| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 8, August 2024, pages 208-214
Anterior Uveitis After Discontinuation of Janus Kinase Inhibitor, Ruxolitinib
Toshihiko Matsuoa, b, c, h, Naoto Ikedad, Yasumasa Monobee, f, Takehiro Tanakag
aGraduate School of Interdisciplinary Science and Engineering in Health Systems, Okayama University, Okayama City 700-8558, Japan
bDepartment of Ophthalmology, Okayama University Hospital, Okayama City 700-8558, Japan
cEye Clinic, Ochiai Hospital, Maniwa City 719-3197, Japan
dDepartment of Internal Medicine, Kaneda Hospital, Maniwa City 719-3193, Japan
eDepartment of Pathology, General Medical Center, Kawasaki Medical School, Okayama City 700-8505, Japan
fOkayama Medical Laboratories, Inc., Kurashiki City 710-0834, Japan
gDepartment of Pathology, Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Okayama University, Okayama City 700-8558, Japan
hCorresponding Author: Toshihiko Matsuo, Regenerative and Reconstructive Medicine (Ophthalmology), Graduate School of Interdisciplinary Science and Engineering in Health Systems, Okayama University, Okayama City 700-8558, Japan
Manuscript submitted June 17, 2024, accepted July 19, 2024, published online July 25, 2024
Short title: Uveitis in Primary Myelofibrosis
doi: https://doi.org/10.14740/jmc4274
| Abstract | ▴Top |
Primary myelofibrosis shows widespread fibrosis in the bone marrow and is part of myeloproliferative neoplasms in which gene mutations in hematopoietic stem cells lead to abnormal clonal expansion of one or more lineage of myeloid and erythroid cells and megakaryocytes. Janus kinase (JAK) inhibitors are the main therapeutic regimen for primary myelofibrosis which harbors gene mutations, resulting in continuous activation of JAK-STAT signaling pathway. Since JAK inhibitors modulate immunological state, the administration would have a potential for uveitis. A 67-year-old patient presented with weight loss of 10 kg in the past 2 years after his retirement. He showed normocytic anemia with anisocytosis and abnormal shape, as well as hepatosplenomegaly. Suspected of hematological malignancy, bone marrow biopsy led to the diagnosis of primary myelofibrosis (grade 2) with bizarre megakaryocytes and relative maintenance of myeloid and erythroid lineage. He started to have blood transfusion. Genomic DNA analysis of the peripheral blood showed a pathogenic variant in the exon 9 of calreticulin (CALR) gene while pathogenic variants in Janus kinase-2 (JAK2), and myeloproliferative leukemia virus oncogene (MPL) were absent. He began to have oral ruxolitinib 10 mg daily at the timepoint of 5 months after the initial visit and the dose was increased to 20 mg daily 8 months later but was discontinued further 4 months later because he showed the limited effect of ruxolitinib. He had blood transfusion every week or every 2 weeks in the following 2 months until he noticed blurred vision in the right eye. The right eye showed thick fibrin membrane formation in the anterior chamber in front of the pupil which prevented the fundus from visualization. The left eye showed no inflammation and optic nerve atrophy, sequel to tuberculous meningitis in childhood. The patient started to use 0.1% betamethasone six times daily and 1% atropine once daily as eye drops. A week later, fibrin membrane disappeared and the pupillary area with total iris posterior synechia was visible in the right eye. He regained the vision in the right eye and did not show relapse of uveitis only with topical 0.1% betamethasone. Uveitis might be related with the administration and discontinuation of ruxolitinib.
