| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 9, September 2024, pages 231-236
Unexpected Rasburicase-Induced Hemolysis in a Patient With Normal Glucose-6-Phosphate Dehydrogenase Activity
Saba Musleh Ud Dina, d, Khine Shanb, Tauseef Ur Rehmanb, Stanislav Ivanovb, Fernando M. Vargas-Maduenoc
aDepartment of Internal Medicine, Memorial Healthcare System, Hollywood, FL, USA
bDepartment of Hematology and Oncology, Memorial Healthcare System, Hollywood, FL, USA
cMoffitt at Memorial Healthcare System, Pembroke Pines, FL, USA
dCorresponding Author: Saba Musleh Ud Din, Department of Internal Medicine, Memorial Healthcare System, Hollywood, FL, USA
Manuscript submitted June 19, 2024, accepted August 5, 2024, published online August 22, 2024
Short title: Rasburicase Hemolysis Without G6PD Deficiency
doi: https://doi.org/10.14740/jmc4277
| Abstract | ▴Top |
Tumor lysis syndrome (TLS) presents significant challenges in oncology, primarily due to metabolic complications such as hyperuricemia, which can lead to acute kidney injury. Rasburicase, a recombinant urate oxidase, is frequently employed to manage hyperuricemia in TLS patients. However, its use is an absolute contraindication in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency due to the risk of hemolysis. In this case, the patient developed hemolytic anemia post-rasburicase administration even though she had normal G6PD activity, which was confirmed on two separate occasions, including during an acute episode and 3 months later. This case is unique as it documents hemolytic anemia induced by rasburicase in a patient without G6PD deficiency, challenging current understandings of the drug’s safety profile. It suggests the need for caution and thorough screening before rasburicase use, even in patients considered low risk for G6PD deficiency. The report highlights the importance of close monitoring for adverse effects and the potential for alternative mechanisms of rasburicase-induced hemolysis.
Keywords: Rasburicase-induced hemolysis; G6PD deficiency; Tumor lysis syndrome; Oxidative stress
| Introduction | ▴Top |
Tumor lysis syndrome (TLS) remains a formidable challenge in the oncological landscape, with its metabolic complications posing serious threats to organ functionality and patient survival. Among these complications, hyperuricemia is central, often culminating in acute kidney injury - a predictor of mortality in the context of TLS [1]. Treatment of TLS requires close monitoring with aggressive intravenous hydration, correction of electrolyte imbalances, and reduction of serum uric acid levels. To mitigate the dire consequences of hyperuricemia, clinicians often resort to rasburicase, a recombinant urate oxidase [2]. While the efficacy of rasburicase is undeniable, its safety profile becomes tenuous in the presence of glucose-6-phosphate dehydrogenase (G6PD) deficiency. Notably, in G6PD deficiency, rasburicase may act as a harbinger of hemolysis and methemoglobin formation, the consequences of which can be as perilous as TLS itself [3]. The onset of acute hemolytic anemia after rasburicase administration is well described in male patients with G6PD deficiency due to its X-linked inheritance pattern [4].
However, here we describe a case of hemolytic anemia in a woman who received rasburicase for TLS. Quantitative activity testing for G6PD deficiency following the hemolytic episode did not demonstrate G6PD deficiency. Therefore, this is being considered a case of rasburicase-induced hemolysis in the absence of G6PD deficiency.
| Case Report | ▴Top |
A 66-year-old African American woman with a history of hypertension and endometrial hyperplasia treated with a hysterectomy 12 years ago, was referred for evaluation of abnormal outpatient laboratories notable for low hemoglobin (Hb). Upon further review, the patient reported episodes of fevers, chills, and night sweats for the past 3 weeks prior to her presentation. These constitutional symptoms were accompanied by loss of appetite and weight loss of approximately 18 pounds in the past 4 months. Physical examination at the time of evaluation was only remarkable for a pale, tired-looking patient.
