| Journal of Medical Cases, ISSN 1923-4155 print, 1923-4163 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Med Cases and Elmer Press Inc |
| Journal website https://www.journalmc.org |
Case Report
Volume 15, Number 11, November 2024, pages 359-366
Intravitreal Fluconazole Injection for Fungal Endophthalmitis as Treatment Option in a Patient With End-Stage Liver and Kidney Diseases
Toshihiko Matsuoa, b, c, h, Yasuyuki Kobayashid, e, Shingo Nishimurad, e, Naoko Yoshiokaf, Yasushi Takahashig, Yasutaka Iguchig
aGraduate School of Interdisciplinary Science and Engineering in Health Systems, Okayama University, Okayama City 700-8558, Japan
bDepartment of Ophthalmology, Okayama University Hospital, Okayama City 700-8558, Japan
cEye Clinic, Ochiai Hospital, Maniwa City 719-3197, Japan
dDepartment of Urology, Okayama University Hospital, Okayama City 700-8558, Japan
eUrology Clinic, Ochiai Hospital, Maniwa City 719-3197, Japan
fDepartment of Gastroenterology and Hepatology, Kawasaki Medical School, Kurashiki City 701-0192, Japan
gDepartment of Internal Medicine, Ochiai Hospital, Maniwa City 719-3197, Japan
hCorresponding Author: Toshihiko Matsuo, Regenerative and Reconstructive Medicine (Ophthalmology), Graduate School of Interdisciplinary Science and Engineering in Health Systems, Okayama University, Shikata-cho 2-5-1, Okayama City 700-8558, Japan
Manuscript submitted July 23, 2024, accepted October 1, 2024, published online October 10, 2024
Short title: Intravitreal Fluconazole Injection
doi: https://doi.org/10.14740/jmc4302
| Abstract | ▴Top |
Endogenous endophthalmitis is an infectious disease of the intraocular tissue that is a consequence of bloodstream infection. The efficacy of intravitreal fluconazole injection to assist low-dose oral fluconazole in fungal endophthalmitis remains unknown in older adults with advanced liver and renal disease. In this case report, a 78-year-old man with hepatitis C virus-related liver cirrhosis and hepatocellular carcinoma who also had end-stage renal disease with temporary nephrostomy noticed blurred vision and showed a large retinal infiltrate with vitreous opacity in the right eye. In the clinical diagnosis of endogenous fungal endophthalmitis, he had an intravitreal injection of 0.1% fluconazole in 0.2 - 0.3 mL every 2 weeks four times in total, in addition to a minimum dose of oral fluconazole. One month before the ophthalmic presentation, he developed a fever and computed tomography scan showed ureterolithiasis with hydronephrosis on the right side, indicating that the renal pelvic stone fell into the ureter. He underwent nephrostomy tube insertion on the right side in the diagnosis of obstructive urinary tract infection. In the course, a potassium hydroxide (KOH) preparation of the urine sediments which were obtained from the nephrostomy tube showed yeast-like fungi, suggestive of Candida, 1 week before the development of eye symptoms. One week after the ophthalmic presentation, the nephrostomy tube at 14 Fr (French gauge) which had been inserted 1 month previously was replaced with a new tube with a larger size at 16 Fr because urine excretion from the tube was reduced. Immediately after the exchange of the nephrostomy tube, a large volume of urine was excreted from the tube. In a week, he had no systemic symptoms and serum C-reactive protein became low. In the meantime, the retinal infiltrate became inactive and vitreous opacity resolved. Intravitreal fluconazole injection is a treatment option for fungal endophthalmitis in the case that a patient cannot undergo vitrectomy and cannot take a maximum dose of fluconazole because of poor renal function.
Keywords: Fungal endophthalmitis; Intravitreal injection; Fluconazole; Nephrostomy; Urinary tract infection; Ureterolithiasis
| Introduction | ▴Top |
Endogenous endophthalmitis is an infectious disease of the intraocular tissue that is a consequence of bloodstream infection [1-5]. Causative agents for the infection are mostly bacteria and fungi [1-6], and also acanthamoeba on rare occasions [7]. The typical manifestation is the yellowish retinal infiltrates with vitreous opacity. Endogenous bacterial endophthalmitis is an ophthalmic emergency which shows rapid deterioration and requires early interventions such as intravitreal injection of antibiotics and vitrectomy [1-5]. The well-known primary sources and common infectious agents for endogenous bacterial endophthalmitis are pneumonia with Streptococcus pneumoniae and liver abscess with Enterococcus faecalis [1-5].
In contrast, endogenous fungal endophthalmitis manifests as isolated and spotty retinal infiltrates with vitreous opacity and usually takes a slower course of deterioration [8]. Frequent primary infectious sources for endogenous fungal endophthalmitis are central line-associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) [9-12]. Urinary tract infection leads to bloodstream infection when the urinary tract has obstruction often by stones as in nephrolithiasis [13-15]. Candida is a most prevalent infectious agent among fungi in bloodstream infection which is precipitated by urinary tract infection [9-15].
