| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 10, October 2024, pages 261-266
Combination Therapy With Asciminib and Ponatinib as a Bridge to Brexucabtagene Autoleucel and Maintenance in a Patient With Relapsed Refractory Philadelphia Positive B-Cell Acute Lymphoblastic Leukemia
Alina Amin Muhammada, Erum Mir Ghazib, Amir Alic, Eric Tamd, Karrune Woand, Preet Chaudharyd, George Yaghmourd, Abdullah Ladhad, e
aDepartment of Medicine, Liaquat National Hospital and Medical College, Karachi, Pakistan
bDepartment of Medicine, Ziauddin University, Karachi, Pakistan
cDepartment of Pharmacy, Alfred E. Mann School of Pharmacy and Pharmaceutical Sciences, University of Southern California, Los Angeles, CA, USA
dJane Anne Nohl Division of Hematology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
eCorresponding Author: Abdullah Ladha, Jane Anne Nohl Division of Hematology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA
Manuscript submitted June 24, 2024, accepted August 17, 2024, published online September 20, 2024
Short title: Asciminib and Ponatinib as a Bridge to Brexu-Cel
doi: https://doi.org/10.14740/jmc4287
| Abstract | ▴Top |
Tyrosine kinase inhibitors (TKIs) have changed the prognosis of Philadelphia-positive B-cell acute lymphoblastic leukemia (ALL); however, relapsed and refractory disease after multiple TKIs continues to be a clinical challenge. Brexucabtagene autoleucel (brexu-cel) is a novel FDA-approved therapy for relapsed and refractory ALL. Given the lengthy manufacturing time, bridging therapy is commonly employed prior to brexu-cel. Here we describe a case of a 75-year-old Hispanic male patient with relapsed/refractory Philadelphia-positive B-cell ALL with extramedullary disease involving abdominal lymph nodes and skin. He was initially treated with chemotherapy in combination with imatinib, and later received dasatinib and subsequently blinatumomab and nilotinib. As the patient progressed, he received ponatinib with low-dose salvage chemotherapy and did not show kinase domain mutation. In a final effort, a novel combination of ponatinib with asciminib was used as a bridge therapy before brexu-cel and later as maintenance therapy after brexu-cel. This novel combination was able to control disease prior to brexu-cel for 2 months and maintained remission for at least 10 months. This report shows that the novel combination of ponatinib and asciminib is tolerable and effective as a bridge and maintenance therapy after brexu-cel.
Keywords: Relapsed acute lymphoblastic leukemia; Asciminib; Ponatinib; Brexucabtagene autoleucel
| Introduction | ▴Top |
B-cell acute lymphoblastic leukemia (ALL) is a malignant hematological disease, with a bimodal age distribution, and patients with advanced age have a worse prognosis [1]. Philadelphia chromosome positivity is found in approximately 20-30% of patients, which is traditionally also considered a poor prognostic marker. Although the addition of tyrosine kinase inhibitors (TKIs) to chemotherapy and immunotherapy in upfront and relapsed/refractory settings has significantly improved survival, the acquisition of resistance to TKIs and development of extramedullary disease needs novel interventions [2-4]. Brexucabtagene autoleucel (brexu-cel) is an FDA-approved chimeric antigen receptor T-cell (CAR-T) therapy, for relapsed/refractory B-cell ALL. Real-world data have shown promising data for the maintenance use of TKI after brexu-cel [5, 6]. Here we present a case of relapsed B-cell ALL with extramedullary disease which progressed through imatinib, dasatinib and ponatinib. The novel combination of ponatinib with asciminib effectively worked as a bridge therapy and post brexu-cel maintenance.
