clinic for the initiation of chemotherapy. Unfortunately, the patient was lost to follow-up and his phone remains unanswered.
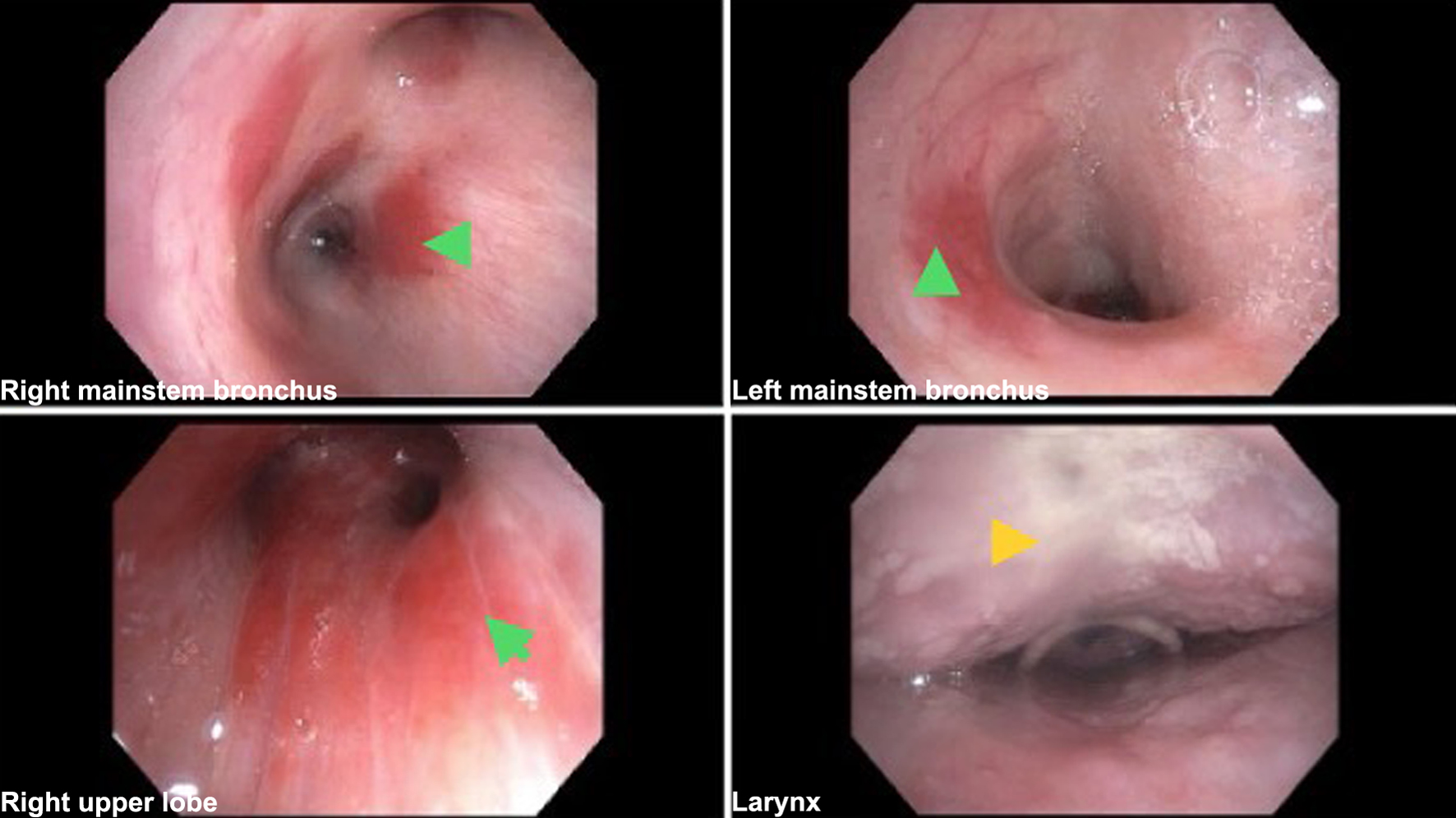 Click for large image | Figure 3. Bronchoscopy images showing scattered erythematous endobronchial lesions and candidiasis in the hypopharynx (arrows). |
Case 2
A 43-year-old male with a history of cocaine use, HIV/AIDS, and medication non-compliance was referred to the ED from an infectious disease (ID) clinic due to concerns for an opportunistic infection. Two months prior to this presentation, the patient was seen in the ID to re-establish care and resume ART. At that time, his CD4 count was 47 cells/mm3 with an HIV viral load of three million copies/mL. ART was resumed, along with azithromycin and trimethoprim-sulfamethoxazole as prophylaxis for opportunistic infections. When seen in the ID clinic for routine follow-up, the patient reported dyspnea on exertion and a dry cough of 2 months’ duration. He also complained of persistent fatigue, watery diarrhea, and intermittent episodes of hemoptysis. He denied having fever, chills, night sweats, chest pain, palpitations, melena, hematochezia, or recent sick contact. A chest X-ray showed bilateral infiltrative changes with associated nodularity and a small right-sided pleural effusion.