Keywords: Janus kinase inhibitor; Ruxolitinib; Anemia; Myelofibrosis; Anterior uveitis
| Introduction | ▴Top |
Myelofibrosis is the condition that shows widespread fibrosis in the bone marrow [1]. Primary myelofibrosis is the part of myeloproliferative neoplasms in which gene mutations occurring in hematopoietic stem cells lead to abnormal clonal expansion of one or more lineage of myeloid cells, erythroid cells, and megakaryocytes. Growth factors and cytokines secreted by anomalous megakaryocytes or monocytes induce widespread fibrosis which results in ineffective hematopoiesis in the bone marrow, in association often with splenomegaly which may have extramedullary hematopoiesis. Red blood cells in abnormal shapes such as tear-drop red blood cells may be found in the peripheral blood. Secondary myelofibrosis is caused mainly by essential thrombocythemia, polycythemia vera, and myelodysplastic syndrome [2], and rarely by systemic inflammatory diseases.
In primary myelofibrosis, mutations in the genes of Janus kinase-2 (JAK2), calreticulin (CALR), myeloproliferative leukemia virus oncogene (MPL), and so on lead to continuous activation of Janus kinase (JAK)-STAT signaling pathway, and hence, JAK2 inhibitors are used as therapeutic regimen for primary myelofibrosis [3]. In this case report, we present a patient with primary myelofibrosis who developed severe anterior uveitis after ruxolitinib, JAK1/2 inhibitor, had been discontinued.
| Case Report | ▴Top |
Investigations and diagnosis
A 67-year-old man noticed chest pain in air intake and visited a regional hospital. In the past history, he experienced tuberculous meningitis in childhood and was told to have hearing disturbance on the left side due to streptomycin since then. Otherwise, he had been healthy and had nothing particular to be noted in annual health checkups until the age of 65 years when he retired from a company as an office worker and moved to this small city. He experienced weight loss of 10 kg in the past 2 years. He did not smoke and was an occasional alcohol drinker. In the past 1 year, he had cough and sputum which became better recently. In a few weeks before the initial visit, he noticed chest pain when he coughed. He had no medications. At the initial visit, physical examinations disclosed hepatomegaly, together with conjunctival anemia. He had neither fever nor skin rashes. The systolic and diastolic blood pressure was 116/76 mm Hg with the pulse rate of 76 per minute. The height was 163 cm and body weight was 58 kg. Complete blood cell counts showed normocytic anemia with anisocytosis and abnormal shape (Table 1). Urinalysis was normal except for 2+ protein. C-reactive protein (CRP) was high at 5.98 mg/dL, serum ferritin was high at 376 ng/mL, and soluble interleukin-2 receptor was high at 757 U/mL. Mycoplasma antibody was not elevated. Antinuclear antibody was elevated to 1,280 folds with speckle staining pattern while other autoantibodies (Sjogren syndrome-A (SS-A), SS-B, autoantibodies against Sm and double-stranded DNA) were all negative. Serological tests for syphilis as well as screening tests for hepatitis B virus antigen, hepatis C virus antibody, and human immunodeficiency virus antigen/antibody were all negative. Coronavirus disease 2019 (COVID-19) antigen in nasal swab was also negative. CRP became non-detectable in several days after blood transfusion was done as described in the following section.
 Click to view | Table 1. Blood Examinations at the Initial Visit and at Cataract Surgery, 22 Months Later From the Initial Visit |
Computed tomographic scan demonstrated reticular and ground glass appearance in the dorsal part of bilateral lower lung field, indicative of interstitial change, with a small volume of pleural effusion on the left side (Fig. 1a) as well as hepatosplenomegaly (Fig. 1b). Suspected of hematological malignancy, he was referred to a hematologist in another hospital for further examinations. Bone marrow aspiration ended up with dry tap and bone marrow biopsy led to the diagnosis of primary myelofibrosis (grade 2) with bizarre megakaryocytes (Fig. 2a-e). He maintained myeloid and erythroid lineage although erythroid islands were small both in size and in number (Fig. 2f, g). He started to have blood transfusion with post-irradiation leukocytes-reduced red blood cells (2 units, equal to 400 mL blood, RBC-LR-2, Japanese Red Cross Society, Tokyo, Japan) every month for 3 months from the initial visit (Fig. 1c). Genomic DNA analysis of the peripheral blood sample showed a pathogenic variant in the exon 9 of CALR gene while pathogenic variants in JAK2 (JAK2-V617F, variants in exon 12) and MPL (variants in exon 10) were absent. He began to have oral ruxolitinib 10 mg daily at the timepoint of 5 months after the initial visit and the dose was increased to 20 mg daily 8 months later but was discontinued further 4 months later because he showed the limited effect of ruxolitinib (Fig. 1c). He had red blood cells transfusion every week or every 2 weeks both in the preceding 2 months and in the following 2 months around ruxolitinib discontinuation until he noticed blurred vision in the right eye (Fig. 1c). Throughout the period, CRP remained non-detectable.