| Case Report | ▴Top |
The patient is a 75-year-old Hispanic male, initially diagnosed with CD19+, CD20+, CD22+, CD34+ and Philadelphia-positive B-cell ALL with 90% blasts in October 2020. Cytogenetics showed XY, t(9;22) (q34.1;q11.2),+17,psu dic(17;17)(q25;q25),-21[12]/46,idem,+X[7]/46,XY[1]. Although P210 transcript was detected, the patient did not have any prior splenomegaly or chronic myeloid leukemia history. Initial treatment included multiagent chemotherapy with imatinib (LALA 94 protocol) [7]. Later imatinib was changed to dasatinib due to persistent BCR-ABL1 positivity but after 2 months of dasatinib, BCR-ABL1 was still detected at 0.5%. BCR-ABL was reported as % BCR-ABL1/ABL1 (IS) quantified by real-time polymerase chain reaction (RT-PCR) amplification. The patient was started on blinatumomab clinical trial, later blinatumomab was continued off trial, and nilotinib was added due to persistent BCR-ABL1 PCR detection after > 6 months of therapy. In September 2021, the patient experienced extramedullary relapse involving right cheek skin and abdominal lymph nodes (Fig. 1). Bone marrow (BM) BCR-ABL1 PCR was 0.01% and ABL kinase mutation was not detected. To address extramedullary relapse, the patient was started on a reduced dose of hyperCVAD-part B. After chemotherapy, the patient developed neutropenic fever and enteritis. Subsequent positron emission tomography (PET) scan and BM biopsy (bx) were negative with the major molecular response. The patient was switched to ponatinib for better extramedullary coverage, and later monthly 1 mg vincristine and prednisone were added to continue as low-intensity therapy along with intrathecal (IT) chemotherapy. The patient was not deemed suitable for an allogeneic stem cell transplant. In November 2022, after 1 year of low-intensity therapy, unfortunately, the patient developed central nervous system (CNS)-3 disease. PET scan showed extramedullary disease with pelvic lymphadenopathy and thoracic spine involvement without compression (Fig. 2). BM bx showed 30-40% cellularity, < 5% blasts, BCR ABL1 PCR was 0.095% and minimal residual disease (MRD) flow cytometry showed 0.24%. ABL kinase mutation was again not detected. For ABL kinase mutation testing, the entire ABL1 kinase domain and activation loop was amplified using RT-PCR. Various factors including time from sample collection to testing, amount of nucleic acid and sample preparation can potentially affect its results. The patient was maintained on serial IT chemotherapy composed of 12 mg methotrexate, 30 mg cytarabine and 25 mg of hydrocortisone. The patient had CNS-3 disease, but with serial IT chemotherapy, he converted to CNS-1. In December 2022, reduced dose hyperCVAD-part B was given, ponatinib was increased to 30 mg daily and asciminib was added to 40 mg twice a day. Follow-up BM bx in January 2023 showed normocellular marrow, < 5% blasts, MRD flow cytometry 0.047% and BCR-ABL1 PCR 0.04%. The patient was continued on ponatinib and asciminib combination only, and subsequent peripheral blood BCR-ABL1 PCR was 0.007%. This combination was continued from January 2023 till March 2023 as a bridge to brexu-cel, which was administered on March 20, 2023 after lymphodepleting chemotherapy (fludarabine/cyclophosphamide). The patient also had at least 1 week washout period off TKIs prior to collection. The patient was started on ponatinib 15 mg daily and asciminib 40 mg bid approximately 3 weeks after brexu-cel. Day +90 PET scan in June 2023 did not show any abnormal findings, and serial peripheral blood BCR-ABL1 PCR was undetected for 10 months. The patient had periodic electrocardiograms (EKGs), which did not show QTc prolongation. Transaminitis or thrombocytopenia was not observed. Table 1 provides details of the treatment timeline and response assessment. In February 2024, unfortunately the patient presented with new thrombocytopenia and BM bx showed 30-40% focal aggregates of residual B-cell ALL, BCR-ABL1 PCR was 12.9% and ABL kinase mutation was not detected. Extramedullary and CNS diseases were not detected. While the patient was referred for a CAR-T trial, a repeated course of salvage blinatumomab was started. The patient was able to attain complete remission with undetectable BCR-ABL1 PCR, and subsequently was also started on nilotinib 200 mg twice a day.