In the ED, the patient’s vital signs were normal and he appeared comfortable. Focused physical examination revealed decreased breath sounds at the right lung base, raised dark purple lesions around the neck area, and bilateral cervical lymphadenopathy. The remainder of the physical examination was unremarkable. The initial blood work was significant for hyponatremia, transaminitis, elevated protein gap, and elevated erythrocyte sedimentation rate (Table 1). The iron profile was consistent with iron-deficiency anemia, and the rest of the rainbow laboratory results were normal. A repeat chest X-ray in the ED showed multifocal opacities with nodularity, which were more pronounced in the right hemithorax. An infectious workup was ordered and the patient was empirically treated with broad-spectrum antibiotics. A CT scan of the abdomen and pelvis without contrast revealed multiple hypodense liver and splenic nodules measuring up to 8 mm in diameter. Multiple para-aortic, bilateral inguinal, common and external iliac lymph nodes were also observed. The imaging findings were concerning for metastatic KS. A high-resolution chest CT scan revealed multiple diffuse pulmonary nodular opacities and moderate right-sided pleural effusion (Fig. 4). Mediastinal and bilateral axillary lymphadenopathy was also observed.
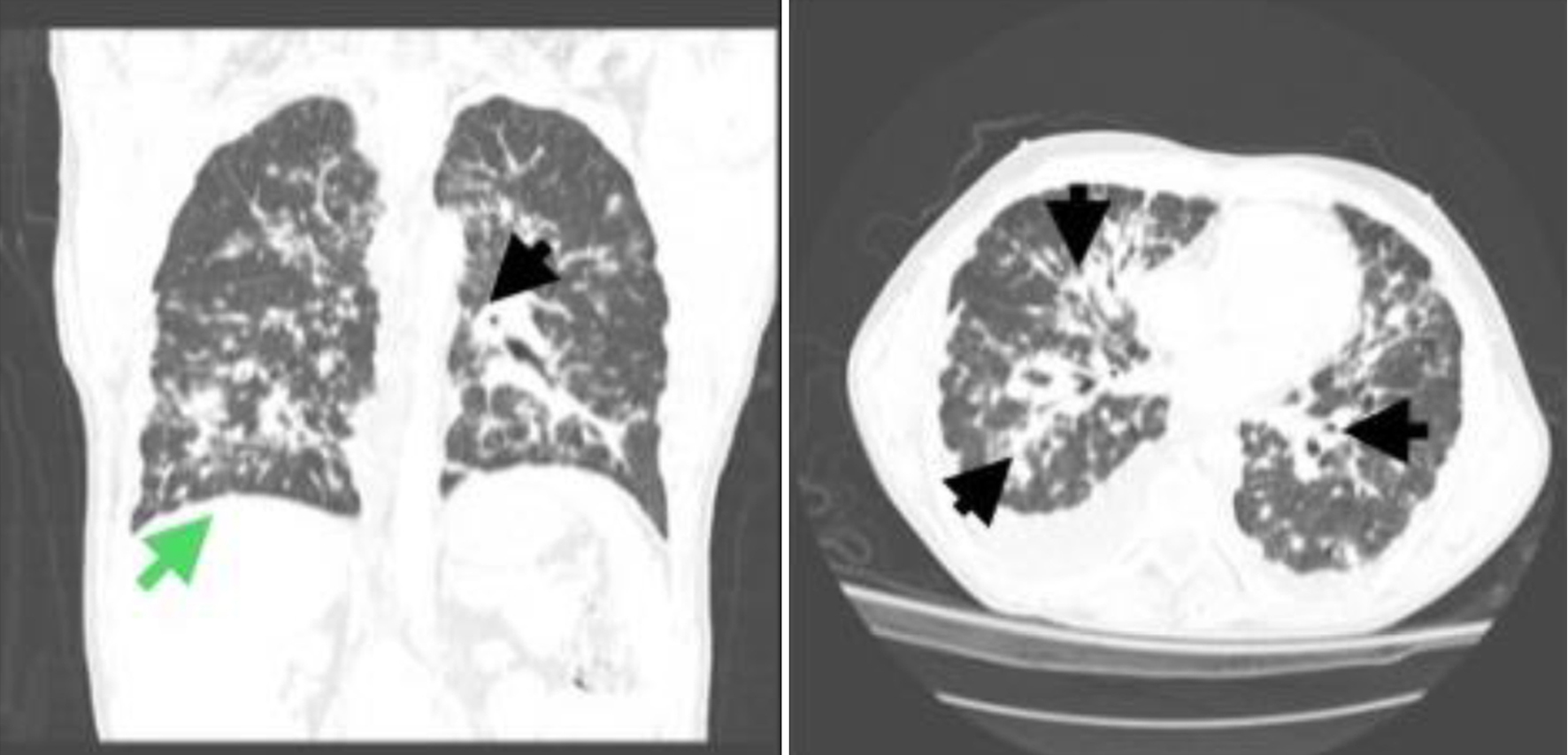 Click for large image | Figure 4. Two views of a high-resolution computed tomography (CT) scan of the chest showing diffuse nodular opacities, with bilateral and roughly symmetrical perilymphatic and peribronchovascular distribution, marked peribronchial thickening, perivascular nodularity, multiple