Urinary tract infection and bloodstream infection by fungi, especially Candida, occur in older adults with systemic illness [9-15]. Under the circumstances, dose escalation in antifungal drug therapy would be limited due to the poor renal function, and surgical intervention such as vitrectomy would not be performed. To assist the systemic antifungal therapy, a local therapy should be designed. In this study, we report intravitreal fluconazole injection to assist a low dose of oral fluconazole due to poor renal function in an old man with end-stage liver and kidney diseases who had temporary insertion of a nephrostomy tube for ureterolithiasis and nephrolithiasis.
| Case Report | ▴Top |
A 78-year-old man noticed foggy vision in the right eye and was referred for ophthalmic examinations. The best-corrected visual acuity in decimals was 0.3 in the right eye and 1.2 in the left eye. The intraocular pressure was 2 mm Hg in the right eye and 8 mm Hg in the left eye. He had intraocular lens implantation in both eyes by cataract surgeries 4 years previously. He showed a small number of aqueous cells and diffuse vitreous opacity with a large yellowish-white fluffy retinal infiltrate in the inferotemporal midperiphery of the fundus in the right eye (Fig. 1a-d), indicative of fungal endophthalmitis. The left eye had nothing abnormal to be noted (Fig. 1e-h). He was ambulatory with good appetite and no fever. The pulse rate was 67/min, and the systolic and diastolic blood pressures were 105 and 62 mm Hg, respectively. He had been followed monthly for hepatitis C virus-related liver cirrhosis and multiple hepatic cancer, coupled with diabetes mellitus in several years. Diabetes was basically controlled with insulin glulisine prior to every meal and insulin glargine once at night. He took oral levothyroxine sodium hydrate 100 µg daily for hypothyroidism.
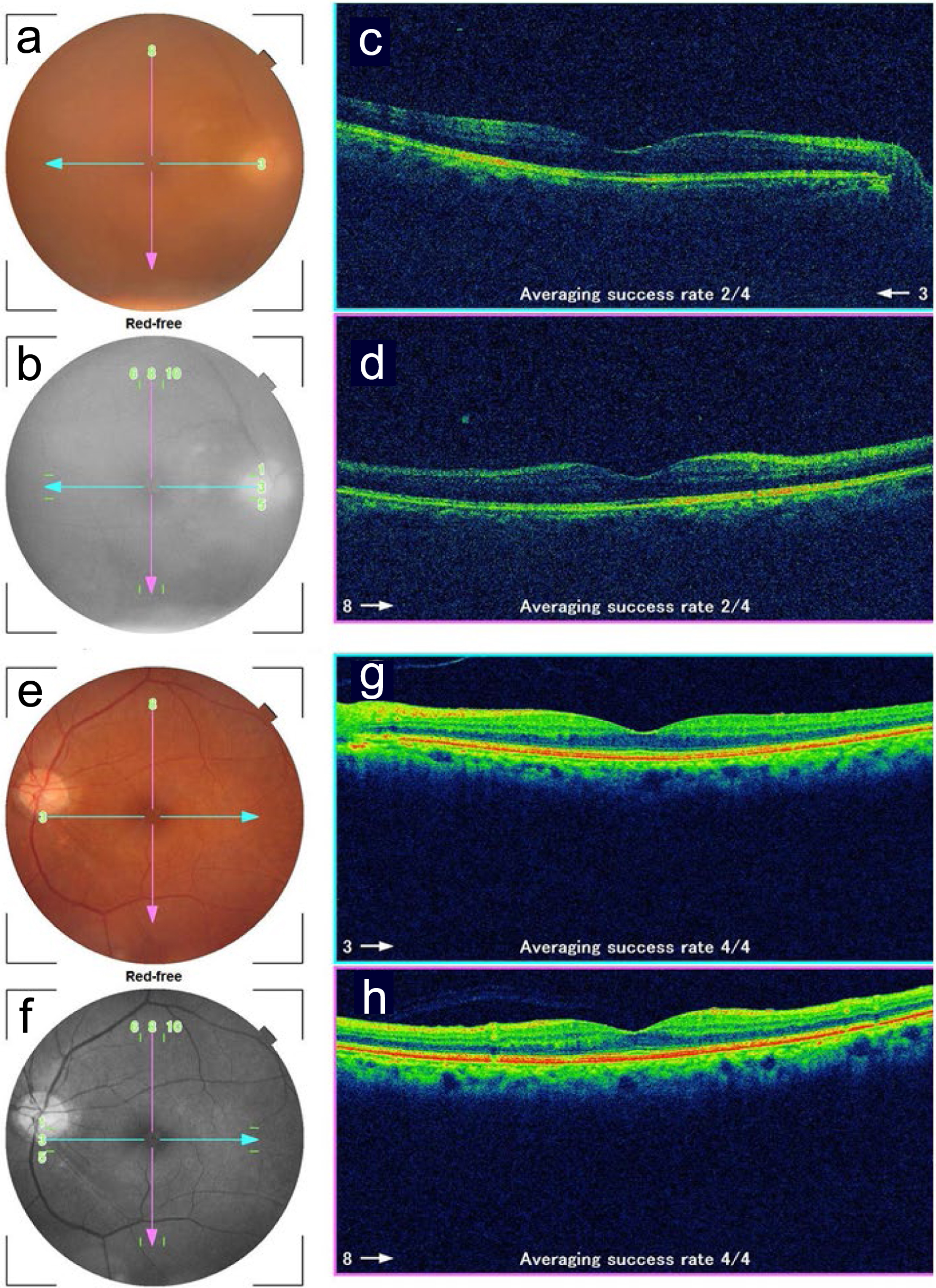 Click for large image | Figure 1. Fundus photographs and optical coherence tomography in the right eye (a-d) and left eye (e-h) at the initial ophthalmic presentation. a and e: color photographs; b and f: red-free photographs; c and g: horizontal sections designated by blue arrows in fundus photographs; d and h: vertical sections designated by pink arrows in fundus photographs. Note blurred images due to vitreous opacity in the right eye (a, c, d). |
In past history, he was diagnosed as diabetes mellitus and hypertension at the age of 57 years. He was diagnosed as hepatocellular carcinoma at the age of 63 years and underwent radiofrequency ablation of the lesions four times, in addition to partial hepatectomy (S8) at the age of 67 years, in the following 7 years. Afterwards in 5 years, he was stationary until the age of 75 years when magnetic resonance imaging (MRI) showed relapse of hepatocellular carcinoma. Oral lenvatinib mesylate (multitarget tyrosine kinase inhibitor) 4 mg daily for hepatocellular carcinoma was started and discontinued in 1.5 years. He then had intravenous bevacizumab twice in 2 months and intravenous atezolizumab (anti-PD-L1) 1,200 mg every 3 weeks eight times in half a year until 3 months before the ophthalmic presentation. He underwent surgeries for diaphragmatic hernia, complicated with intestinal perforation, 4 years previously.