Initial lab evaluation revealed elevated white blood cell (WBC) count at 24.2 × 103/µL (3.5 - 10 × 103/µL) with absolute lymphocyte count of 14.76 × 103/µL (1.16 - 3.18 × 103/µL), absolute neutrophil count of 1.72 × 103/µL (2.00 - 7.15 × 103/µL), Hb of 6.0 g/dL (11.4 - 15.4 g/dL), mean corpuscular volume (MCV) of 91.0 fL (80.0 - 95.0 fL), and platelets of 144 × 103/µL (150 - 450 × 103/µL). Lactate dehydrogenase (LDH) was > 5,000 U/L (120 - 246 U/L) and was noted to have normal haptoglobin of 120 mg/dL (30 - 200 mg/dL) with a reticulocyte count of 2.1% (0.5-2.0%) at admission. Her coagulation studies were normal at activated partial thromboplastin time (APTT) of 25.6 s (23.0 - 31.0 s), prothrombin time (PT) of 12.6 s (9.5 - 11.5 s), and international normalized ratio (INR) of 1.2 (0.9 - 1.1) with elevated fibrinogen at 408 mg/dL (180 - 400 mg/dL). A review of her metabolic profile noted high uric acid of 0.91 mmol/L (0.15 - 0.37 mmol/L), high phosphorous of 1.78 mmol/L (0.81 - 1.45 mmol/L), and low potassium of 2.5 mmol/L (3.5 - 5.1 mmol/L). Her creatinine and calcium levels were within the normal range. Folate level was low at 2.61 ng/mL (2.76 - 20 ng/mL) and vitamin B12 at 255 pg/mL (239 - 931 pg/mL). Peripheral blood smear showed atypical immature lymphocytes concerning for leukemia or lymphoma. Subsequent flow cytometry revealed an atypical CD19+/CD10+ population comprising 60% of the WBC, suggesting B lymphoblasts/B-lymphoblastic leukemia.
In the setting of these laboratory anomalies, a concern for spontaneous TLS was present based on Cairo-Bishop criteria [5]. She was started on intravenous fluids and allopurinol 300 mg twice a day. Given no familial history of G6PD and female gender, the patient was assigned a “low risk” for G6PD deficiency. The patient was subsequently given a single dose of rasburicase (6 mg) intravenously. She was also transfused with two units of packed red blood cells (pRBCs) which improved her Hb to 8.0 g/dL. She was started on folate and vitamin B12 supplementation. Patient was taking hydrochlorothiazide, losartan, and omeprazole daily at home.
Over the next 24 h, her Hb decreased from 8.0 g/dL to 6.1 g/dL. Two days after she received rasburicase, her Hb level further worsened to 5.3 g/dL and her bilirubin went up to 7.8 mg/dL with direct bilirubin 1.35 mg/dL. Haptoglobin was down to ≤ 20 mg/dL. Platelets also trended down to 95 × 103/µL. Her direct antiglobulin test was negative and her G6PD level tested in whole blood sample was 13.0 U/g Hb (7.0 - 20.5 U/g Hb). Since the patient was at risk for methemoglobinemia, arterial blood gas was collected, which showed normal oxygen saturation. She was given two units pRBCs which improved her Hb to 7.2, but the next day it was found to be low again to 6.1. She received another unit of pRBC and Hb improved to 7.9. It continued to improve over the next 3 days and stayed stable thereafter. Other hemolysis parameters continued to improve to normal over the next 3 days. Table 1 shows comparison of lab values pre- and post-rasburicase administration. Figure 1 shows Hb trend during admission, and Figure 2 shows haptoglobin trend during admission.