nodules along interlobar fissures, and thickening of interlobular septa (black arrows). Prominent mediastinal lymph nodes and an enlarged necrotic right upper paratracheal lymph node measuring 1.7 cm were also noted. A moderate right-sided pleural effusion could also be seen on the CT scan (green arrow). |
With a CD4 count of 163 cells/mm3, viral load of 25,011 copies/mL, and 19,798 copies of HHV-8, the patient was continued on ART and prophylactic medications. The test results for TB and other fungal etiologies were negative. Flexible bronchoscopy revealed multiple flat erythematous lesions throughout the tracheobronchial tree (Fig. 5). Transbronchial biopsies were obtained from the lesions in the RLL and left upper lobes (LUL). Bronchial washes from these sites were negative for malignant cells. However, biopsy of the LUL revealed positive immunostaining for HHV-8 with focal scattered positive spindle cells, supporting a diagnosis of PKS. Stains for fungal organisms and AFB were negative. A punch biopsy of the left postauricular lesion was also performed, and histopathology revealed KS with positive HHV-8. The patient was seen in the oncology clinic 3 weeks after discharge, and he reported an improvement in his dyspnea. A positron emission tomography (PET) scan revealed moderate right and mid left pleural effusion. The study also showed diffuse patchy nodular infiltrates throughout the lungs with bronchial wall thickening. The patient was started on liposomal doxorubicin (20 mg/m2) every 3 weeks and is tolerating it well. Thus far, the patient has completed three cycles of chemotherapy and the cutaneous lesions have decreased in size.