Three months before the ophthalmic presentation, computed tomography (CT) scan from the chest, abdomen to pelvis showed a nodular lesion in right upper lobe (Fig. 2a) as sequelae to pulmonary abscess which he had experienced 1.5 years previously, in addition to multiple low-density areas in both lobes of cirrhotic liver (Fig. 2b), indicative of stable hepatocellular carcinoma, and bilateral nephrolithiasis (Fig. 2c). Two months before the ophthalmic presentation, he experienced bleeding from the duodenal ulcer. He had no medication for hepatocellular carcinoma.
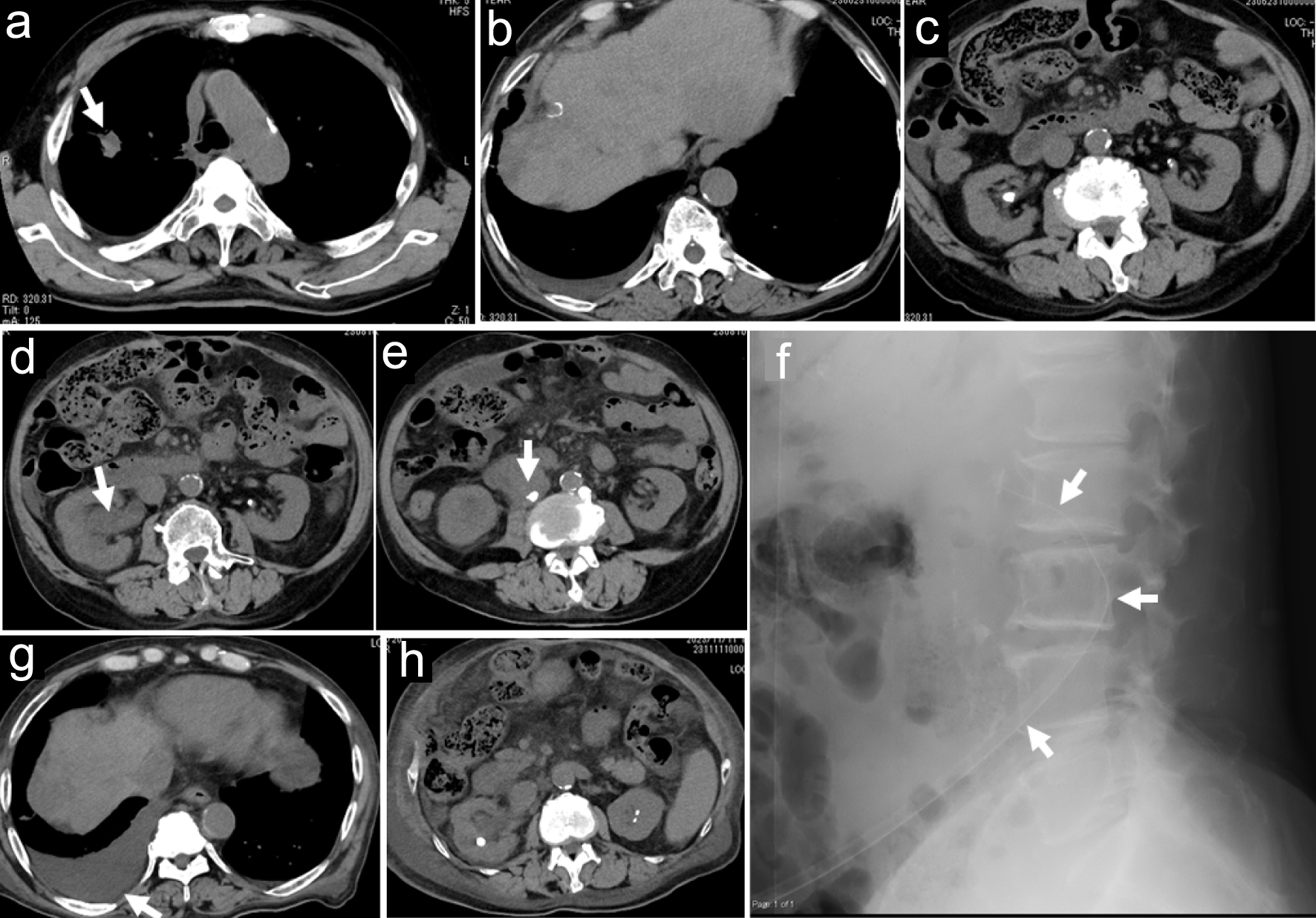 Click for large image | Figure 2. Axial images of computed tomography scans. Nodular lesion in right upper lobe (arrow in a), multiple low-density areas in cirrhotic liver (b), and bilateral nephrolithiasis (c) 3 months before the initial ophthalmic presentation. Ureterolithiasis (arrow in e) with hydronephrosis (arrow in d) on the right side at the time of fever onset, a month before the initial ophthalmic presentation. Lateral view of abdomen X-ray (f) at replacement of a nephrostomy tube (arrows in f) a week after the initial ophthalmic presentation. Stable hepatic cancer in cirrhotic liver (g) and subsiding hydronephrosis (h) 2 months after the initial ophthalmic presentation. |
One month before the ophthalmic presentation, he developed a fever up to 37.6 °C and was hospitalized for urinary tract infection. Repeat CT scan showed ureterolithiasis with hydronephrosis on the right side (Fig. 2d, e), and indicated that the renal pelvic stone fell into the ureter, suggestive of obstructive urinary tract infection. The pulmonary nodule on the right side and multiple low-density areas of the cirrhotic liver were stable. The patient had a ureter stent placed on the right side. However, 10 days later, because of poor drainage efficiency, he underwent nephrostomy tube insertion on the right side at a different large hospital.
At that time of fever onset, white blood cell count was 9,720/µL with differentials of 1.5% stab neutrophils, 88.5% segmented neutrophils, 7% lymphocytes, and 3% monocytes. Red blood cell count was 3.01 × 106/µL, and platelets were low at 8.9 × 104/µL. Hemoglobin was 9.7 g/dL. The casual blood glucose was 141 mg/dL, and hemoglobin A1c was 6.9%. Total protein was 7.0 g/dL, while albumin was low at 2.5 g/dL. Urinary nitrogen was high at 46.2 mg/dL, creatinine was high at 2.92 mg/dL, and estimated glomerular filtration rate was reduced to 17 mL/min/1.73 m2. The other liver function tests were in the normal range. C-reactive protein (CRP) was elevated to 10.39 mg/dL. Urinalysis showed 100 or more leukocytes per high power field, together with 4+ glucose and 2+ protein. Urinary culture was negative for aerobic and anaerobic bacteria and also for fungi. He was given intravenous ceftriaxone 1 g daily for a week, meropenem 1 g daily, together with fosfomycin 2 g daily for the following 2 weeks, and then, meropenem 0.5 g daily for the additional 1 week as empirical treatment. In the course, a potassium hydroxide (KOH) preparation of the urine sediments which were obtained from the nephrostomy tube showed yeast-like fungi, suggestive of Candida, 1 week before the development of eye symptoms.