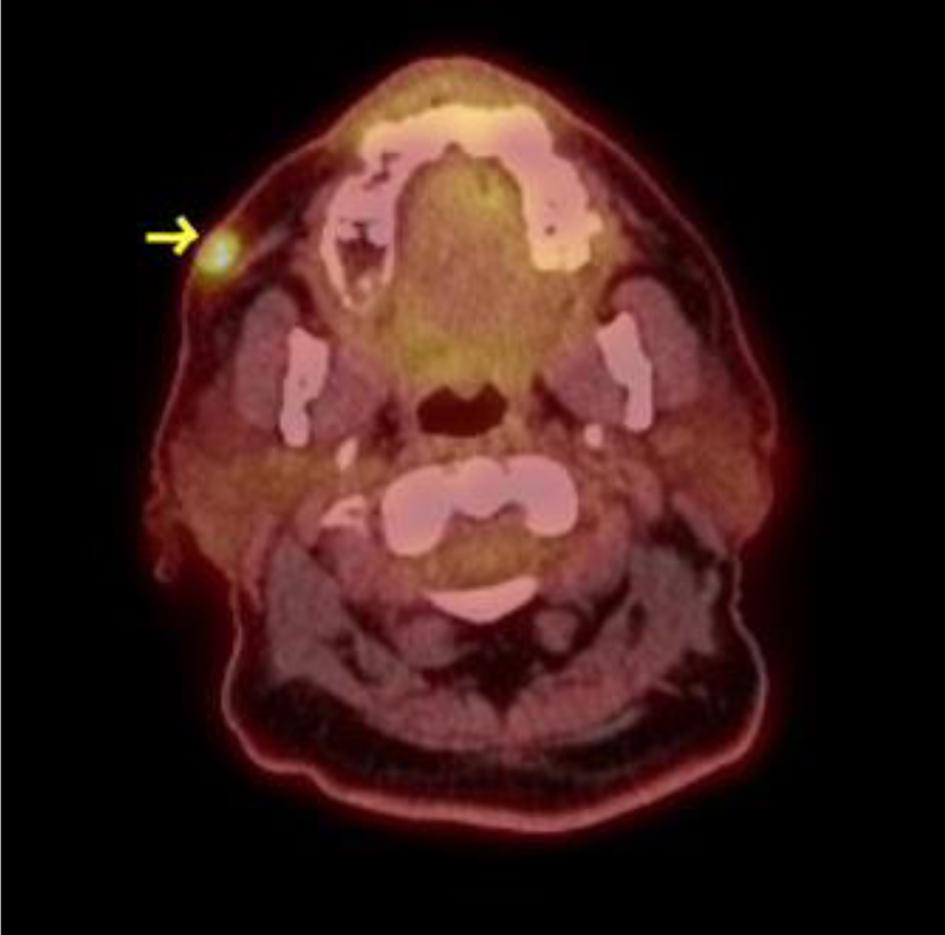 Click for large image | Figure 1. Right cheek involvement with Ph+ B-cell ALL. Arrow indicates 11 mm right cheek skin involvement with Ph+ B-cell ALL, showing hypermetabolic activity on PET scan (SUV 5.9). ALL: acute lymphoblastic leukemia; PET: positron emission tomography; SUV: standard uptake value. |
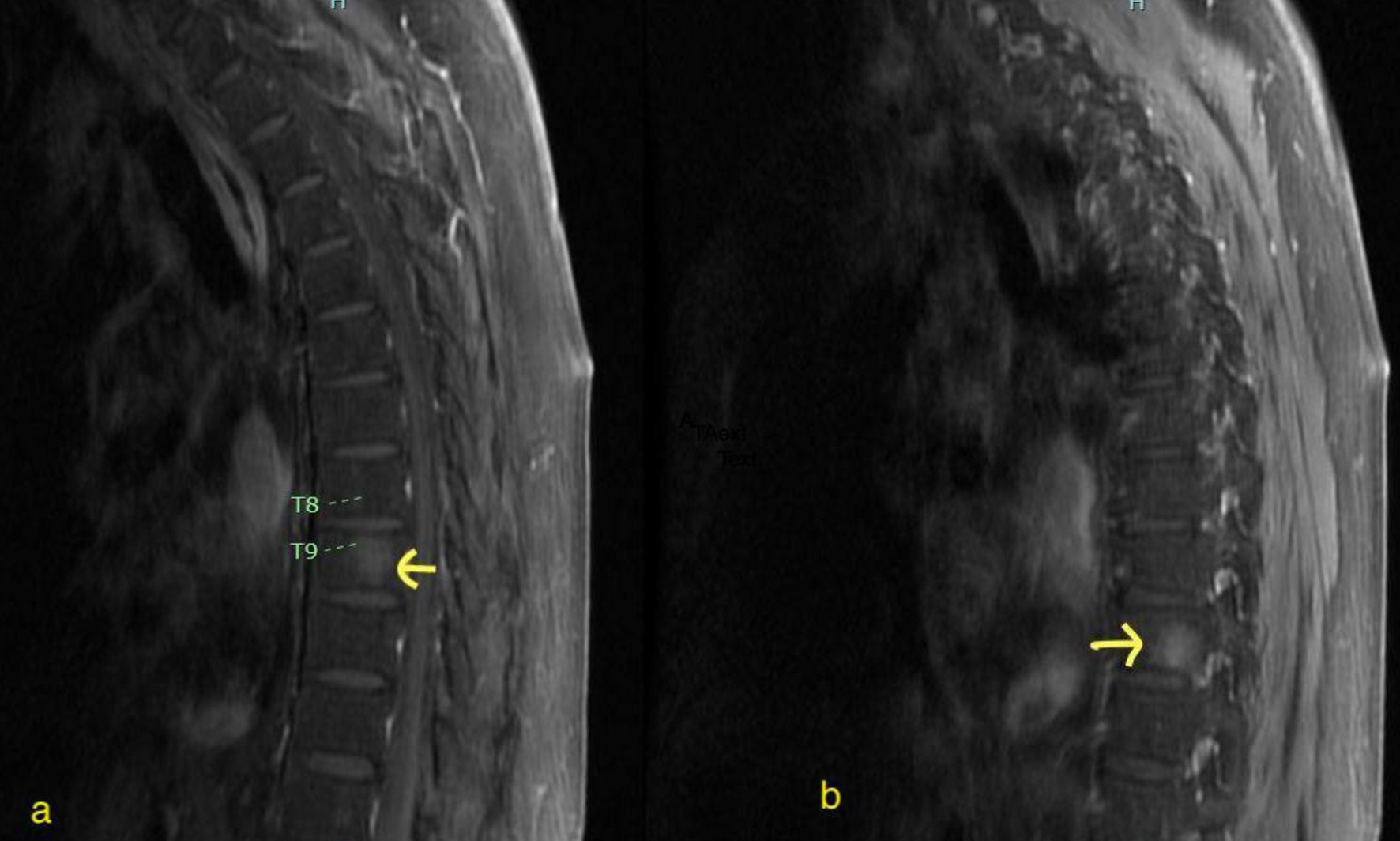 Click for large image | Figure 2. Extramedullary disease involving T9 vertebral body (a) and T10 vertebral body (b). Arrows indicate hyperintense enhancing lesions on MRI, involving T9 (right) and T10 (left) vertebral body with Ph+ B-cell ALL. ALL: acute lymphoblastic leukemia; MRI: magnetic resonance imaging. |
 Click to view | Table 1. Timeline of Treatment and Response Assessment |
| Discussion | ▴Top |
TKIs play a key role in the treatment of Ph+ B-cell ALL but its clinical use around administration of brexu-cel is not standardized. In this case, the patient had progressed with multiple TKIs including imatinib, dasatinib and ponatinib and later developed extramedullary disease. Asciminib has been shown to overcome ponatinib resistance in pre-clinical studies, and we combined ponatinib with asciminib as a bridge to brexu-cel and also used this combination as maintenance therapy after brexu-cel. This novel combination safely maintained remission for 10 months after brexu-cel. This case report highlights the tolerability and effectiveness of combining ponatinib with asciminib around the use of brexu-cel in a patient with relapsed/refractory extramedullary disease.