 Click to view | Table 1. Patient Lab Results Pre- and Post-Rasburicase Administration |
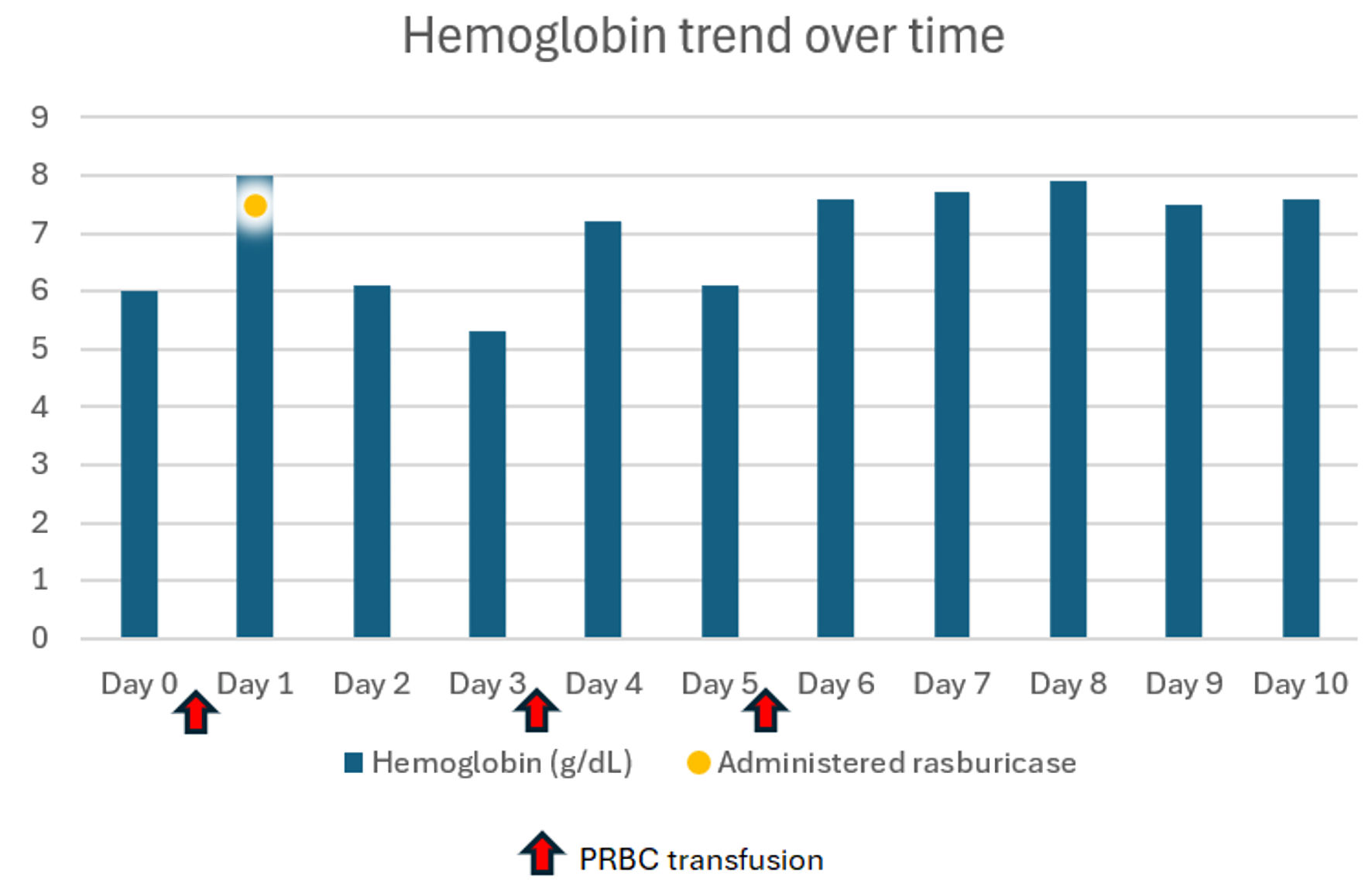 Click for large image | Figure 1. Hemoglobin trend during admission. PRBC: packed red blood cells. |
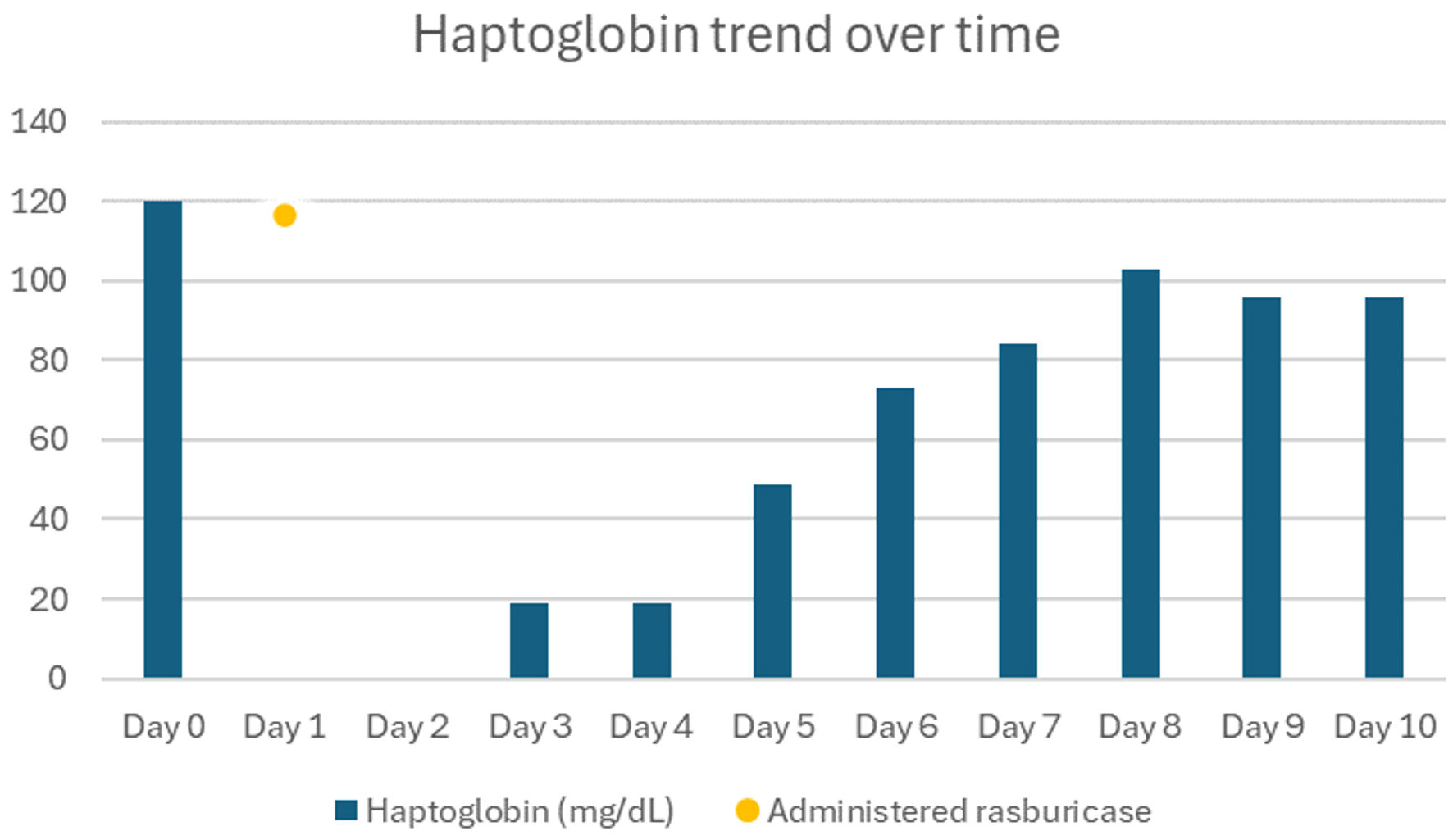 Click for large image | Figure 2. Haptoglobin trend during admission. |
Meanwhile, she underwent further evaluation and treatment of her leukemia. A bone marrow biopsy was performed which demonstrated B-lymphoblastic leukemia. Flow cytometry performed on the bone marrow aspirate showed 95% B lymphoblasts. BCR/ABL1 translocation t(9;22) was not detected. Cytogenetics showed abnormal female karyotype 46, XX, del(1)(q32q42)[15]/46, XX[5]. Computed tomography (CT) chest, abdomen, and pelvis showed multiple cervical lymphadenopathies, with a spleen size at the upper limit of normal. Lumbar puncture was notable for the positive central nervous system (CNS).