At the time of ophthalmic presentation, 1 month later from the fever onset, he had neither general fatigue nor fever. White blood cell count was 3,190/µL and CRP was 2.96 mg/dL. Serum β-D-glucan was markedly elevated to 146.6 pg/mL. He started to have intravenous fluconazole 50 mg daily for 2 weeks as a minimal dose because of end-stage renal disease. As topical treatment for the right eye, he began to have eye drops of 0.1% fluconazole (50 mg/50 mL injection solution), eight times daily, 0.1% betamethasone four times daily, 0.5% levofloxacin four times daily, and 0.1% bromfenac twice daily. One week after the ophthalmic presentation, he had low-grade fever and appetite loss. CRP was elevated again to 5.38 mg/dL. The nephrostomy tube at 14 Fr (French gauge) which had been inserted 1 month previously was replaced with a new tube with a larger size at 16 Fr (removable funnel catheter, Nephrostomy Balloon Catheter, Create Medic Co., Ltd., Yokohama, Japan, Fig. 2f) because urine excretion from the tube was reduced. Immediately after the exchange of the nephrostomy tube, a large volume (50 mL) of urine was excreted from the tube. In a week, he had no systemic symptoms and CRP became low at 1.94 mg/dL.
Meanwhile, the retinal infiltrate became enlarged with high activity and the vitreous opacity increased in the right eye in 2 weeks, leading to the decrease of visual acuity in the right eye to counting fingers. He thus, underwent intravitreal fluconazole injection (50 mg/50 mL injection solution) at 0.2 mL in the right eye. The daily intravenous fluconazole was switched to oral fluconazole 50 mg daily, and he was discharged based on his wish to go back home. Additional intravitreal fluconazole injection 0.2 - 0.3 mL was repeated every 2 weeks in 2 months four times in total, resulting in the recovery of visual acuity in the right eye to 0.1. In 2 months after the ophthalmic presentation, he showed gradual clearing of the vitreous opacity to allow the visualization of the enlarged retinal infiltrate (Fig. 3a-h). Oral fluconazole was discontinued. At this time, the nephrostomy tube which had been in the right place for the preceding 2 months was found to be spontaneously dislodged probably by body movement with its tip located out of the kidney in the subcutaneous tissue. The reinsertion was tried but was difficult to be passed through the renal capsule, and was thus decided to be removed since hydronephrosis on the right side had subsided on CT scan (Fig. 2g, h).