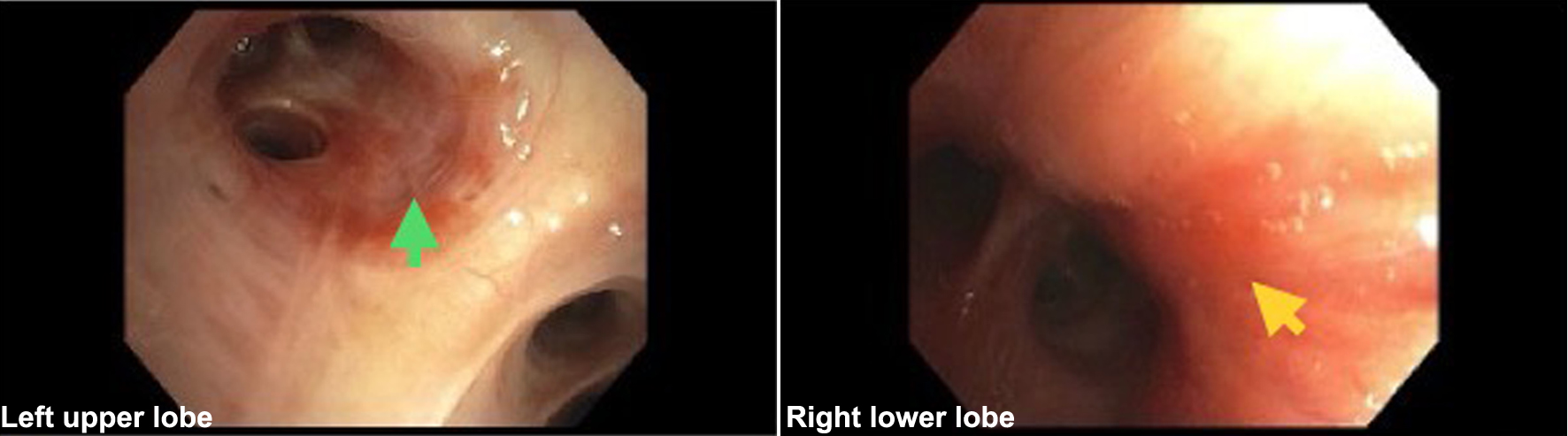 Click for large image | Figure 5. Bronchoscopy images showing flat erythematous lesions in the left upper lobe and right lower lobe (arrows). |
| Discussion | ▴Top |
First described by Moritz Kaposi in 1872, KS is a slow-growing angioproliferative tumor involving the skin and lymph nodes [1, 2, 5]. HHV-8 targets spindle cells of the endothelium, causing angiogenesis through the production of VEGF molecules, cytokines, interleukin (IL)-6, and fibroblast growth factor 2 (FGF2) [2-4]. There are four major subtypes of KS: classic KS is seen in elderly males of Mediterranean or Eastern European descent; endemic KS is seen in immunocompetent young individuals in Sub-Saharan Africa; iatrogenic KS is seen in organ transplant recipients on immunosuppressive therapy; and epidemic KS, which is an AIDS-defining illness [5, 9-11]. Prior to the widespread adoption of ART as a treatment for HIV-AIDS, the incidence of KS was 1,500 - 2,500 cases per 100,000 person-years [5]. Three decades later, and the incidence rate has significantly decreased to 500 cases per 100,000 person-years, and survival rates have improved. Most of the reported cases are in low-income countries, where ART availability is limited. PKS remains an exceedingly rare clinical entity, and is typically observed in patients with epidemic KS. In this series, we share our experience in diagnosing and treating two patients with PKS. We also provide a concise review of the epidemiology, signs and symptoms, diagnosis, and management of this rare condition.
Patients with PKS often present with nonspecific signs and symptoms such as dyspnea, cough, fever, and weight loss [2, 5, 6, 9, 12, 13]. Hemoptysis, wheezing, and chest pain have been observed in some cases [2]. PKS often turns into a clinical conundrum due to its vague presentation. Thus, a high index of suspicion is warranted, especially in patients with HIV/AIDS and a history of medication non-compliance. In addition to a comprehensive history and detailed physical examination, blood tests such as HIV, viral load, viral hepatitis panel, complete blood count, comprehensive metabolic panel, bacterial pneumonia panel, viral respiratory panel, procalcitonin, lactic acid, and HHV-8 polymerase chain reaction can help narrow the differential diagnosis. Chest radiographs typically show bilateral infiltrates and unilateral pleural effusion [5, 7]. In a cohort of 35 patients with PKS, 34 patients had an abnormal chest X-ray, with bilateral pulmonary infiltrates being the most common finding [5]. CT scans often depict interlobular septal thickening and irregular consolidations in a peribronchovascular distribution [5]. Similar findings were observed in case 2, raising concern for metastatic KS. Hilar adenopathy has been observed in some cases, but it is a nonspecific finding with a broad differential diagnosis [2, 5, 11]. A high-resolution CT scan has a higher sensitivity and specificity for PKS diagnosis [5].