Brexu-cel is approved for relapsed Ph+ B-cell ALL, but bridging therapy for frail older patients can be challenging, especially when patients present with extramedullary involvement. In our report, the patient had significant toxicities from reduced dose chemotherapy and was already exposed to various BCR-ABL TKIs including imatinib, dasatinib and ponatinib. When the patient developed extramedullary disease on 15 mg daily ponatinib, we used reduced dose chemotherapy (mini-hyperCVD-part B), increased ponatinib to 30 mg daily, and later added asciminib 40 mg twice a day. The novel combination of ponatinib and asciminib effectively controlled the disease for 2 months as a bridge to brexu-cel and later the patient was maintained on low-dose ponatinib 15 mg daily with asciminib 40 mg twice daily and maintained 10 months in remission.
Asciminib is a myristoyl pocket inhibitor, it does not affect ATP binding site, which is responsible for most of the resistant mutations, and also it is not affected by TKI efflux transporters [8]. It can work synergistically to overcome resistance against available TKI [9, 10]. Clinical efficacy of this combination resulted in long-term remission of approximately 17 months in a case report of relapsed/refractory disease [11]. Another case report demonstrated the effectiveness of ponatinib with less intense chemotherapy as a bridge to cell therapy and as a subsequent maintenance [12]. The patient in our report had progressed with extramedullary disease while on 15 mg ponatinib/vincristine/prednisone, and we decided to add asciminib to ponatinib based on preclinical efficacy of combination, which exhibited strong inhibitory effects on the growth and survival of BCR-ABL1+ cell lines, including leukemic cells with compound mutations [9, 10]. ZUMA-3, a phase 2 trial, assessed the efficacy of brexu-cel in relapsed/refractory disease with a median relapse-free survival of 11.6 months, and only 11% of patients had extramedullary disease. Multicenter real-world data showed a median progression-free survival (PFS) of 8.6 months [6, 13]. We report 10 months of remission on novel combination maintenance post brexu-cel in a patient with relapsed/refractory extramedullary disease.
In vitro studies have shared concerns that second-generation TKIs like dasatinib and ponatinib inhibit dual ABL/Src pathway leading to T-cell exhaustion and reducing CAR-T and blinatumomab efficacy [14, 15]. On the other hand, asciminib is a BCR-ABL1 fusion protein inhibitor specifically targeting the ABL myristoyl pocket (STAMP) and it is not shown to be associated with T-cell exhaustion [16]. Interestingly, a preclinical study showed that dasatinib exposure to CAR-T during ex vivo expansion inhibited T-cell activation signaling and reduced CAR-T cell differentiation and exhaustion, which resulted in increased in vivo efficacy and CAR-T persistence. At the same time, simultaneous administration of dasatinib and CAR-T cells resulted in reduced in vitro and in vivo efficacy [17]. These preclinical findings are not consistently replicated in human clinical trials. A phase 2 clinical trial combining immunotherapy (blinatumomab) with ponatinib has shown a complete molecular response rate of 87% and 79% in newly diagnosed Ph+ B-cell ALL and refractory disease, respectively [4]. Also, in the real-world studies comprising multiple transplant centers, maintenance post brexu-cel including the use of TKI was associated with improved PFS [6].