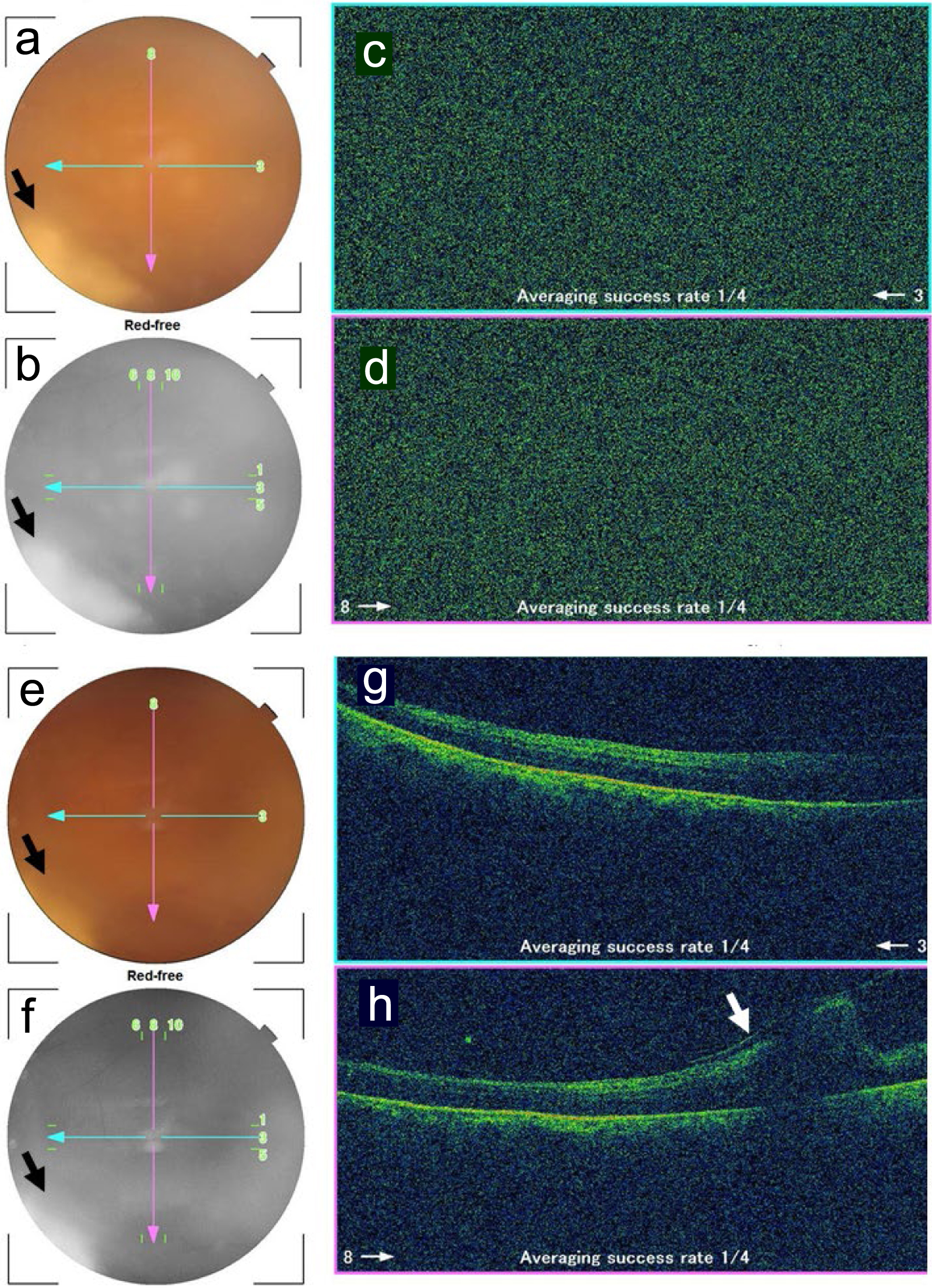 Click for large image | Figure 3. Fundus photographs and optical coherence tomography in the right eye 1.5 months (a-d) and 2 months (e-h) after the initial ophthalmic presentation. a and e: color photographs; b and f: red-free photographs; c and g: horizontal sections designated by blue arrows in fundus photographs; d and h: vertical sections designated by pink arrows in fundus photographs. No images are depicted by optical coherence tomography due to vitreous opacity (c, d) while fluffy retinal infiltrate is visualized in the inferotemporal midperiphery (arrows in a, b, e, f). Note retinal fold (arrow in h) inferior to the macula, caused by vitreoretinal traction. |
Three months after the ophthalmic presentation, he showed relatively clear vitreous to visualize that the retinal infiltrate became inactive in terms of infection. The scarring retinal lesion in the inferotemporal quadrant had vitreoretinal adhesion and caused a retinal fold by vitreoretinal traction which passed inferior to the macula (Fig. 4a-d). One month later, 4 months after the ophthalmic presentation, the retinal fold became flat to maintain the macular attachment (Fig. 4e-h). He was healthy and followed every 2 weeks in outpatient clinics of the regional hospital. At the last follow-up visit, 5 months after the ophthalmic presentation, he maintained the visual acuity of 0.05 in the right eye and had no active intraocular inflammation in the right eye with eye drops of 0.1% fluconazole four times daily, 0.1% betamethasone twice daily, and 0.1% bromfenac twice daily. The left eye was normal with the visual acuity of 1.2 throughout the course. Later in a month, he died of liver failure at home.