Bronchoscopy is performed for direct visualization of KS lesions and enables the operator to biopsy the lesions. A presumptive diagnosis can be made after the appearance of violaceous or reddish maculopapular endobronchial lesions, which are pathognomonic for PKS and are typically observed in the lower airway mucosa [1, 2, 4, 5, 7, 14]. However, in the absence of classic lesions, hyperemia and edema of the lower airways may be the only bronchoscopic evidence of PKS. While a biopsy of the lesions can be sent for immunostaining, the highly vascularized tissue is associated with a bleeding risk [2]. In both our cases, a bronchoscopy with biopsy was performed despite the risk of bleeding to rule out tuberculous and fungal infections, and lymphoproliferative disorder. To minimize the risk, a bronchoalveolar lavage (BAL) sample can be analyzed and detects HHV-8 in up to 80% of cases [14]. Although BAL samples in both cases were positive for HHV-8, no malignant cells were detected in either case. This could be because KS lesions reside in the submucosa and interstitium of the lungs, areas not well-sampled by BAL. The lesions are also vascular and may shed few cells, which further reduces the likelihood of detecting malignant cells in the lavage fluid.
In rare instances in which PKS is highly suspected, yet the characteristic endobronchial lesions are absent, thallium and gallium scintigraphy can aid in confirming the diagnosis as PKS has delayed uptake of thallium without abnormal uptake of gallium [15]. On histopathology, KS demonstrates spindle cell proliferation, inflammatory cells, and angiogenesis, which give rise to the reddish appearance of the endobronchial lesions and the increased bleeding risk during biopsy [1, 2, 4, 5, 7, 14]. Immunohistochemical staining for HHV-8 latent nuclear antigen-1 (LNA-1) is highly sensitive and specific for diagnosing KS, making it widely considered the gold standard [2, 4]. Ultimately, PKS must be differentiated from other AIDS-defining illnesses, infections, and inflammatory conditions, including primary effusion lymphoma (PEL) and Kaposi sarcoma inflammatory cytokine syndrome (KICS) [16, 17]. PKS, PEL, and KICS are all associated with HHV-8 infection but differ in their clinical presentation and etiopathogenesis [5, 7, 18]. PKS typically affects the lungs and presents with respiratory symptoms, while PEL manifests as fluid accumulation in body cavities without a solid tumor [5, 16, 17, 19]. KICS usually presents as a systemic inflammatory condition with high cytokine levels, often without visible tumors or effusions [16-18]. Distinctive histological features, in conjunction with immunohistochemical analysis, aid in distinguishing KS from PEL and KICS. In our case series, the negative BAL samples excluded PEL and the histopathology confirmed PKS. Serum cytokine and IL levels were not obtained in either case.
ART has led to a decline in the incidence and prevalence of KS and PKS in HIV-infected patients [6, 7]. Isolated cases continue to be reported in patients who are non-compliant with their HIV treatment. Because of its rarity and vague presentation, KS is often missed in clinical practice, leading to a delay in treatment [6]. KS treatment depends on the patient’s functional status, number of cutaneous lesions, and the extent of the disease spread [2, 18]. Isolated skin lesions can be treated with surgical excision, cryotherapy, oral etoposide, topical alitretinoin gel, or intralesional chemotherapy using vincristine [2, 18]. Lesions in the lower extremities tend to be complicated by pain, edema, and bleeding, and can be managed with localized radiation therapy [2, 18]. Amputation is a last resort intervention in patients with persistent lesions and skeletal involvement [2].
The management of PKS is a multi-pronged approach consisting of ART and systemic chemotherapy [6, 7, 18]. In developing countries where the availability of chemotherapy is limited, ART is often the only form of therapy for disseminated KS [18]. Owing to its rarity, no prospective studies have assessed the optimal time for initiating chemotherapy. Due to concerns for immune reconstitution inflammatory syndrome (IRIS), some clinicians often delay the initiation of chemotherapy until the CD4 count reaches a set threshold. In our case series, both patients were discharged on ART and antibiotics for PCP prophylaxis, with plans to start chemotherapy as an outpatient. The patient described in case 1 was lost to follow-up, whereas the patient in case 2 has started cancer-directed therapy. Mann et al [8] recommend tailoring the treatment plan to the individual patients to ensure better outcomes. The NCCN guidelines recommend pegylated liposomal doxorubicin and paclitaxel as first-line agents for the treatment of disseminated KS in HIV-seropositive patients [8]. These cytotoxic agents cause a reduction in the size and number of cutaneous lesions and prevent the spread of the disease to other visceral organs [2, 6, 8]. In patients with lung involvement, KS presents a diagnostic and treatment challenge warranting more innovative approaches in some cases [6]. Endoscopic laser resection, bronchial stenting, and radiotherapy have been associated with better outcomes in PKS [6]. Research on immune checkpoint inhibitors and VEGF inhibitors is still nascent but preliminary studies show promising results [2, 6].