She was diagnosed with Ph chromosome-negative B-cell CD20 positive acute lymphoblastic leukemia (Ph- B-ALL) with 1q deletion. She was initiated on therapy with dose-adjusted HyperCVAD (arm A: cyclophosphamide, vincristine, doxorubicin, and dexamethasone; arm B: methotrexate and cytarabine) with growth factor support.
Since the initial evaluation of her G6PD levels was obtained in the context of an acute episode of hemolysis, repeat levels were obtained 3 months later which were 10.0 U/g Hgb. WBC was noted to be 3.6 × 103/µL. The results were within the reference ranges. The patient did not experience any further episodes of hemolysis during the remainder of her treatment.
| Discussion | ▴Top |
The manuscript presents a case of rasburicase-induced hemolytic anemia in a woman without evident G6PD deficiency, as assessed by two normal enzyme assays. This case is unique due to the absence of a diagnosed G6PD deficiency despite the occurrence of acute hemolytic anemia.
Rasburicase is a recombinant urate oxidase enzyme that converts uric acid to allantoin, an inactive and soluble metabolite that is readily excreted in urine. It is used for the treatment of hyperuricemia associated with TLS [6, 7]. TLS is a fatal condition characterized by hyperkalemia, hyperphosphatemia, hyperuricemia, and hypocalcemia due to the abrupt release of cellular components into the bloodstream from cellular turnover. Uric acid, produced during purine breakdown, is a key contributor to the presentation of acute renal failure by causing crystal precipitation within renal tubules. It is commonly seen in highly proliferative tumors, such as high-grade lymphomas and acute leukemias, and can occur before or during the administration of chemotherapy [8]. Severe TLS can even lead to death in 20-40% of cases, necessitating prompt recognition and emergent intervention to prevent harmful effects on vital organs [9]. According to the “Guideline for the Management of Pediatric and Adult Tumor Lysis Syndrome”, TLS should be treated with aggressive hydration and allopurinol or recombinant urate oxidase (rasburicase) [6]. Although rasburicase is generally a safe and effective treatment, it can be associated with the rare and potentially severe complication of hemolytic anemia and methemoglobinemia in G6PD deficient patients [3].
G6PD enzyme plays a vital role in protecting red blood cells (RBCs) from oxidative damage by generating nicotinamide adenine dinucleotide phosphate (NADPH), as a reducing agent. Since RBCs lack alternative pathways for NADPH production, they rely heavily on G6PD to maintain their integrity under oxidative stress [10]. G6PD deficiency is the most common red blood cell enzyme deficiency, impacting around 400 million people globally. This X-linked inherited disorder predominantly affects individuals of African, Southeast Asian, and Mediterranean descent, with a gene frequency ranging from 5% to 25% [11]. In the United States, a notable fraction of the African American male population is afflicted with this deficiency, recording a prevalence rate of almost 10% [12]. However, it is rarely observed in the female population. The risk of severe hemolytic episodes in G6PD-deficient individuals is heightened when they are exposed to certain drugs, foods, or infections [11]. Rasburicase should not be administered to patients with G6PD deficiency because its conversion of uric acid to allantoin generates hydrogen peroxide. Patients with G6PD deficiency cannot detoxify hydrogen peroxide, leading to oxidative damage to the red blood cell membranes. Other etiologies for rasburicase-induced hemolysis have been proposed including an immune-mediated mechanism [13].