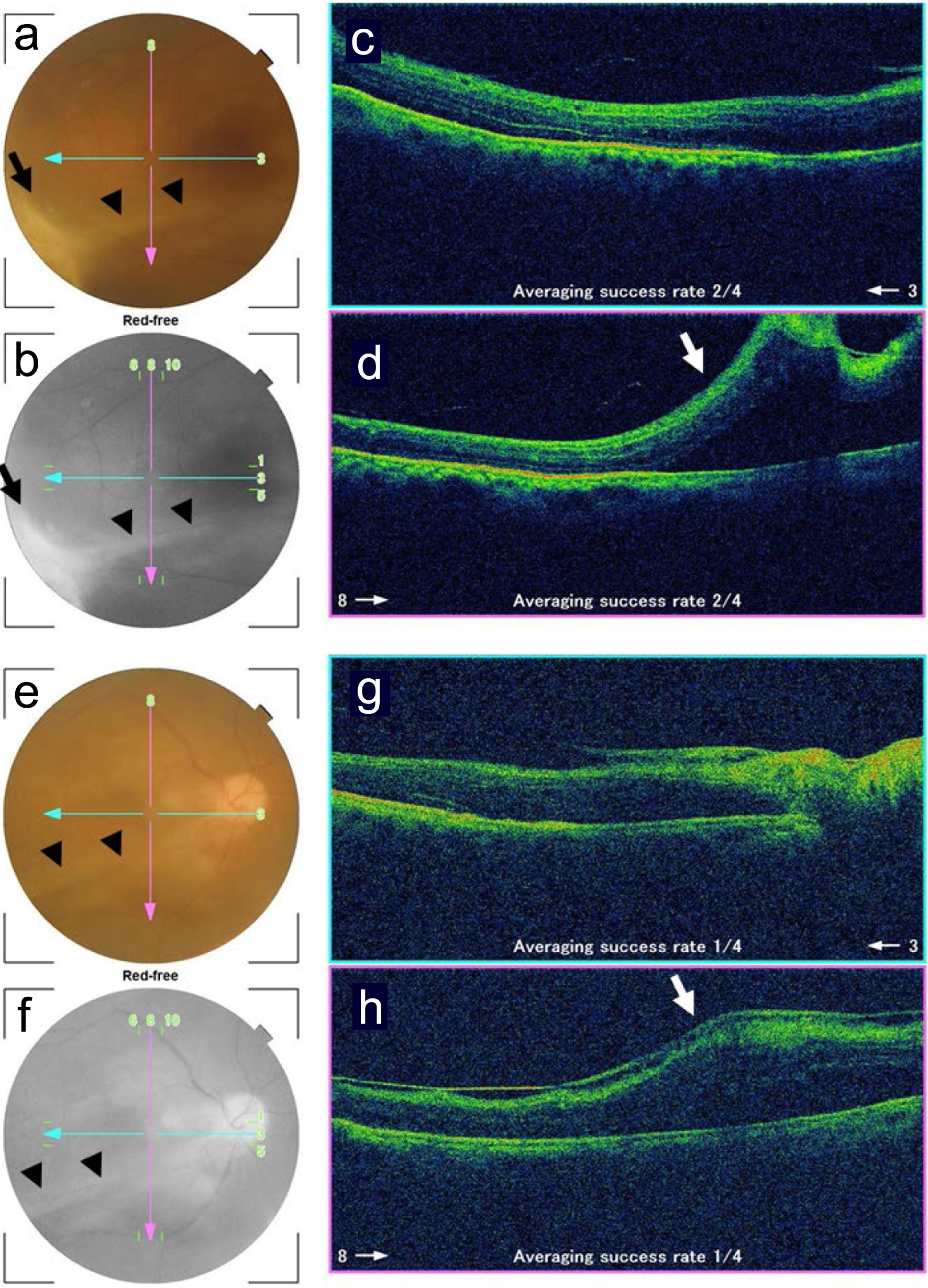 Click for large image | Figure 4. Fundus photographs and optical coherence tomography in the right eye 3.5 months (a-d) and 5 months (e-h) after the initial ophthalmic presentation. a and e: color photographs; b and f: red-free photographs; c and g: horizontal sections designated by blue arrows in fundus photographs; d and h: vertical sections designated by pink arrows in fundus photographs. Note clear vitreous and scarring retinal infiltrate (arrows in a, b) with vitreoretinal adhesion resulting in retinal fold (arrowheads in a, b, e, f, arrows in d, h) inferior to the macula. The retinal fold (arrowheads in e, f) becomes flat in the time course. |
| Discussion | ▴Top |
In the clinical diagnosis of endogenous fungal endophthalmitis in the right eye, he first had a 2-week course of intravenous fluconazole 50 mg daily and then took oral fluconazole 50 mg daily as a minimal dose in the end-stage liver and kidney diseases. In the ophthalmic aspect, the retinal infiltrate remained active and became larger, and the vitreous opacity increased in the right eye in the time course of oral fluconazole administration. He was using fluconazole eye drops eight times daily, together with anti-inflammatory eye drops, 0.1% betamethasone and 0.1% bromfenac. The deteriorating condition of fungal endophthalmitis raised a suggestion that vitrectomy could be a better intervention for local control of the closed-space infection and hence the protection of the vision. Vitrectomy could not be done at this regional hospital which was equipped only with a cataract surgery machine [16]. Furthermore, the patient did not want to be referred to a large hospital with vitrectomy machine. We thus, decided to proceed to intravitreal fluconazole injection every 2 weeks, concurrent with the nephrostomy tube exchange at this regional hospital.