Although kinase domain mutational analysis was negative, there are reports of kinase domain independent mechanisms for resistance to ponatinib including changes in influx and efflux transporters, deregulation of PI3K, JAK-STAT pathway and increased expression of AXL kinase pathways [18, 19]. We did not find any kinase domain mutations at relapses, thus we speculate that the disease progressed due to a kinase domain-independent mechanism and the addition of asciminib was clinically effective in this scenario. Clinical trials are underway to study novel TKI combinations (NCT03595917) [20]. There is a lack of standardization regarding the use of TKI maintenance after brexu-cel. We used asciminib in combination with ponatinib to potentially restore the efficacy of ponatinib, and asciminib itself can also be an effective option for bridge therapy. Further clinical trials and real-world studies will shed light on preferred single agent or combination TKIs-based maintenance and sequencing of TKI with CAR-T.
Learning points
This case shows that patients can become resistant to different lines of TKIs without mutations in tyrosine kinase domain. A novel combination of TKIs with different targets on leukemia cells can be effective in controlling disease. Ponatinib is pan-BCR-ABL TKI while asciminib targets ABL myristoyl pocket. Their combination can synergistically inhibit ABL1 kinase activity to effectively control disease and maintain remission after brexu-cel.
Acknowledgments
None to declare.
Financial Disclosure
Chaudhary: Oncotartis: Consultancy; Pancella: Consultancy; Angeles Therapeutics: Consultancy, Current equity holder in private company; Moderna: Current equity holder in publicly-traded company; Celldex: Current equity holder in publicly-traded company; TCR2: Current equity holder in publicly-traded company; Allogene: Current equity holder in publicly-traded company; Athelas: Consultancy. Yaghmour: Takeda: Consultancy, Speakers Bureau; Astellas: Speakers Bureau; Alexion: Speakers Bureau; BMS: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Agios: Consultancy, Speakers Bureau; Jazz: Speakers Bureau. Ladha: Pfizer: Honoraria.
Conflict of Interest
Authors do not have any competing interest pertaining to this submission.
Informed Consent
Institutional guidelines were followed and informed consent was obtained from the patient.
Author Contributions
Writing - original draft: Alina Amin Muhammad, Erum Mir Ghazi, Amir Ali, Eric Tam, Karrune Woan, Preet Chaudhary, George Yaghmour, Abdullah Ladha. Writing - review and editing: Alina Amin Muhammad, Erum Mir Ghazi, Amir Ali, Eric Tam, Karrune Woan, Preet Chaudhary, George Yaghmour, Abdullah Ladha. All authors certify that he or she has participated sufficiently in the intellectual content. Each author has reviewed the final version of the manuscript and approves it for publication.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet. 2020;395(10230):1146-1162.
doi pubmed - Kantarjian H, Short NJ, Jain N, Sasaki K, Huang X, Haddad FG, Khouri I, et al. Frontline combination of ponatinib and hyper-CVAD in Philadelphia chromosome-positive acute lymphoblastic leukemia: 80-months follow-up results. Am J Hematol. 2023;98(3):493-501.
doi pubmed - Foa R, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo MC, Canichella M, et al. Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383(17):1613-1623.
doi pubmed - Jabbour E, Short NJ, Jain N, Huang X, Montalban-Bravo G, Banerjee P, Rezvani K, et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: a US, single-centre, single-arm, phase 2 trial. Lancet Haematol. 2023;10(1):e24-e34.
doi pubmed - Bouchkouj N, Lin X, Wang X, Przepiorka D, Xu Z, Purohit-Sheth T, Theoret M. FDA approval summary: brexucabtagene autoleucel for treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Oncologist. 2022;27(10):892-899.
doi pubmed pmc - Roloff GW, Aldoss I, Kopmar NE, Lin C, Dekker SE, Gupta VK, Jeyakumar N, et al. Brexucabtagene autoleucel in adults with relapsed/refractory B-Cell ALL: outcomes and novel insights from the real-world outcomes collaborative of CAR T in adult ALL (ROCCA). Blood. 2023;142:1030.
- Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, Kovacsovics T, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22(20):4075-4086.
doi pubmed - Deeks ED. Asciminib: first approval. Drugs. 2022;82(2):219-226.
doi pubmed - Gleixner KV, Filik Y, Berger D, Schewzik C, Stefanzl G, Sadovnik I, Degenfeld-Schonburg L, et al. Asciminib and ponatinib exert synergistic anti-neoplastic effects on CML cells expressing BCR-ABL1 (T315I)-compound mutations. Am J Cancer Res. 2021;11(9):4470-4484.
pubmed pmc - Eide CA, Zabriskie MS, Savage Stevens SL, Antelope O, Vellore NA, Than H, Schultz AR, et al. Combining the allosteric inhibitor asciminib with ponatinib suppresses emergence of and restores efficacy against highly resistant BCR-ABL1 mutants. Cancer Cell. 2019;36(4):431-443.e435.
doi pubmed pmc - Canale FA, Pitea M, Alati C, Porto G, Pratico G, Utano G, Germano J, et al. Case Report: CAR-T cells and subsequent maintenance with ponatinib in an adult Philadelphia acute lymphoblastic leukemia patient with hematological and extramedullary relapse after allogeneic stem cell transplantation. Eur J Haematol. 2024;112(1):137-140.
doi pubmed - Zerbit J, Tamburini J, Goldwirt L, Decroocq J, Cayuela JM, Chapuis N, Contejean A, et al. Asciminib and ponatinib combination in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leuk Lymphoma. 2021;62(14):3558-3560.
doi pubmed - Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, Leguay T, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502.
doi pubmed - Kauer J, Marklin M, Pflugler M, Horner S, Hinterleitner C, Tandler C, Jung G, et al. BCR::ABL1 tyrosine kinase inhibitors hamper the therapeutic efficacy of blinatumomab in vitro. J Cancer Res Clin Oncol. 2022;148(10):2759-2771.
doi pubmed pmc - Leonard JT, Kosaka Y, Malla P, LaTocha D, Lamble A, Hayes-Lattin B, Byrd K, et al. Concomitant use of a dual Src/ABL kinase inhibitor eliminates the in vitro efficacy of blinatumomab against Ph+ ALL. Blood. 2021;137(7):939-944.
doi pubmed pmc - Haselbarth L, Karow A, Mentz K, Bottcher M, Roche-Lancaster O, Krumbholz M, Jitschin R, et al. Effects of the STAMP-inhibitor asciminib on T cell activation and metabolic fitness compared to tyrosine kinase inhibition by imatinib, dasatinib, and nilotinib. Cancer Immunol Immunother. 2023;72(6):1661-1672.
doi pubmed pmc - Zhang H, Hu Y, Shao M, Teng X, Jiang P, Wang X, Wang H, et al. Dasatinib enhances anti-leukemia efficacy of chimeric antigen receptor T cells by inhibiting cell differentiation and exhaustion. J Hematol Oncol. 2021;14(1):113.
doi pubmed pmc - Dufies M, Jacquel A, Belhacene N, Robert G, Cluzeau T, Luciano F, Cassuto JP, et al. Mechanisms of AXL overexpression and function in Imatinib-resistant chronic myeloid leukemia cells. Oncotarget. 2011;2(11):874-885.
doi pubmed pmc - Quentmeier H, Eberth S, Romani J, Zaborski M, Drexler HG. BCR-ABL1-independent PI3Kinase activation causing imatinib-resistance. J Hematol Oncol. 2011;4:6.
doi pubmed pmc - National Library of Medicine. ClinicalTrial.gov [internet]. ABL001 + Dasatinib + Prednisone + Blinatumomab in BCR-ABL+ B-ALL or CML; 2023 [Cited March, 2024]. https://www.clinicaltrials.gov/study/NCT03595917.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