The G6PD gene is located on the X chromosome; thus, females can be homozygous or heterozygous, but males can only be hemizygous for the gene. Through lyonization (inactivation of one X chromosome), heterozygous women can express two distinct red blood cell populations, each with varying levels of G6PD activity corresponding to the alleles on the active X chromosome. The relative ratio of the two RBC populations determines the G6PD activity of the female. The resulting overall levels of G6PD enzyme activity in heterozygous females mainly range from 30% to 80% of normal G6PD activity; values within this range are considered intermediate [4].
The gold standard for determining G6PD status is through direct measurement of G6PD activity in lysate from whole RBC with either quantitative or qualitative assays. Qualitative assays like the fluorescent spot test (FST) are more accessible but may struggle to discern intermediate enzyme levels accurately. As a result, these assays cannot accurately identify female patients who are heterozygous for G6PD [14]. These women may be at risk of hemolysis when exposed to oxidative stress. Microscopy or flow cytometry-based assays that assess G6PD levels in individual RBCs can provide valuable insights into the mosaic expression of G6PD alleles in heterozygous female patients [15]. G6PD genotyping overcomes the possibility of a false negative/positive diagnosis in quantitative methods when a change in G6PD activity occurs due to various hematological factors. G6PD genotyping can be performed based on methods such as restriction fragment length polymorphism (RFLP), amplification-refractory mutation system (ARMS), and sequencing. However, these methods are time-consuming, as many steps are required to complete the genotyping process [16].
Additionally, the G6PD screening test can be falsely negative during acute hemolytic episodes, and when the testing is done after blood transfusion, as donor RBCs can mask G6PD deficiency. During acute hemolysis, reticulocytes increase in number. They have higher G6PD enzyme activity than mature RBCs thus giving falsely normal results. Our patient’s reticulocyte counts during acute hemolysis were within normal limits, suggesting hyperproliferation, which was secondary to folate and vitamin B12 deficiency. Another factor to be taken into consideration is the high WBC count which can contribute to the overestimation of G6PD activity in both the reference assay and the standard G6PD test [17]. In our patient, WBC were high during acute hemolysis, but they were low-normal in subsequent G6PD testing thus eliminating the possibility of overestimation. In heterozygous females, the impact of selective hemolysis of G6PD-deficient red cells may compound the effect of reticulocytosis, and the enzyme activity of the residual non-deficient cells can mask the diagnosis of G6PD deficiency. Therefore, it is recommended that when G6PD deficiency is clinically suspected, a repeat assay should be done at 2 - 3 months after resolution of the hemolytic episode [18].
A study was conducted to determine if measuring the ratio of G6PD to pyruvate kinase (PK) was able to differentiate G6PD-heterozygous individuals from the normal population. This additional assay is a reliable diagnostic parameter for mass screening for G6PD deficiency [19].
Diagnosis of hemolytic anemia demands a high index of suspicion, especially with normal G6PD screening during acute hemolysis. Classical findings include decreased Hb, low haptoglobin, indirect hyperbilirubinemia, and elevated LDH. The prevalence of rasburicase-induced hemolytic anemia and methemoglobinemia are estimated to be below 1%, which may be underreported in high-risk ethnicities due to limited studies. Given the Food and Drug Administration (FDA) warning, screening for G6PD deficiency before rasburicase use could be beneficial. However, challenges such as false-negative results and logistical constraints of the long turnaround time of G6PD screening results may preclude patients from successfully being screened before the administration of rasburicase. Due to the high morbidity and mortality associated with delayed treatment of TLS, it is not advisable to delay treatment with rasburicase if necessary, although in such cases close monitoring for adverse effects post-rasburicase initiation remains paramount [20]. A retrospective analysis of G6PD testing for oncology patients before receiving rasburicase was conducted in a tertiary care hospital. The lab test utilized was performed on whole blood samples using a visual semi-quantitative dye-reduction biochemical with a turnaround time of 4 h (240 min) of sample collection [21]. Availability of pre-emptive rapid testing for G6PD deficiency can improve patient safety and reduce the overall expense and suffering associated with caring for oncology patients.