Regarding the dose of intravitreal injection of fluconazole, fluconazole intravenous injection solution (50 mg/50 mL = 1 mg/1 mL) was administered in the volume of 0.2 - 0.3 mL since he had a low level of intraocular pressure. The amount of fluconazole in the vitreous cavity was calculated to be 0.2 - 0.3 mg, which is within the limit of safety [17]. Fluconazole is known to have high penetration to the tissue, and thus was used concurrently as eye drops to expect the penetration into the vitreous through the conjunctiva and sclera [18]. The concentration of topical fluconazole was based on empirical practice for fungal and amoebic keratitis [19]. Recently, recommended treatment for fungal endophthalmitis is intravitreal voriconazole injection which is repeated every 24 h [20]. Under the circumstances, repeating intravitreal fluconazole injection every 2 weeks would be a low-dose treatment and might not be recommended to other clinicians just on the base that it worked for this particular patient.
This patient was physically independent in terms of activities in daily living and wished to live together with his family at home in the rural area. He was stable in end-stage liver disease with multiple hepatic cancer as a consequence of hepatitis C virus-related liver cirrhosis. He sometimes developed ascites and underwent peritoneal puncture. About 2 years before the present episodes of fever, he experienced pulmonary abscess which left behind a nodular shadow in the right upper lobe. Immunosuppressive state caused by hepatic cancer and diabetes mellitus predisposed this patient to develop pulmonary abscess. These predisposing factors also contributed to the development of bloodstream fungal infection.
The sequence of events is summarized in the present episodes of fever. In the first phase, 1 month before the development of eye symptoms, he suffered from ureterolithiasis and had a nephrostomy tube inserted at a different large hospital to control urinary tract infection probably of bacterial origin. In the second phase at the time of eye symptoms, urinary tract infection by fungi would result in bloodstream infection due probably to the displacement of the nephrostomy tube. Candida species, as a probable pathogen in this patient, are part of resident microbiota in the urinary tract [21, 22] and would become pathogenic when urinary flow has been once obstructed. Unfortunately, in this patient, fungi including Candida species were not cultured from urine and antifungal sensitivity test was not done. He had low-grade fever and appetite loss, together with elevated CRP again. Based on his wish to be cared at a regional hospital, exchange and reinsertion of a new nephrostomy tube were performed by a urologist at this regional hospital. This intervention led successfully to better urinary flow, and thus to his better physical status.
The patient became physically healthy and regained the appetite by nephrostomy tube replacement to control urinary tract infection and bloodstream infection. Oral fluconazole 50 mg daily for a month could eradicate the bloodstream infection probably by Candida and did not hurt anymore the poor status of end-stage kidney disease. The retinal infiltrate could not respond to the low dose of oral fluconazole but responded well to repeated intravitreal fluconazole injection. The vitreous opacity cleared completely and the retinal infiltrate resulted in retinal degeneration with localized retinal folds caused by vitreoretinal adhesion. In the case that the dose of oral fluconazole cannot be increased due to the poor renal function, intravitreal injection of fluconazole is a treatment option to assist the systemic administration.
Conclusions
The course in the present patient illustrates the role of a regional hospital in the rural area. The patient was basically healthy at home with family members and was followed by internists at the regional hospital for the care and treatment for liver cirrhosis with multiple hepatic cancer and diabetes mellitus. This regional hospital has urology clinic and eye clinic with a part-time urologist and ophthalmologist, respectively. The urologist exchanged the nephrostomy tube, leading to better urine excretion, and hence, to better control of systemic infection while the ophthalmologist repeated intravitreal fluconazole injection to control local infection in the right eye. The regional hospital is equipped with CT scan, X-rays, MRI, and clinical laboratories. Based on the patient’s wish to be at home and to be treated at this regional hospital, internists and part-time doctors with specialties collaborated to accomplish better care and treatment.
Learning points
Intravitreal fluconazole injection for fungal endophthalmitis, particularly in patients with end-stage liver and kidney diseases, represents a unique and challenging treatment option. Fungal endophthalmitis is a rare but severe infection that can cause significant visual loss. It often occurs in patients with predisposing factors such as intravenous drug abuse, indwelling venous access devices, or prolonged use of antibiotics, corticosteroids, or immunosuppressive drugs. Intravitreal fluconazole injection, as a valuable treatment option for fungal endophthalmitis, should be carefully evaluated and managed in consultation with a multidisciplinary team to ensure the best possible outcomes for the patient.