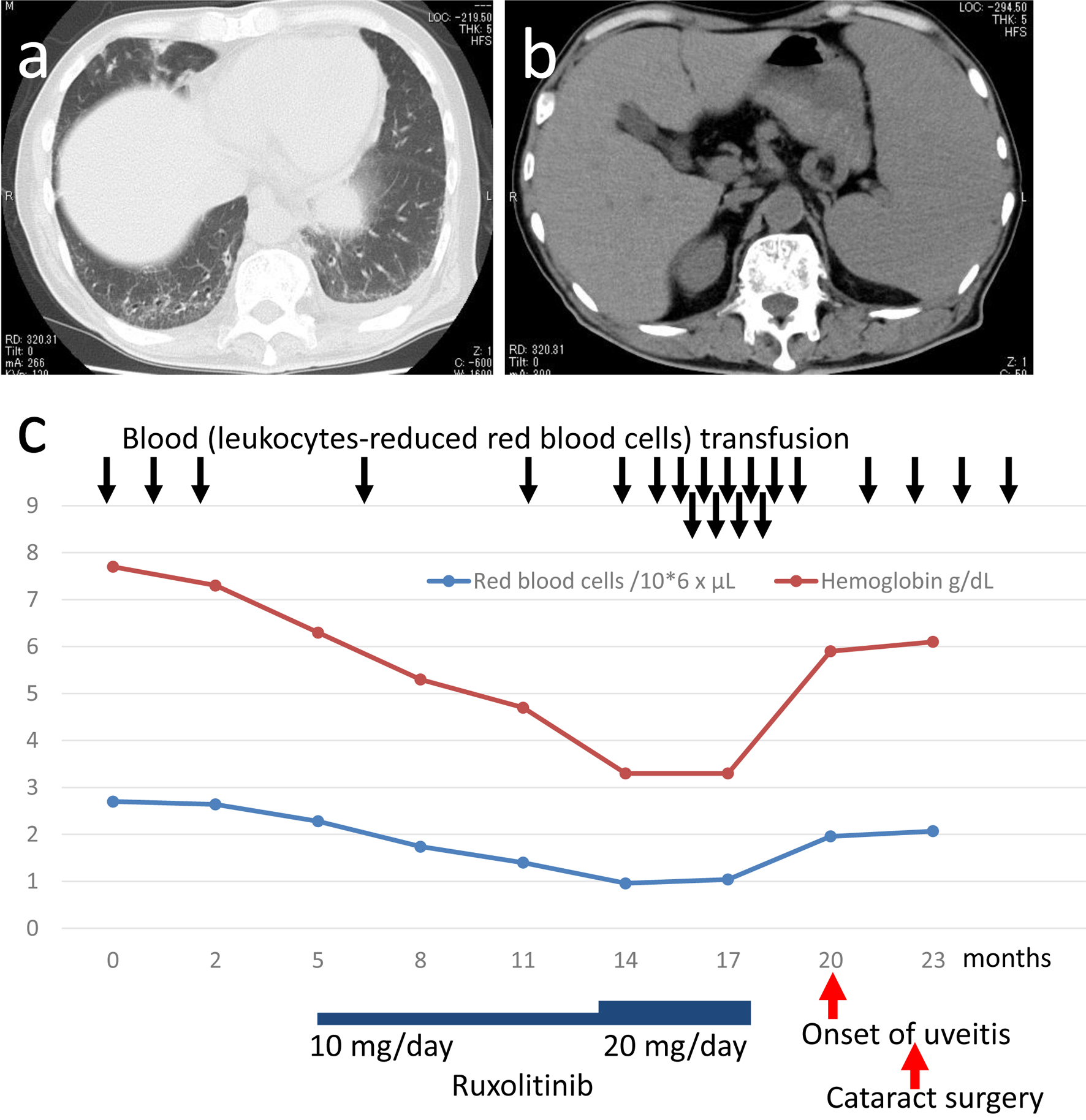 Click for large image | Figure 1. Computed tomographic scans (a, b) at the initial visit and a chart for the entire course (c). Reticular and ground glass appearance, indicative of interstitial change, in bilateral lower dorsal lung fields with a small amount of pleural effusion on the left side (a). Hepatosplenomegaly (b). |
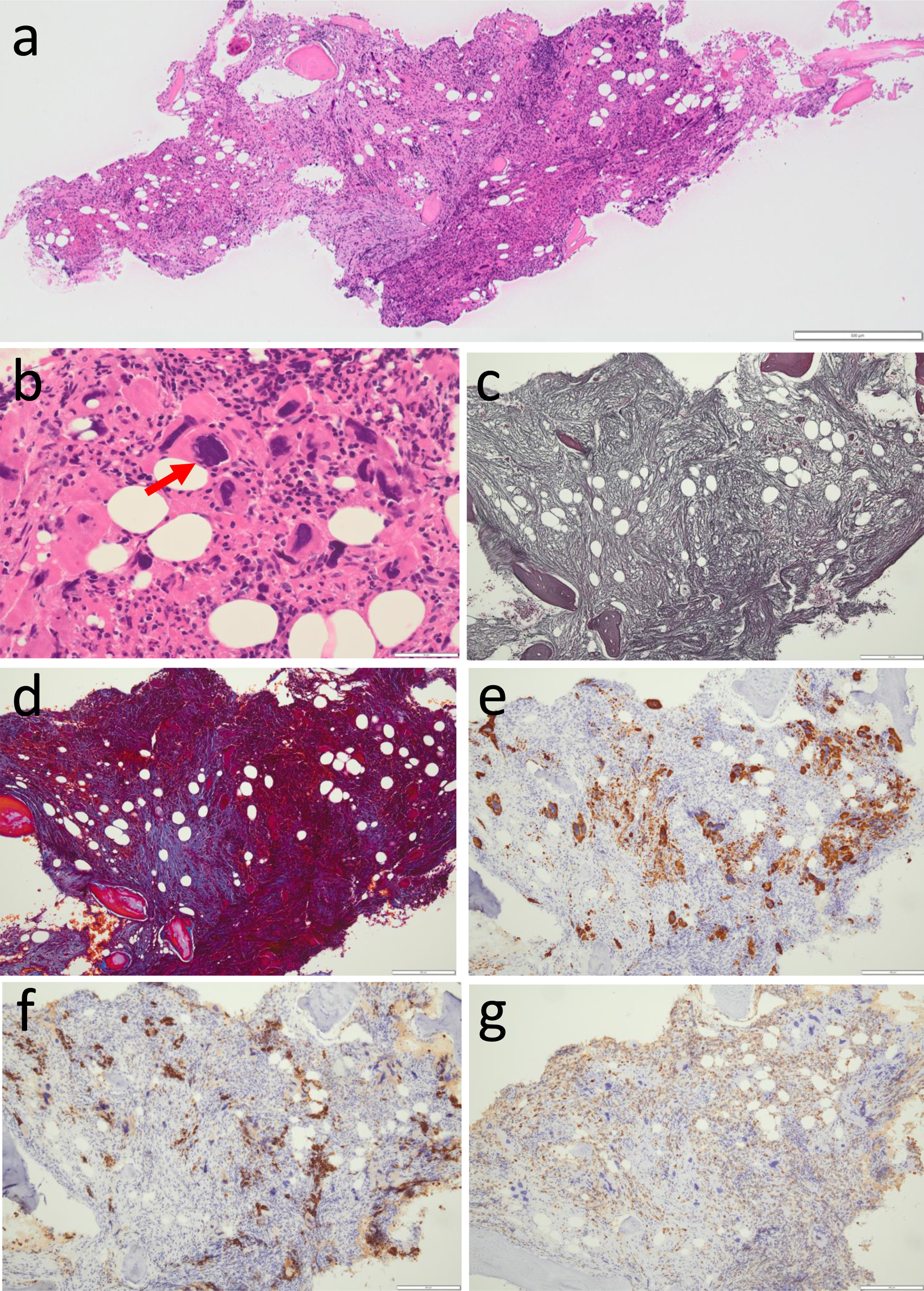 Click for large image | Figure 2. Bone marrow biopsy to support the diagnosis of myelofibrosis. Conspicuous fibrosis with osteosclerosis and reduced amount of adipose tissue in low magnification (a), and cluster of megakaryocytes with bizarre nuclei (arrow, b) in high magnification by hematoxylin-eosin stain. Silver impregnation stain reveals diffusely increased reticulin fibers (c). Masson trichrome stain highlights collagen fibrosis (d). By immunostaining, clusters of CD42b-positive bizarre megakaryocytes (e), CD71-positive erythroid lineage cells in erythroid islands which are in reduced number and in smaller size (f), and myeloperoxidase (MPO)-positive myeloid lineage cells in the normal process of maturation (g). Scale bar = 500 µm in A, 50 µm in B, 200 µm in c-g. |