In this case, the patient developed hemolysis despite having normal G6PD levels on two separate occasions including measurements done a long time after the acute episode. A G6PD quantitative assay can be affected by skewed X-inactivation in females with a heterozygous mutation. This phenomenon leads to variability in the expression of the G6PD enzyme among heterozygous females, causing a broad range of enzyme activity levels from normal to deficient [22]. Since females can have a sub-clinical deficiency of G6PD activity despite having “normal” lab values, there is a need to validate the existing lab assays specifically in women. It can also be argued if the hemolytic anemia in this case was truly caused by rasburicase administration and not by a different etiology. Indeed, hematological malignancies including acute leukemia can also often trigger acute hemolysis, usually by immune-mediated pathways. However, there was a very clear temporal relation between the medication administration and the onset of hemolysis. No other clear cause of hemolysis was identified including medications.
Our case highlights the risks of rasburicase administration, spotlighting a woman who developed hemolytic anemia despite normal G6PD levels. This underscores the need for rigorous G6PD deficiency screening, especially in high-risk groups, before initiating rasburicase therapy. The medical community must balance the urgency of treating TLS with ensuring patient safety, recognizing that females, typically considered low risk for G6PD deficiency, can still be vulnerable. Physicians should be aware that rasburicase can induce hemolytic anemia even in patients with normal G6PD levels, indicating possible alternative mechanisms of hemolysis. They must thoroughly screen and closely monitor patients before administering rasburicase, regardless of perceived risk.
Acknowledgments
None to declare.
Financial Disclosure
The case report was not funded.
Conflict of Interest
All authors have no conflict of interest to declare.
Informed Consent
Signed consent has been obtained from the patient.
Author Contributions
Saba Musleh Ud Din and Khine Shan: data collection and article writing. Tauseef Ur Rehman and Stanislav Ivanov: editing and reviewing. Fernando M. Vargas-Madueno: critical revision of the manuscript. All authors reviewed and approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available within the article.
Abbreviations
TLS: tumor lysis syndrome; G6PD: glucose-6-phosphate dehydrogenase; MCV: mean corpuscular volume; LDH: lactate dehydrogenase; Ph- B-ALL: Ph chromosome-negative B-cell CD20 positive acute lymphoblastic leukemia; HyperCVAD: cyclophosphamide, vincristine, doxorubicin, and dexamethasone; CNS: central nervous system; FST: fluorescent spot test
| References | ▴Top |
- Darmon M, Guichard I, Vincent F, Schlemmer B, Azoulay E. Prognostic significance of acute renal injury in acute tumor lysis syndrome. Leuk Lymphoma. 2010;51(2):221-227.
doi pubmed - Cortes J, Moore JO, Maziarz RT, Wetzler M, Craig M, Matous J, Luger S, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28(27):4207-4213.
doi pubmed pmc - Akande M, Audino AN, Tobias JD. Rasburicase-induced hemolytic anemia in an adolescent with unknown glucose-6-phosphate dehydrogenase deficiency. J Pediatr Pharmacol Ther. 2017;22(6):471-475.
doi pubmed pmc - Domingo GJ, Advani N, Satyagraha AW, Sibley CH, Rowley E, Kalnoky M, Cohen J, et al. Addressing the gender-knowledge gap in glucose-6-phosphate dehydrogenase deficiency: challenges and opportunities. Int Health. 2019;11(1):7-14.