Acknowledgments
None to declare.
Financial Disclosure
The authors receive no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent
Written consent was obtained from the patient for his anonymized information to be published in this article.
Author Contributions
TM, as an ophthalmologist, examined and treated the patient and wrote the manuscript. YK and SN, as urologists, exchanged nephrostomy tubes, NY, YT, and YI, as internists, followed and treated the patient. All authors approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Birnbaum F, Gupta G. Endogenous endophthalmitis: diagnosis and treatment. EyeNext Magazine. 2016;June:33-35.
- Gurnani B, Kaur K. Endogenous endophthalmitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024.
pubmed pmc - Durand ML. Bacterial and fungal endophthalmitis. Clin Microbiol Rev. 2017;30(3):597-613.
doi pubmed pmc - Sadiq MA, Hassan M, Agarwal A, Sarwar S, Toufeeq S, Soliman MK, Hanout M, et al. Endogenous endophthalmitis: diagnosis, management, and prognosis. J Ophthalmic Inflamm Infect. 2015;5(1):32.
doi pubmed pmc - Gajdzis M, Figula K, Kaminska J, Kaczmarek R. Endogenous endophthalmitis - the clinical significance of the primary source of infection. J Clin Med. 2022;11(5):1183.
doi pubmed pmc - Matsuo T, Nakagawa H, Matsuo N. Endogenous aspergillus endophthalmitis associated with periodontitis. Ophthalmologica. 1995;209(2):109-111.
doi pubmed - Matsuo T, Notohara K, Shiraga F, Yumiyama S. Endogenous amoebic endophthalmitis. Arch Ophthalmol. 2001;119(1):125-128.
pubmed - Haseeb AA, Elhusseiny AM, Siddiqui MZ, Ahmad KT, Sallam AB. Fungal endophthalmitis: a comprehensive review. J Fungi (Basel). 2021;7(11):996.
doi pubmed pmc - Berdal JE, Haagensen R, Ranheim T, Bjornholt JV. Nosocomial candidemia; risk factors and prognosis revisited; 11 years experience from a Norwegian secondary hospital. PLoS One. 2014;9(7):e103916.
doi pubmed pmc - Fisher JF, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infections—treatment. Clin Infect Dis. 2011;52(Suppl 6):S457-S466.
doi pubmed - Peman J, Ruiz-Gaitan A. Candidemia from urinary tract source: the challenge of candiduria. Hosp Pract (1995). 2018;46(5):243-245.
doi pubmed - Odabasi Z, Mert A. Candida urinary tract infections in adults. World J Urol. 2020;38(11):2699-2707.
doi pubmed - Abdeljaleel OA, Alnadhari I, Mahmoud S, Khachatryan G, Salah M, Ali O, Shamsodini A. Treatment of renal fungal ball with fluconazole instillation through a nephrostomy tube: case report and literature review. Am J Case Rep. 2018;19:1179-1183.
doi pubmed pmc - Lieb MW, Dennison JJ, Mubarik A. Fungal pyelonephritis and fungemia due to obstructive uropathy. WMJ. 2022;121(2):E27-E30.
pubmed - Elbaz M, Chikly A, Meilik R, Ben-Ami R. Frequency and clinical features of Candida bloodstream infection originating in the urinary tract. J Fungi (Basel). 2022;8(2):123.
doi pubmed pmc - Matsuo T, Iguchi M, Morisato N, Murasako T, Hagiya H. Are prophylactic systemic antibiotics required in patients with cataract surgery at local anesthesia? Int J Environ Res Public Health. 2022;19(23):15796.
doi pubmed pmc - Schulman JA, Peyman G, Fiscella R, Small G, Coats M, Wajszczuk CP, Steahly L. Toxicity of intravitreal injection of fluconazole in the rabbit. Can J Ophthalmol. 1987;22(6):304-306.
pubmed - Abbasoglu OE, Hosal BM, Sener B, Erdemoglu N, Gursel E. Penetration of topical fluconazole into human aqueous humor. Exp Eye Res. 2001;72(2):147-151.
doi pubmed - Matsuo T, Nose M. A simple method for culturing Acanthamoeba from soft contact lens at a clinical laboratory of a hospital: case report of Acanthamoeba keratitis. Clin Case Rep. 2023;11(11):e8248.
doi pubmed pmc - Dave VP, Pappuru RR, Pathengay A, Gupta R, Joseph J, Sharma S, Das T. Aspergillus endophthalmitis: clinical presentations and factors determining outcomes. Asia Pac J Ophthalmol (Phila). 2020;9(1):9-13.
doi pubmed pmc - Perez-Carrasco V, Soriano-Lerma A, Soriano M, Gutierrez-Fernandez J, Garcia-Salcedo JA. Urinary microbiome: yin and yang of the urinary tract. Front Cell Infect Microbiol. 2021;11:617002.
doi pubmed pmc - Ackerman AL, Underhill DM. The mycobiome of the human urinary tract: potential roles for fungi in urology. Ann Transl Med. 2017;5(2):31.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Medical Cases is published by Elmer Press Inc.