Ophthalmic presentation
He was thus referred to an ophthalmologist in the first regional hospital. The best-corrected visual acuity in decimals was 0.01 in the right eye and 0.05 in the left eye. He said his left vison had been poor from youth. The intraocular pressure was 13 mm Hg in the right eye and 20 mm Hg in the left eye. The right eye showed thick fibrin membrane formation in the anterior chamber in front of the pupil (Fig. 3a) which prevented the fundus from visualization. No mydriasis was obtained by topical 0.1% phenylephrine and 0.4% tropicamide application, indicative of iris posterior synechia. Ultrasonographic examination demonstrated no retinal detachment in the right eye. The left eye had no aqueous inflammation but with mild cataract. Fundus examination in the left eye showed the normally appearing retina with optic nerve atrophy.
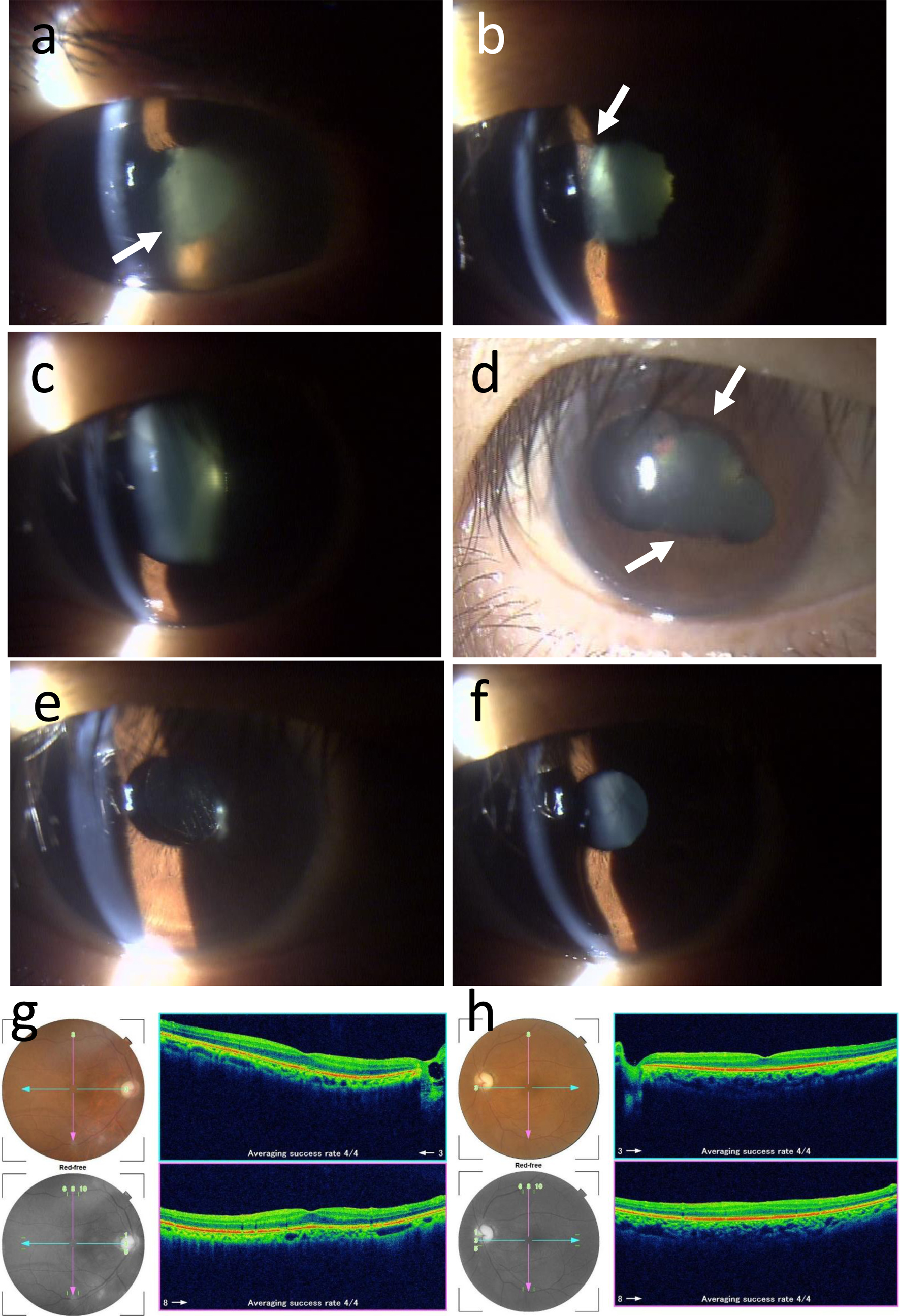 Click for large image | Figure 3. Slit-lamp photographs at ophthalmic presentation (right eye, a), a week later (right eye, b), 3 weeks later (right eye, c, d), 2 months later (right eye, e; left eye, f) from the first ophthalmic visit. Fundus photographs and optical coherence tomography (g, h) 2 months later. Fluffy fibrin deposition in the pupillary area (arrow in a) of anterior chamber has disappeared a week later (b) only with topical 0.1% betamethasone eye drops six times daily, leaving iris posterior synechia (arrow in b). Upper and lower iris posterior synechia (arrows in d) under mydriasis with 1% atropine eye drops 3 weeks later (c, d). No inflammation after intraocular lens implantation by cataract surgery in the right eye (e), and also in the left eye with anterior and posterior subcapsular cataract (f). Ocular fundus photographs and optical coherence tomographic images in the right eye (g) and left eye (h) appear normal except for temporal pallor of the optic disc in the right eye and total atrophy of the optic disc in the left eye. Each panel in g and h shows color fundus photograph (top left), red-free photograph (bottom left), horizontal section (shown in blue arrows in photographs) of the image from the nasal to the temporal side (top right), and vertical section (shown in pink arrows in photographs) of the image from the superior to the inferior side (bottom right). |
Treatment
The patient started to use 0.1% betamethasone six times daily and 1% atropine once daily as eye drops. A week later, fibrin membrane disappeared and the pupillary area with total iris posterior synechia (Fig. 3b) was visible in the right eye. Fundus examination showed normally appearing retina with temporal pallor of the optic disc in the right eye. The visual acuity in the right eye returned to 0.1. Topical 0.1% betamethasone was changed to four times daily. Further 2 weeks later, iris posterior synechia was partly resolved to result in mydriasis (Fig. 3c, d) by daily topical atropine. The intraocular pressure was 17 mm Hg in the right eye and 19 mm Hg in the left eye.