doi pubmed pmc - Cheson BD, Heitner Enschede S, Cerri E, Desai M, Potluri J, Lamanna N, Tam C. Tumor lysis syndrome in chronic lymphocytic leukemia with novel targeted agents. Oncologist. 2017;22(11):1283-1291.
doi pubmed pmc - Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778.
doi pubmed - Yu X, Liu L, Nie X, Li J, Zhang J, Zhao L, Wang X. The optimal single-dose regimen of rasburicase for management of tumour lysis syndrome in children and adults: a systematic review and meta-analysis. J Clin Pharm Ther. 2017;42(1):18-26.
doi pubmed - Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis. 2014;21(1):18-26.
doi pubmed pmc - Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127(1):3-11.
doi pubmed - Ng JS, Edwards EM, Egelund TA. Methemoglobinemia induced by rasburicase in a pediatric patient: a case report and literature review. J Oncol Pharm Pract. 2012;18(4):425-431.
doi pubmed - Elyassi AR, Rowshan HH. Perioperative management of the glucose-6-phosphate dehydrogenase deficient patient: a review of literature. Anesth Prog. 2009;56(3):86-91.
doi pubmed pmc - Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Physician. 2005;72(7):1277-1282.
pubmed - Ahmed M, Sanchez T, Norgbe S, Picking CR, Millner PG. Rasburicase-Induced Methemoglobinemia. Cureus. 2021;13(4):e14406.
doi pubmed pmc - LaRue N, Kahn M, Murray M, Leader BT, Bansil P, McGray S, Kalnoky M, et al. Comparison of quantitative and qualitative tests for glucose-6-phosphate dehydrogenase deficiency. Am J Trop Med Hyg. 2014;91(4):854-861.
doi pubmed pmc - Bancone G, Kalnoky M, Chu CS, Chowwiwat N, Kahn M, Malleret B, Wilaisrisak P, et al. The G6PD flow-cytometric assay is a reliable tool for diagnosis of G6PD deficiency in women and anaemic subjects. Sci Rep. 2017;7(1):9822.
doi pubmed pmc - Chamchoy K, Sudsumrit S, Wongwigkan J, Petmitr S, Songdej D, Adams ER, Edwards T, et al. Molecular characterization of G6PD mutations identifies new mutations and a high frequency of intronic variants in Thai females. PLoS One. 2023;18(11):e0294200.
doi pubmed pmc - Pal S, Myburgh J, Bansil P, Hann A, Robertson L, Gerth-Guyette E, Ambler G, et al. Reference and point-of-care testing for G6PD deficiency: Blood disorder interference, contrived specimens, and fingerstick equivalence and precision. PLoS One. 2021;16(9):e0257560.
doi pubmed pmc - Roper D, Layton M, Rees D, Lambert C, Vulliamy T, De la Salle B, D'Souza C, et al. Laboratory diagnosis of G6PD deficiency. A British Society for Haematology Guideline. Br J Haematol. 2020;189(1):24-38.
doi pubmed - Tagarelli A, Piro A, Tagarelli G, Bastone L, Paleari R, Mosca A. G6PD/PK ratio: a reliable parameter to identify glucose-6-phosphate dehydrogenase deficiency associated with microcytic anemia in heterozygous subjects. Clin Biochem. 2004;37(10):863-866.
doi pubmed - Wossmann W, Schrappe M, Meyer U, Zimmermann M, Reiter A. Incidence of tumor lysis syndrome in children with advanced stage Burkitt's lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82(3):160-165.
doi pubmed - Ganapathi M, Campbell P, Ofori K, Aggarwal V, Francis RO, Kratz A. Impact of pre-emptive rapid testing for glucose-6-phosphate dehydrogenase deficiency prior to rasburicase administration at a tertiary care centre: A retrospective study. Br J Clin Pharmacol. 2022;88(9):4163-4170.
doi pubmed - Luzzatto L, Ally M, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Blood. 2020;136(11):1225-1240.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