PKS has a poor prognosis with an estimated median survival of 1.6 years [6, 8, 16, 19]. Early diagnosis and prompt treatment can prevent progression to a fulminant form [8]. Mann et al [8] reported an interesting case of a 32-year-old male patient with a recent diagnosis of PKS who died 1 month after diagnosis after defaulting on ART and doxorubicin-based chemotherapy. Similarly, Tidwell et al [6] described a unique case of PKS in a male patient with a history of HIV-AIDS who transitioned to hospice care due to a poor prognosis. Recurrent pleural effusion is a common complication in patients with PKS and can be managed with serial thoracentesis [7]. An indwelling pleural catheter may be inserted for symptom palliation in some cases [7]. Tajarernmuang et al [20] reported a unique case of PKS complicated by intractable pleural effusion and persistent dyspnea that was managed with an indwelling pleural catheter. Alexander et al [10] shared a rare case of metastatic KS complicated by chylous pleural effusions refractory to serial thoracentesis and pleural drains. Despite all efforts by the medical team, the patient ultimately died of cardiopulmonary arrest. PKS is a complicated clinical entity that requires a multidisciplinary approach to ensure improved patient outcomes.
Learning points
KS is a slow-growing tumor that affects the skin and lymph nodes via angiogenesis. It is caused by HHV-8, and visceral involvement is rare, especially in the age of ART. PKS occurs when KS affects the tracheobronchial tree, lung parenchyma, and/or lung pleura. Patients typically present with a cough, hemoptysis, pleuritic chest pain, fever, or weight loss. Bronchoscopy with biopsy provides a definitive diagnosis. PKS has a poor prognosis, even when treated with a combination of ART and cancer-directed therapy. This case series sheds light on the diagnostic and therapeutic complexities of PKS in HIV-infected patients.
Acknowledgments
We are grateful to the patients and their family for allowing us to share this interesting case series with the rest of the medical community.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The patients consented for the publication of this case series.
Author Contributions
MAP and LB conceptualized the idea for this case series. AA, KV, MM, AR, NM, and HM helped in writing the paper and collecting pertinent patient information. MI and PM supervised the patient’s care, and edited and proofread the final manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
KS: Kaposi sarcoma; HHV-8: human herpesvirus-8; PKS: pulmonary KS; ART: antiretroviral therapy; VEGF: vascular endothelial growth factor; NCCN: National Comprehensive Cancer Network; ED: emergency department; ECG: electrocardiogram; IV: intravenously; BNP: B-type natriuretic peptide; CT: computed tomography; PCP: Pneumocystis carinii pneumonia; AFB: acid-fast bacilli; RLL: right lower lobe; ID: infectious disease; LUL: left upper lobe; PET: positron emission tomography; FGF2: fibroblast growth factor 2; BAL: bronchoalveolar lavage; LNA-1: latent nuclear antigen-1; PEL: primary effusion lymphoma; KICS: Kaposi sarcoma inflammatory cytokine syndrome
| References | ▴Top |
- Zou RH, Nguyen VD-B, Zigrossi PA, Beckham JM, Smith BJ, Gorgone MJ, Zia H, et al. Non-invasive diagnosis of pulmonary Kaposi sarcoma: a case report. J Infect Dis Epidemiol. 2020;6:161.
- Zeinaty PE, Lebbe C, Delyon J. Endemic Kaposi’s sarcoma. Cancers (Basel). 2023;15(3):872.
doi pubmed pmc - Hamm PG, Judson MA, Aranda CP. Diagnosis of pulmonary Kaposi’s sarcoma with fiberoptic bronchoscopy and endobronchial biopsy. A report of five cases. Cancer. 1987;59(4):807-810.
doi pubmed - Komitova R, Chudomirova K, Abadjieva T. Pulmonary Kaposi’s sarcoma - initial presentation of HIV infection. Folia Med (Plovdiv). 2019;61(4):643-649.
doi pubmed - Nwabudike SM, Hemmings S, Paul Y, Habtegebriel Y, Polk O, Mehari A. Pulmonary Kaposi sarcoma: an uncommon cause of respiratory failure in the era of highly active antiretroviral therapy - case report and review of the literature. Case Rep Infect Dis. 2016;2016:9354136.