Follow-up and outcomes
Two months later from the ophthalmic presentation, he maintained no inflammation with topical 0.1% betamethasone four times daily and underwent cataract surgery with intraocular lens implantation in the right eye (Fig. 3e). The best-corrected visual acuity in the right eye became 0.4 in the right eye. He continued to have no relapse of inflammation only with topical 0.1% betamethasone twice daily at the latest visit 4 months after cataract surgery. No inflammation was also noted in the left eye without any topical medication (Fig. 3f). The retina in both eyes were normal with optic nerve atrophy in the left eye (Fig. 3g, h). Systemically, he was ambulatory and stable in health with blood transfusion once a month.
| Discussion | ▴Top |
The patient developed anterior uveitis in the unilateral eye roughly 2 months after he had discontinued oral ruxolitinib, as a JAK1/2 inhibitor, because of no effect while he continued to have blood transfusion to alleviate symptoms caused by anemia (Fig. 1c). The unilateral anterior uveitis in this patient would be attributed to: 1) primary myelofibrosis in itself [4]; 2) adverse event of oral ruxolitinib [4]; 3) discontinuation of oral ruxolitinib; or less likely 4) frequent blood transfusion. A previous report described an 83-year-old woman with unilateral anterior uveitis who was diagnosed as myeloproliferative disease in systemic examinations for a possible cause of uveitis [4]. Another previous case report described a 64-year-old man who developed unilateral uveitis with hypopyon and vitreous opacity in the course of fedratinib, JAK2 inhibitor, toward myelofibrosis [5]. The uveitis in the latter patient was considered as adverse event of fedratinib [5]. Ruxolitinib has been shown to be effective in patients with COVID-19 triggered hyperinflammation [6]. Experimentally, ruxolitinib has been demonstrated to alleviate uveitis caused by Salmonella typhimurium endotoxin in rats [7]. These clinical and experimental studies suggest that uveitis in the present patient might be related with rebound inflammation which might be induced by activation of JAK-STAT signaling pathway when oral ruxolitinib has been discontinued. The present patient received frequent blood transfusion before the onset of anterior uveitis (Fig. 1c) but did not develop well-known allergic reaction to blood transfusion, such as fever, urticaria and itching, in that period. A previous case report described a 79-year-old woman with myelodysplastic syndrome who developed bilateral anterior uveitis after she received blood transfusion and darbepoetin alpha [8]. The present patient did not have darbepoetin alpha in the course.
The patient had hearing disturbance from childhood. In addition, he said he had poor vision in the left eye from youth. He had been told to develop hearing disturbance due to streptomycin administration for tuberculous meningitis. Under the circumstances, the temporal pallor of the optic disc in the right eye and optic nerve atrophy in the left eye of the present patient would be attributed to tuberculous meningitis in itself or neurotoxicity of streptomycin in childhood. Reactivation of old tuberculosis might cause uveitis but would be less likely since the present patient did not show other symptoms throughout the course.
The diagnosis of primary myelofibrosis in this patient was based on widespread fibrosis with bizarre megakaryocytes detected in bone marrow biopsy. Anomalous lineage of megakaryocytes was further supported by the detection of a pathogenic variant of the gene, CALR, 3 months later. Hepatosplenomegaly might be associated with extramedullary hematopoiesis. His main problem was anemia caused by insufficient hematopoietic function in the bone marrow with myelofibrosis even in the situation that erythroid islands for red blood cell production were relatively maintained. Anemia would be also attributed to inflammatory cytokine-induced wasting syndrome which was evident by weight loss and hepatosplenomegaly in this patient. Platelets and white blood cells in the peripheral blood remained at the normal level, and thus, the patient did not fit with the diagnosis of essential thrombocythemia. Autoimmune diseases as a possible cause for secondary myelofibrosis could be excluded by the absence of other systemic signs and symptoms in the background of a high level of antinuclear antibody.