doi pubmed pmc - Tidwell J, Van Antwerp S, Bihag ZA. A race against time: rapidly progressing pulmonary Kaposi sarcoma. Cureus. 2023;15(6):e40019.
doi pubmed pmc - Mohamed A, Saad E, Babkir A, Khtab K, Abdalla M. Pulmonary Kaposi sarcoma as an unusual etiology of acute hypoxemic respiratory failure in the era of highly active antiretroviral therapy: a case report. Cureus. 2022;14(5):e25014.
doi pubmed pmc - Mann BK, D’Assumpcao C, Okumoto L, Aboaid S, Abooed A, Cobos E, Heidari A. Spurious presentation of pulmonary Kaposi sarcoma as unresolved pneumonia led to fatal outcome. J Investig Med High Impact Case Rep. 2023;11:23247096231208996.
doi pubmed pmc - Bhattarai B, Lamichhane J, Mandal A, Datar P, Mukhtar O, Alhafidh O, Lixon A, et al. Pulmonary Kaposi sarcoma: an uncommon presentation in HIV heterosexual female on antiretroviral therpay. J Community Hosp Intern Med Perspect. 2020;10(2):158-161.
doi pubmed pmc - Alexander R, Rizer M, Burke W, Ciment L. Chylothorax in a patient with metastatic Kaposi sarcoma: differential diagnostic considerations. Radiol Case Rep. 2015;10(2):1098.
- Gasparetto TD, Marchiori E, Lourenco S, Zanetti G, Vianna AD, Santos AA, Nobre LF. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis. 2009;4:18.
doi pubmed pmc - Iqbal H. Kaposi Sarcoma with bilateral chylothorax responsive to octreotide. Southwest J Pulm Crit Care Sleep. 2022;25(5):69-72.
- Ramos AL, Granado J, Calderon AI, Andre S, Nogueira F. Pulmonary Kaposi’s sarcoma - an atypical clinical presentation. Int J Infect Dis. 2022;115:185-188.
doi pubmed - Yoo DJ, Lee KH, Munderi P, Shin KC, Lee JK. Clinical and bronchoscopic findings in Ugandans with pulmonary Kaposi’s sarcoma. Korean J Intern Med. 2005;20(4):290-294.
doi pubmed pmc - Nguyen P, Knapp-Wachsner A, Hsieh CG, Kamangar N. Pulmonary Kaposi sarcoma without mucocutaneous involvement: the role of sequential thallium and gallium scintigraphy. J Clin Imaging Sci. 2019;9:12.
doi pubmed pmc - Dumic I, Radovanovic M, Igandan O, Savic I, Nordstrom CW, Jevtic D, Subramanian A, et al. A fatal case of Kaposi sarcoma immune reconstitution syndrome (KS-IRIS) complicated by Kaposi sarcoma inflammatory cytokine syndrome (KICS) or multicentric castleman disease (MCD): a case report and review. Am J Case Rep. 2020;21:e926433.
doi pubmed pmc - Polizzotto MN, Uldrick TS, Wyvill KM, Aleman K, Marshall V, Wang V, Whitby D, et al. Clinical features and outcomes of patients with symptomatic Kaposi sarcoma herpesvirus (KSHV)-associated inflammation: prospective characterization of KSHV inflammatory cytokine syndrome (KICS). Clin Infect Dis. 2016;62(6):730-738.
doi pubmed pmc - Micali C, Russotto Y, Facciola A, Marino A, Celesia BM, Pistara E, Caci G, et al. Pulmonary Kaposi sarcoma without respiratory symptoms and skin lesions in an HIV-naive patient: a case report and literature review. Infect Dis Rep. 2022;14(2):228-242.
doi pubmed pmc - Bangar S, Vashisht R, Sonar P, Ghule K, Rawat L, Mane A, Kadam A, et al. Kaposi sarcoma (KS) with primary effusion lymphoma in HIV infected MSM (men having sex with men) co-infected with pulmonary tuberculosis and syphilis: a case report from India. AIDS Res Ther. 2022;19(1):36.
doi pubmed pmc - Tajarernmuang P, Fiset PO, Routy JP, Beaudoin S. Intractable pleural effusion in Kaposi sarcoma following antiretroviral therapy in a Caucasian female infected with HIV. BMJ Case Rep. 2020;13(2):e233335.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