The cause for the interstitial changes in the dorsal part of bilateral lung fields remained to be elucidated. He had past history of tuberculosis in childhood and the lung field changes might be sequel to old tuberculosis, although tuberculosis typically tends to involve the upper lungs. COVID-19 would show ground glass appearance in the dorsal part of the lower lung field but the patient was negative for COVID-19 antigen on the nasal swab at the initial visit. He might have a preceding episode of non-symptomatic COVID-19 to leave pulmonary interstitial changes as a consequence. At the initial visit, he also showed relatively high levels of CRP, serum ferritin, and soluble interleukin-2 receptor, and IgG in polyclonal increase (Table 1), which altogether might indicate the presence of chronic inflammation of unknown origin. Other differential diagnoses include atypical pneumonia caused by Mycoplasma [9, 10] and sarcoidosis with atypical presentation [11]. In this patient, CRP returned non-detectable after blood transfusion, suggesting that poor systemic condition caused by anemia and myelofibrosis might be the background for chronic inflammation. Mycoplasma antibody was not elevated at the initial visit. No further workup for the interstitial lung change was made since the patient did not have respiratory symptoms anymore.
Learning points
The patient with anemia caused by primary myelofibrosis developed severe anterior uveitis 2 months after oral ruxolitinib, JAK1/2 inhibitor, had been discontinued. The uveitis showed good response to topical corticosteroid eye drops. Uveitis would be related with the administration and discontinuation of a JAK1/2 inhibitor.
Acknowledgments
None to declare.
Financial Disclosure
The authors receive no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent
Written consent was obtained from the patient for his anonymized information to be published in this article.
Author Contributions
TM, as an ophthalmologist, treated and followed the patient, and wrote the manuscript. NI, as an internist (hematologist), treated and followed the patient. YM and TT made pathological diagnosis. All authors approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Tefferi A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(5):801-821.
doi pubmed - Garcia-Manero G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(8):1307-1325.
doi pubmed - Scherber RM, Mesa RA. Management of challenging myelofibrosis after JAK inhibitor failure and/or progression. Blood Rev. 2020;42:100716.
doi pubmed pmc - Ben-Simon GJ, Barequet IS. Anterior uveitis as presenting sign of myeloproliferative disease. Ann Ophthalmol. 2003;35:62-64.
- Evans W, Mathew C, Richardson-May J, Arora R. A case report of fedratinib-associated uveitis. Cureus. 2024;16(1):e52373.
doi pubmed pmc - Hammersen J, Birndt S, Dohner K, Reuken P, Stallmach A, Sauerbrey P, La Rosee F, et al. The JAK1/2 inhibitor ruxolitinib in patients with COVID-19 triggered hyperinflammation: the RuxCoFlam trial. Leukemia. 2023;37(9):1879-1886.
doi pubmed pmc - Du L, Yip YWY, Ng HK, Ho BM, He JN, Chan SO, Pang CP, et al. Ruxolitinib alleviates uveitis caused by salmonella typhimurium endotoxin. Microorganisms. 2021;9(7):1481.
doi pubmed pmc - Li J, Orlin SE, Revere KE, Kempen JH. Acute onset anterior uveitis after darbepoetin alfa infusion. J Ophthalmic Inflamm Infect. 2015;5(1):31.
doi pubmed pmc - Dueck NP, Epstein S, Franquet T, Moore CC, Bueno J. Atypical pneumonia: definition, causes, and imaging features. Radiographics. 2021;41(3):720-741.
doi pubmed - Miyashita N. Atypical pneumonia: Pathophysiology, diagnosis, and treatment. Respir Investig. 2022;60(1):56-67.
doi pubmed - Criado E, Sanchez M, Ramirez J, Arguis P, de Caralt TM, Perea RJ, Xaubet A. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30(6):1567-1586.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


